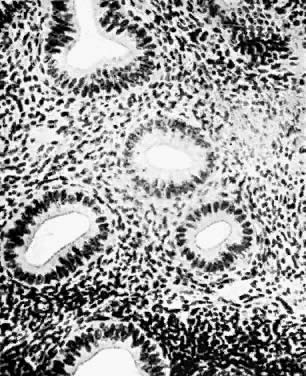
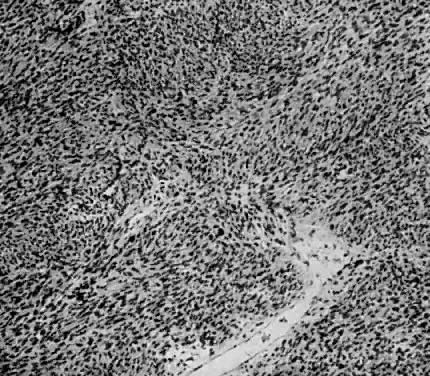
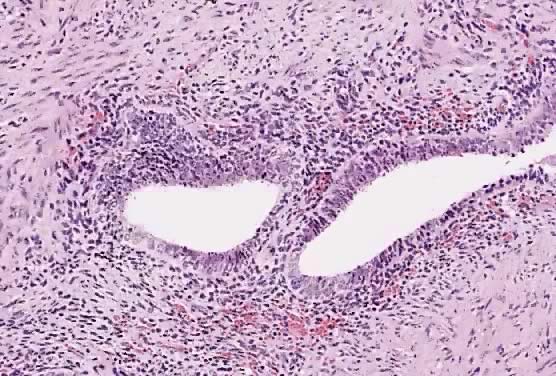
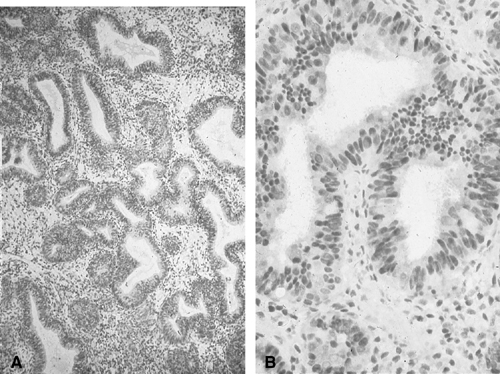
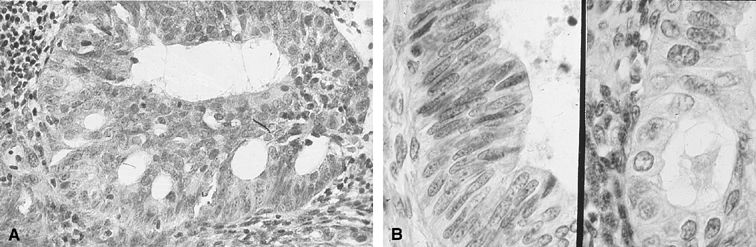
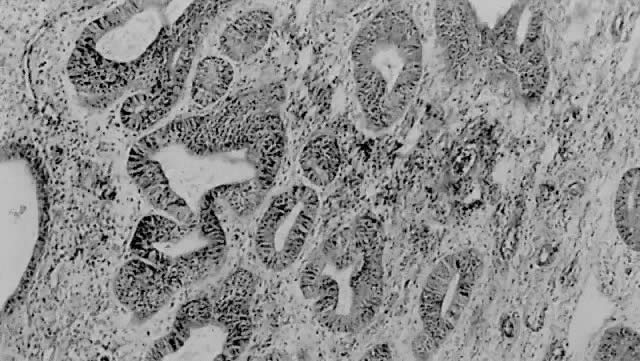
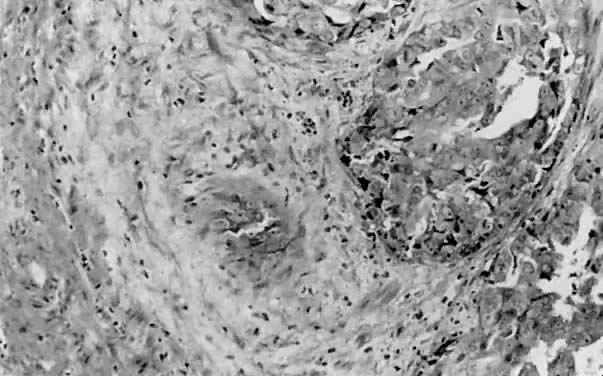
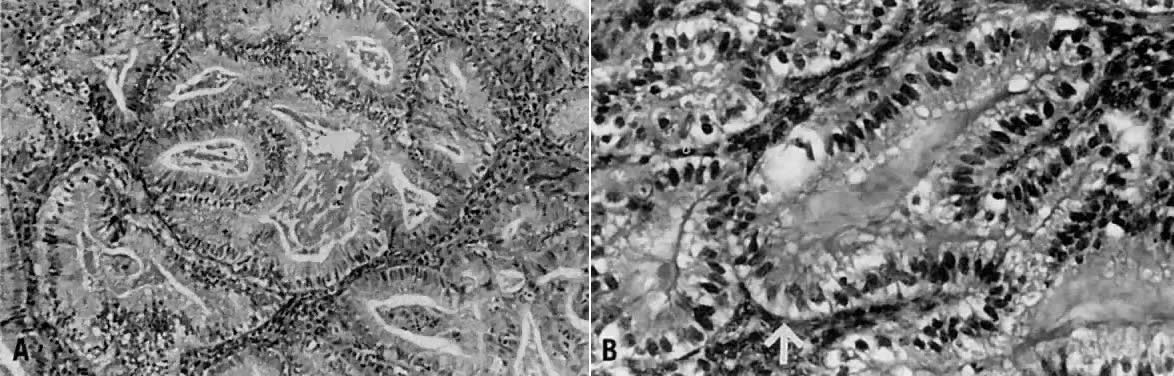
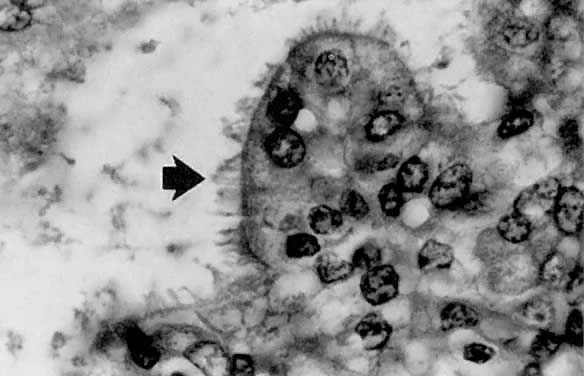
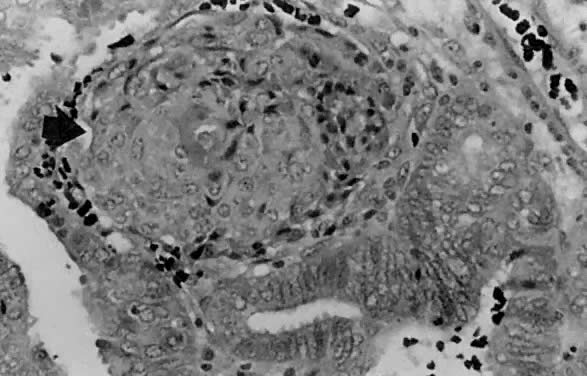
