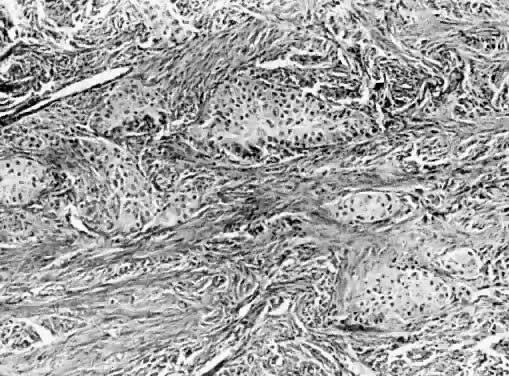
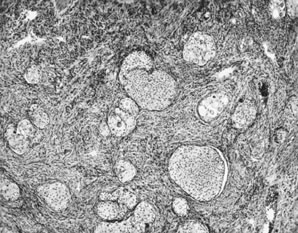
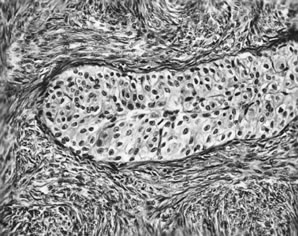
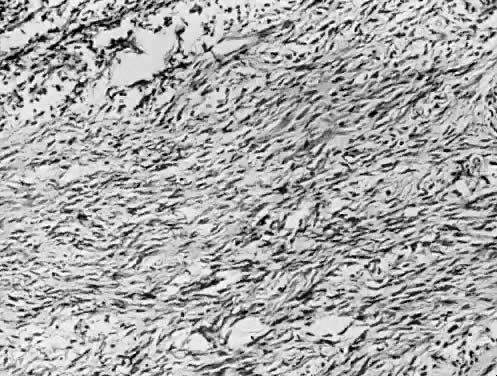
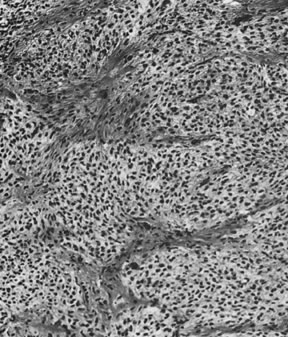
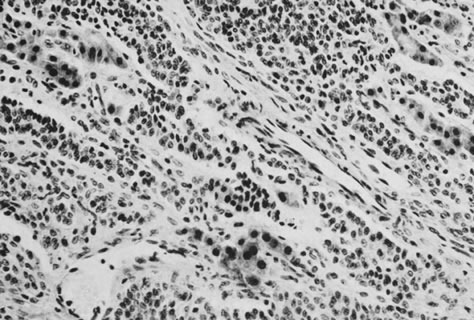
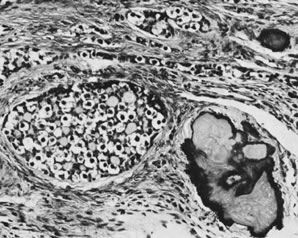
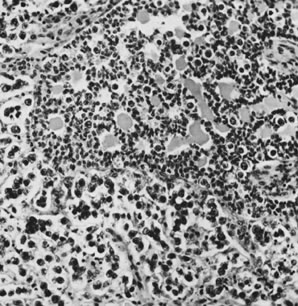
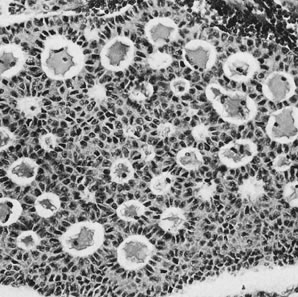
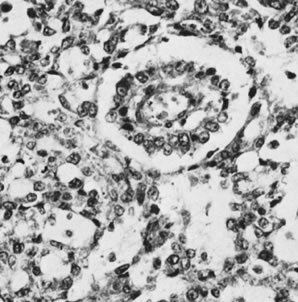