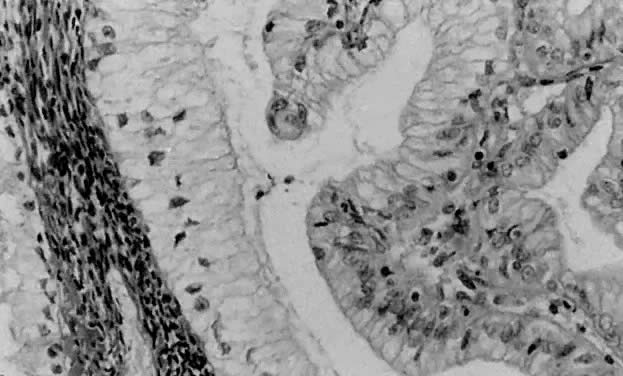
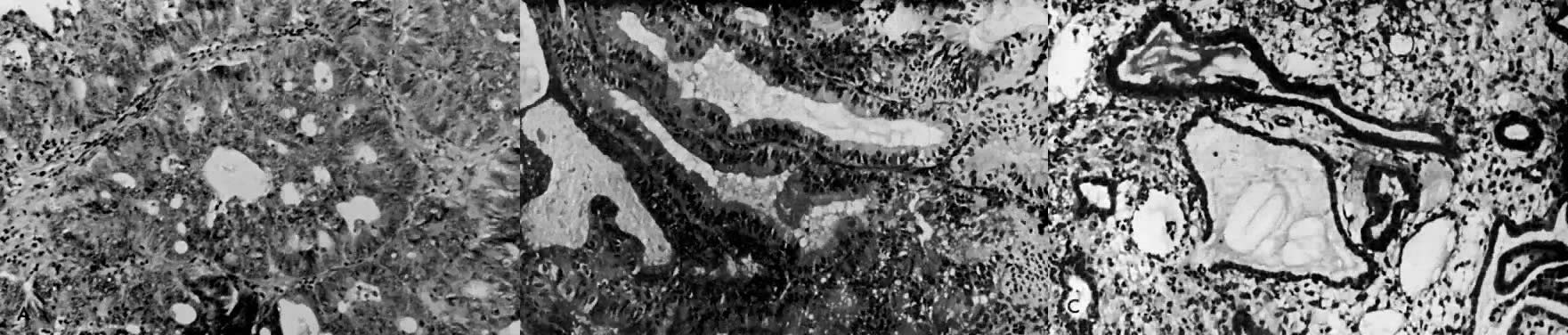
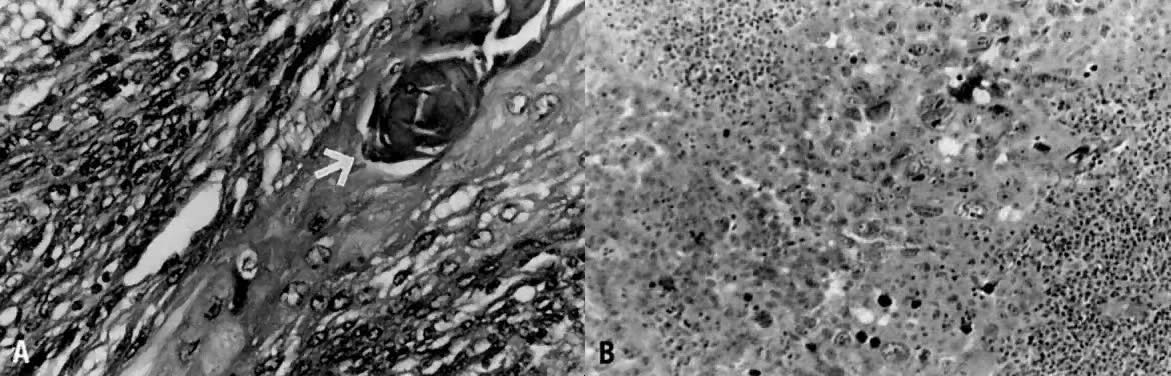
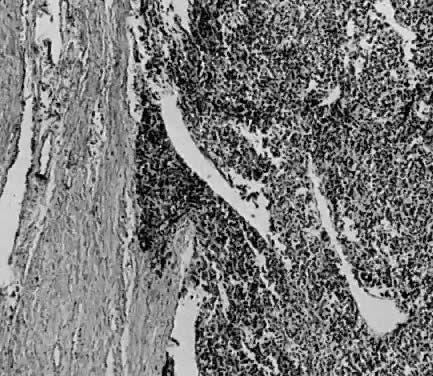
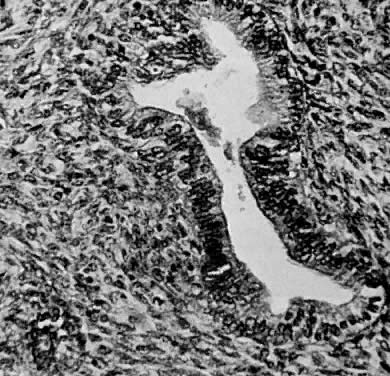
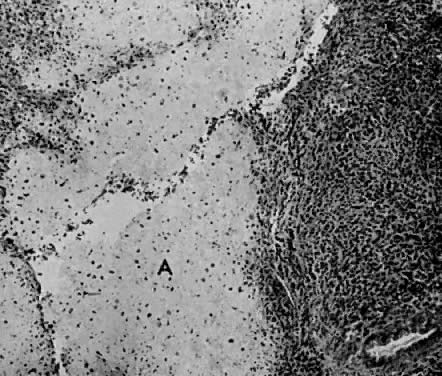
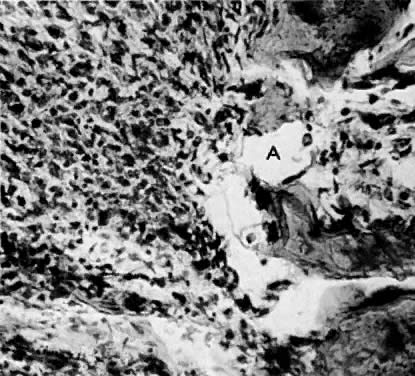
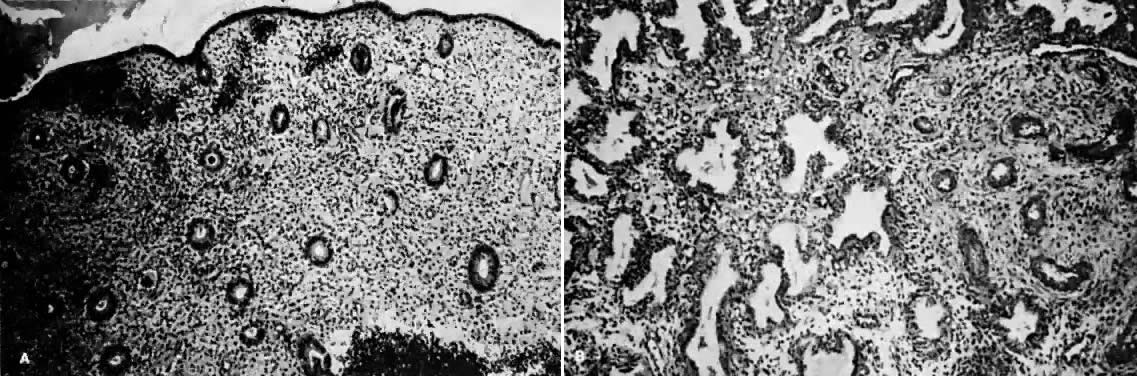
 Fig. 1. A. Early proliferative endometrium (days 3–6).
Surface epithelium is intact. Glands are straight and tubular without
mitotic figures or pseudostratification. This normal endometrium was exposed
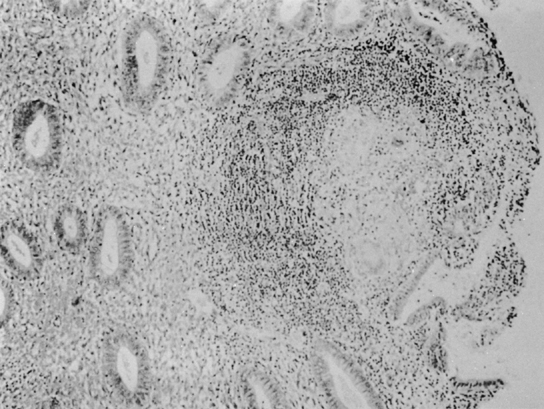
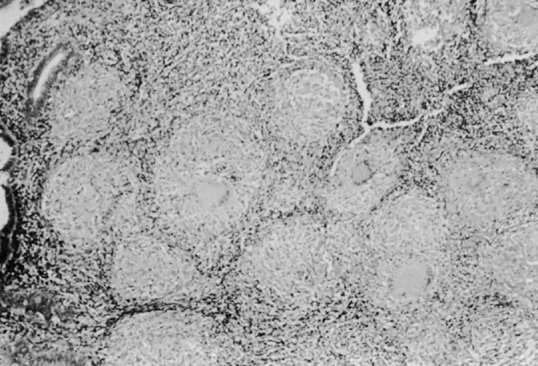
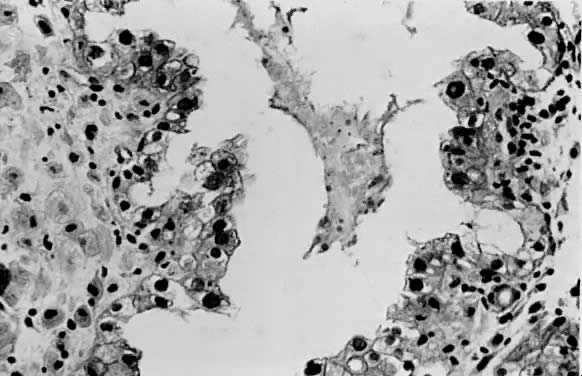
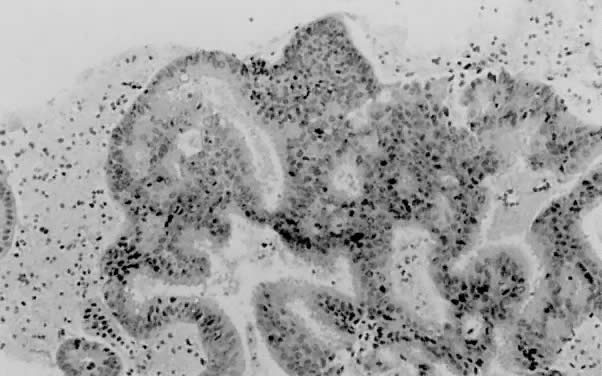
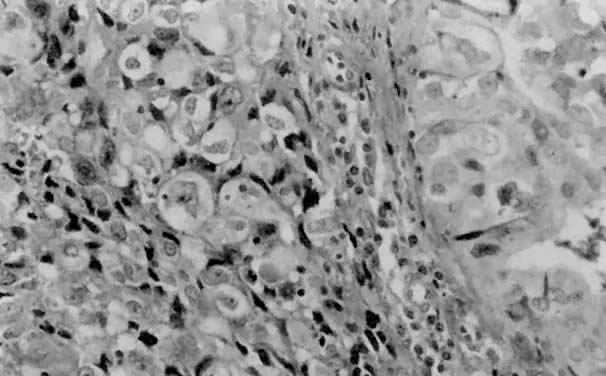
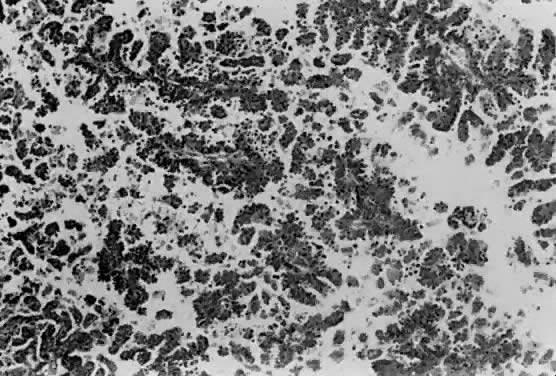
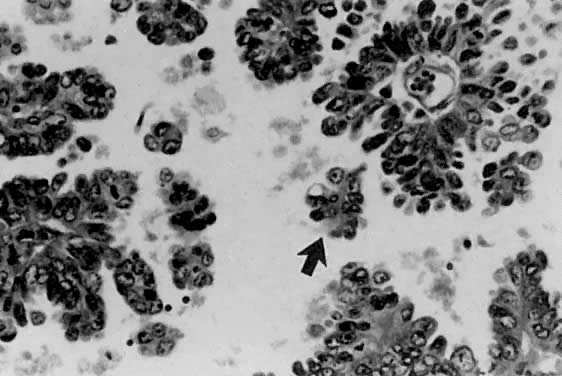