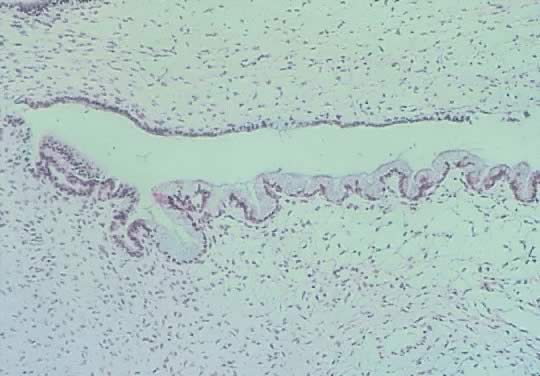
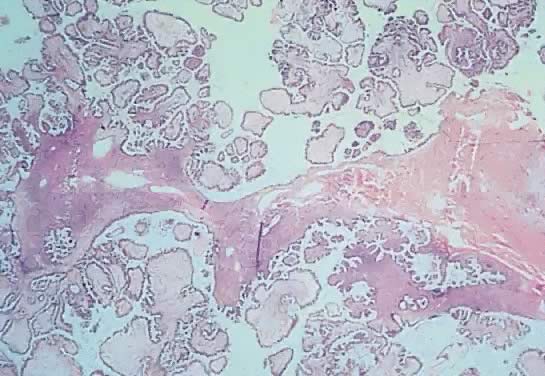
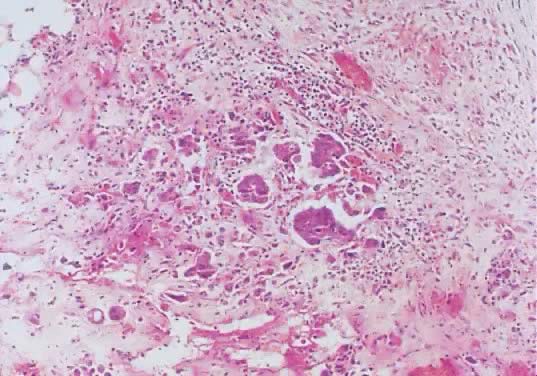
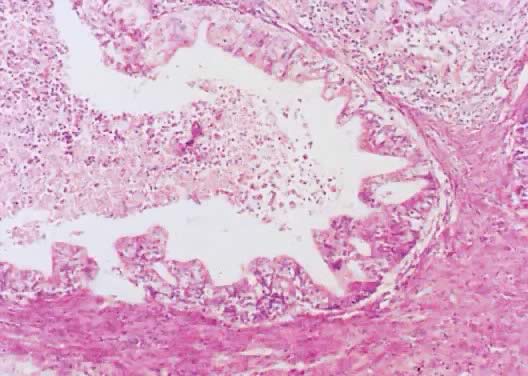
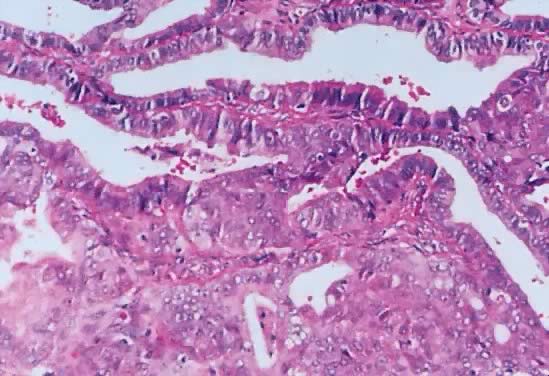
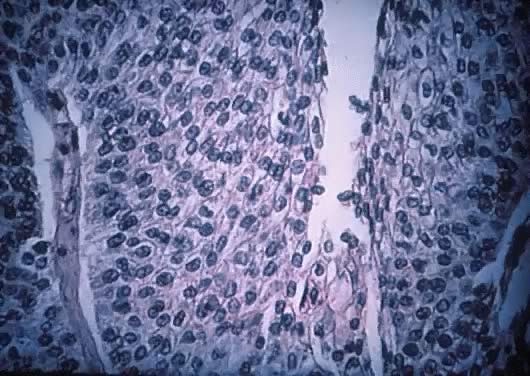
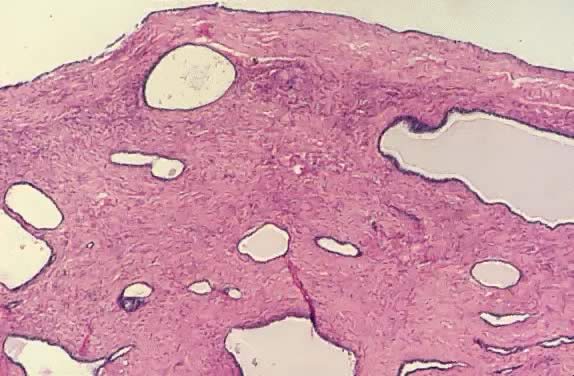
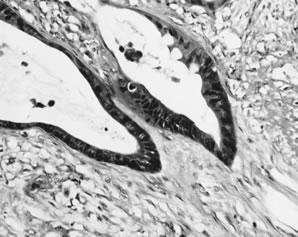
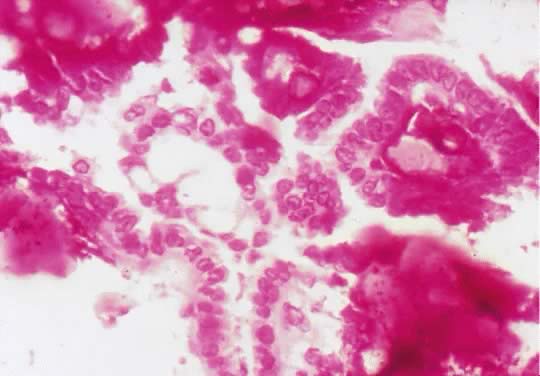