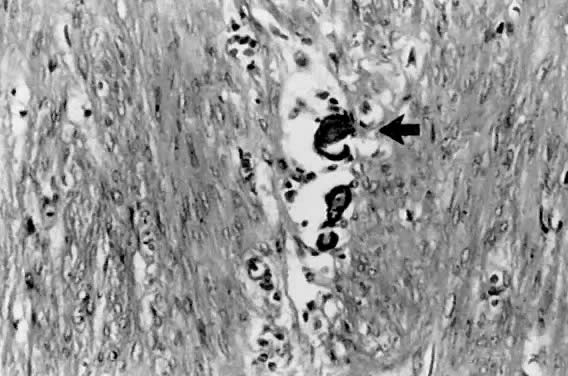
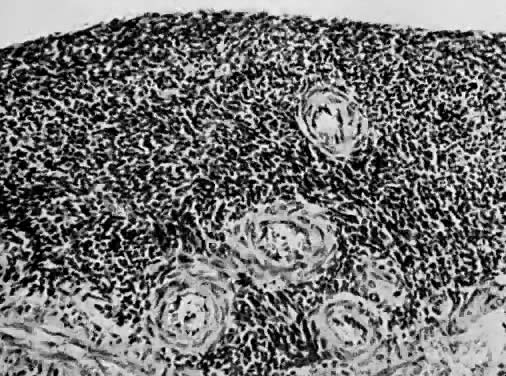
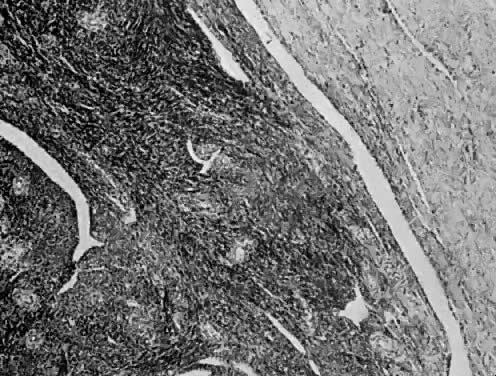
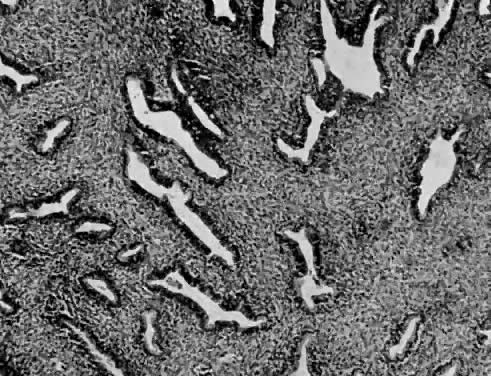
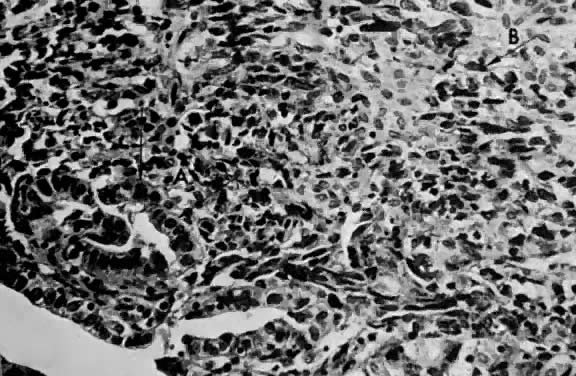
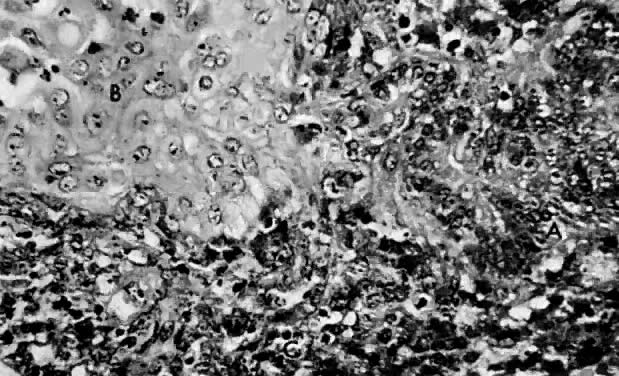
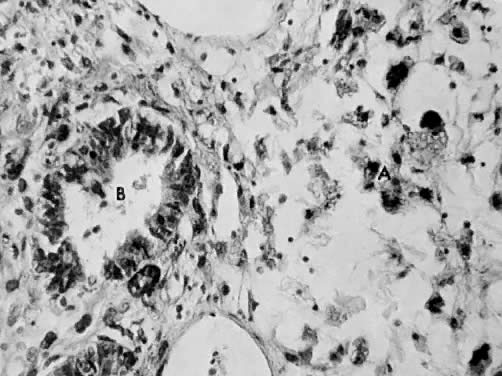
only to estrogen stimulation at the time of biopsy. B. Late secretory
endometrium (days 25–26) in a normal menstrual cycle. Tissue has
been predominantly stimulated by progesterone for 11 to 12 days. Glands
are convoluted and have expended most of their secretory products. The
stroma has undergone an extensive decidual reaction. Volume
4, Chapter 14
Fig. 1. A. Early proliferative endometrium (days 3–6).
Surface epithelium is intact. Glands are straight and tubular without
mitotic figures or pseudostratification. This normal endometrium was exposed
only to estrogen stimulation at the time of biopsy. B. Late secretory
endometrium (days 25–26) in a normal menstrual cycle. Tissue has
been predominantly stimulated by progesterone for 11 to 12 days. Glands
are convoluted and have expended most of their secretory products. The
stroma has undergone an extensive decidual reaction. Volume
4, Chapter 14