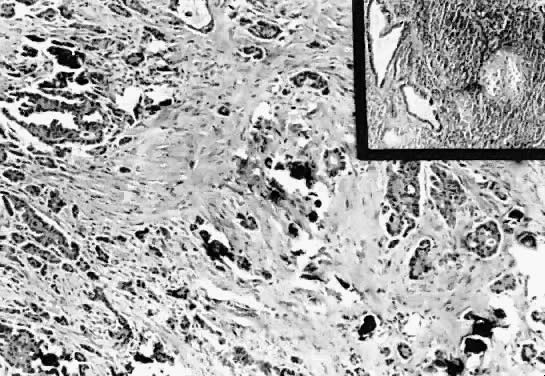
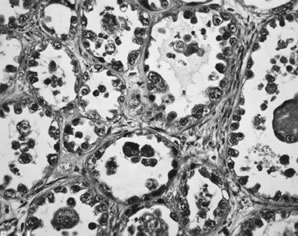
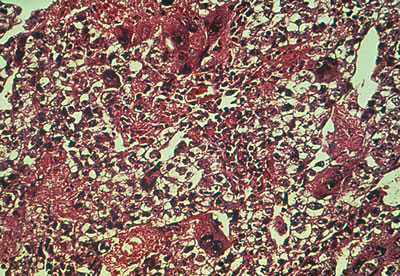
 Fig. 13. Photomicrograph (low power) of the cortex of the ovary of a human infant. The
cortex of the ovary has numerous primordial germ cells with relatively
little stroma. The ovarian stroma is more abundant in the medulla, where
the larger follicles are seen. Volume 1, Chapter 2.
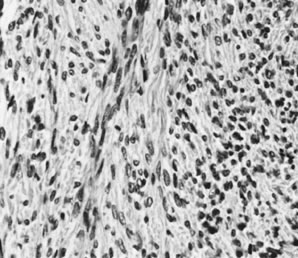
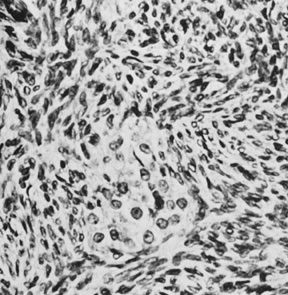
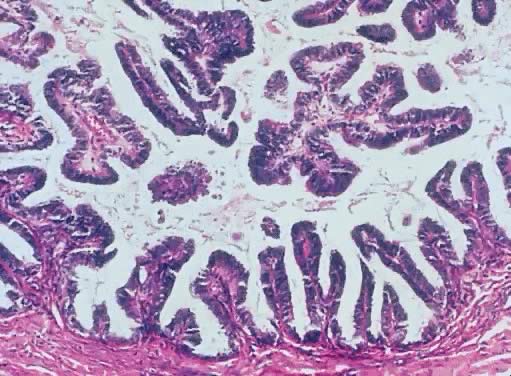
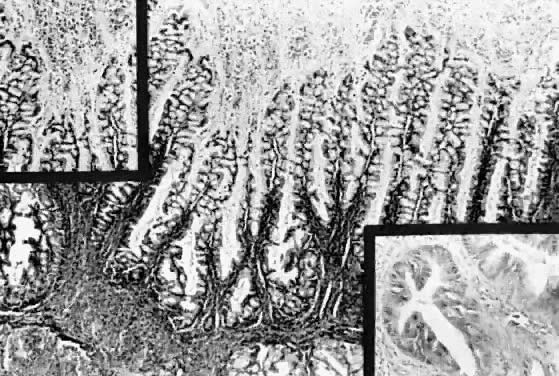
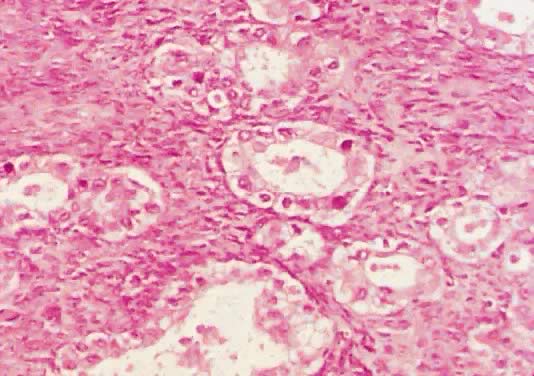
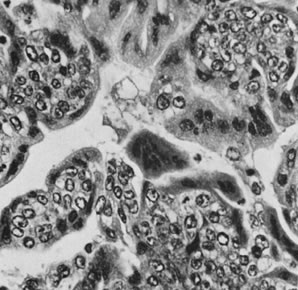
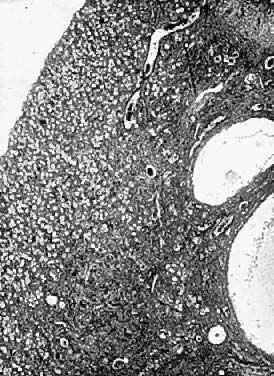
Fig. 13. Photomicrograph (low power) of the cortex of the ovary of a human infant. The
cortex of the ovary has numerous primordial germ cells with relatively
little stroma. The ovarian stroma is more abundant in the medulla, where
the larger follicles are seen. Volume 1, Chapter 2.
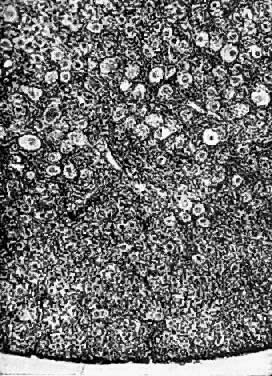
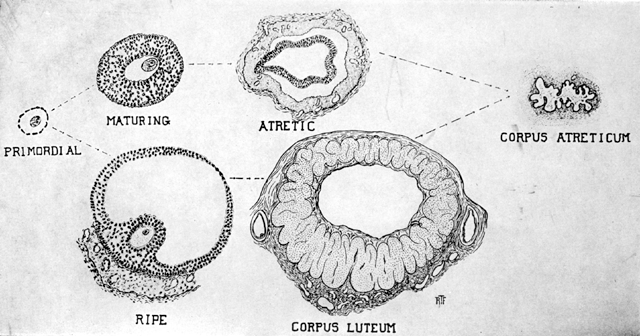
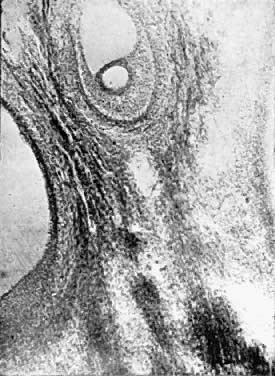
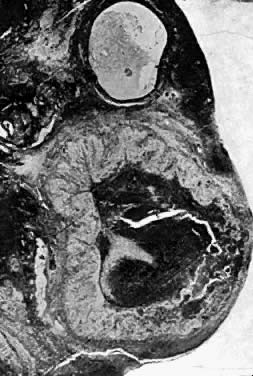
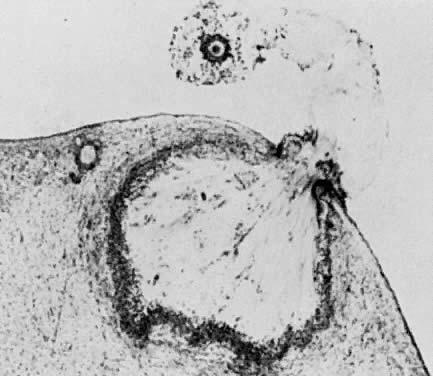

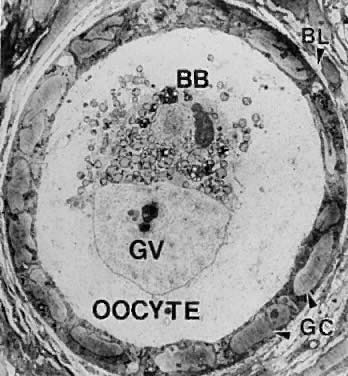
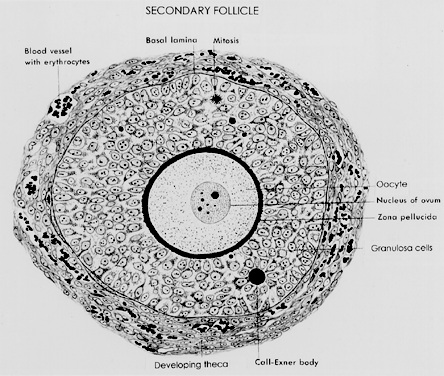
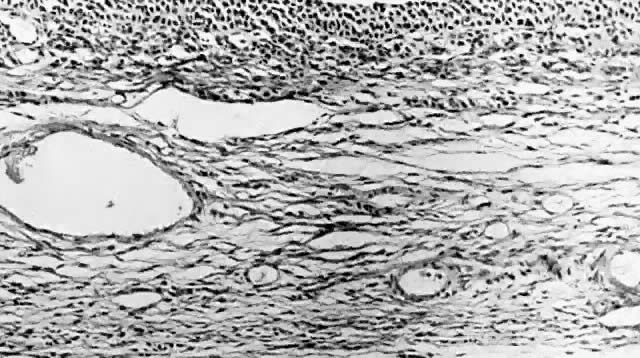
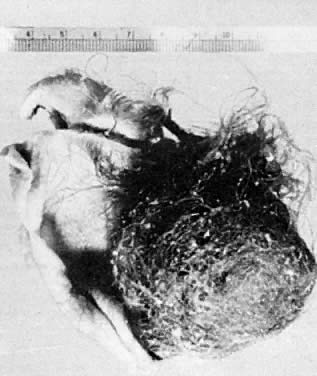
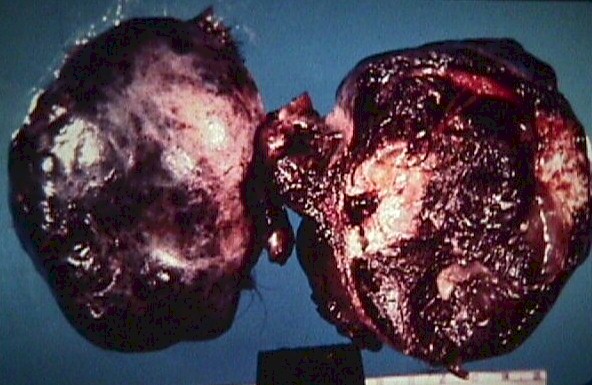
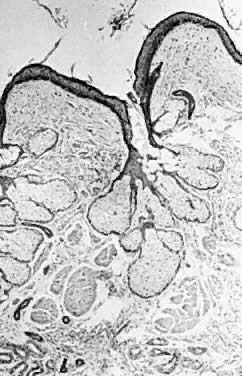
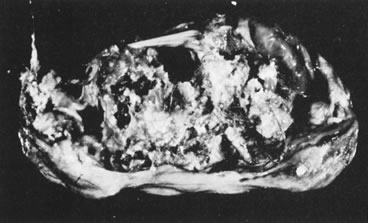
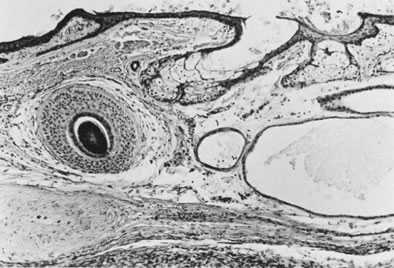
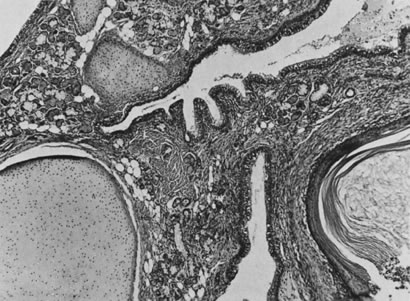
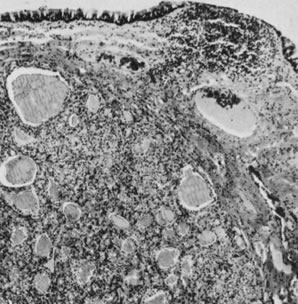
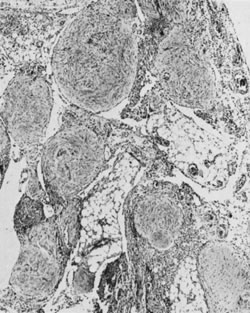
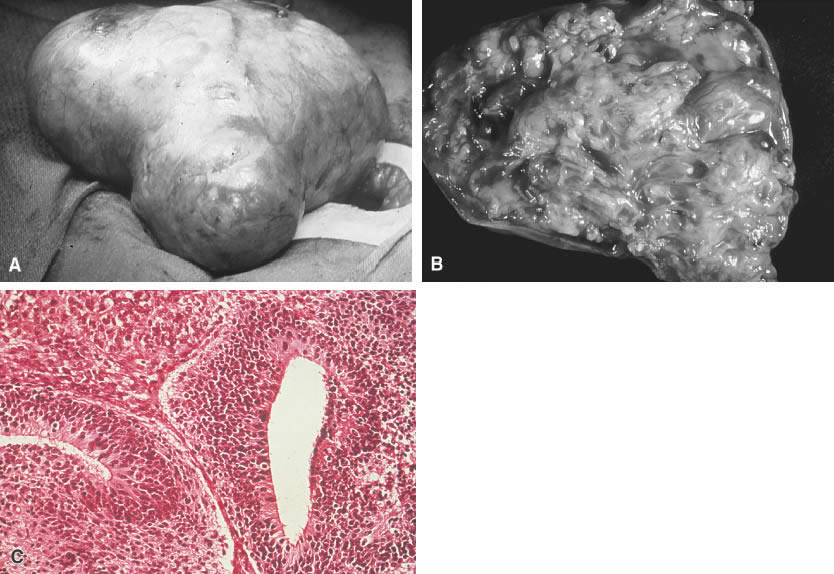
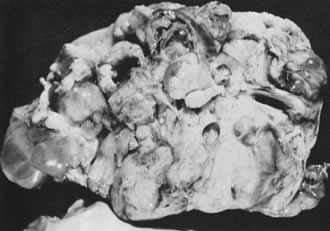
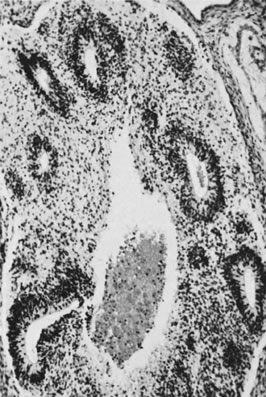
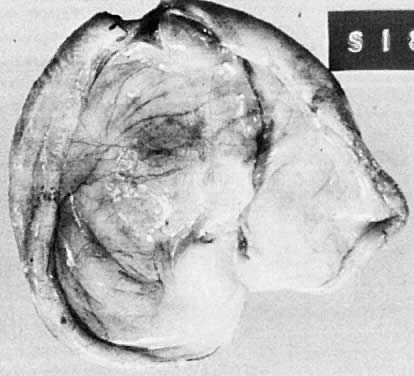
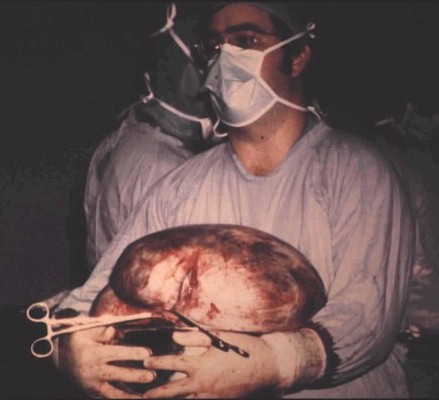
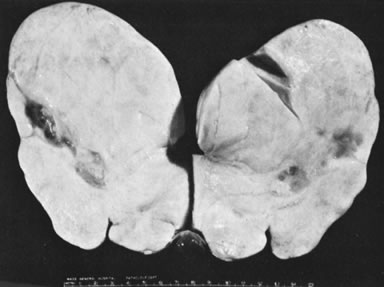
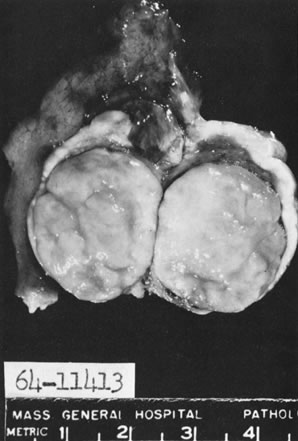
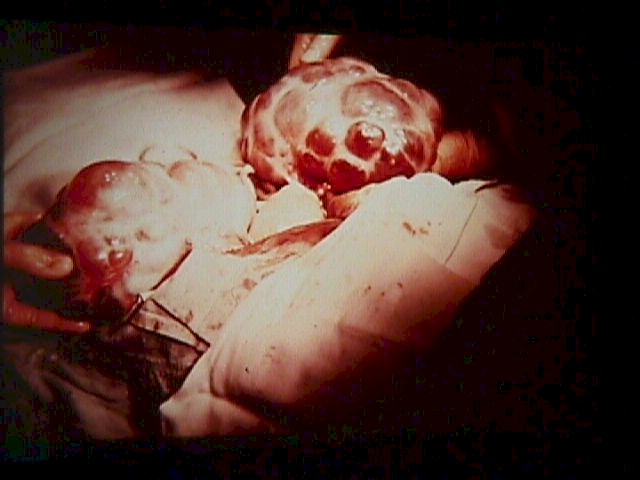
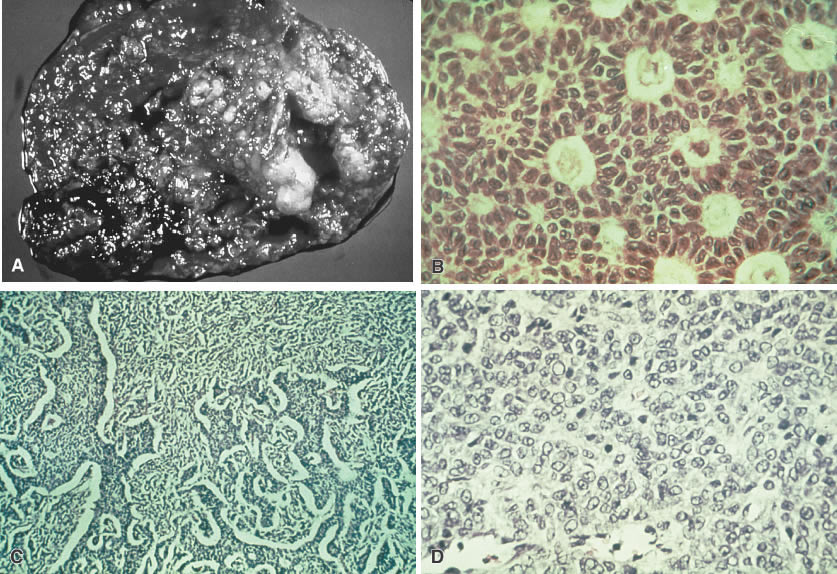
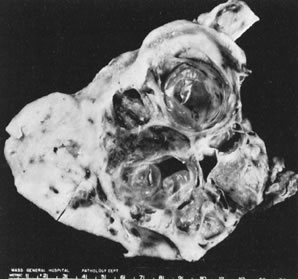
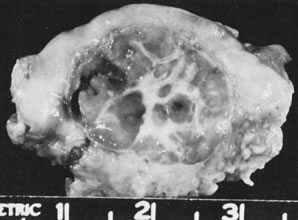
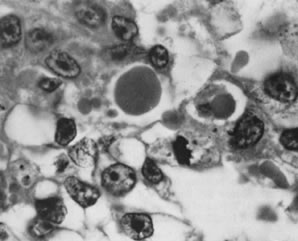
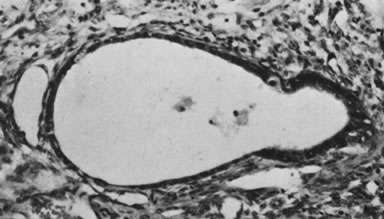
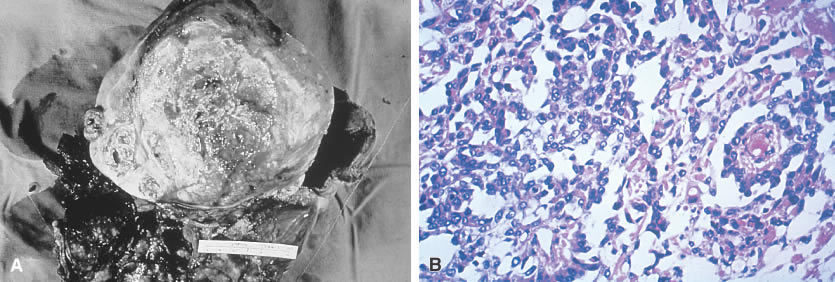
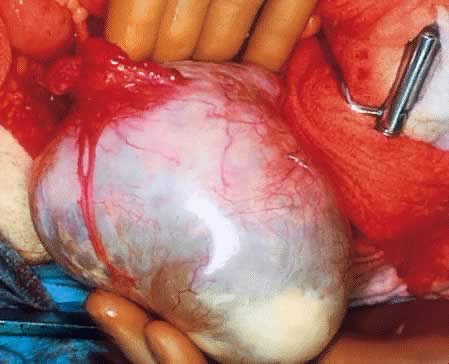
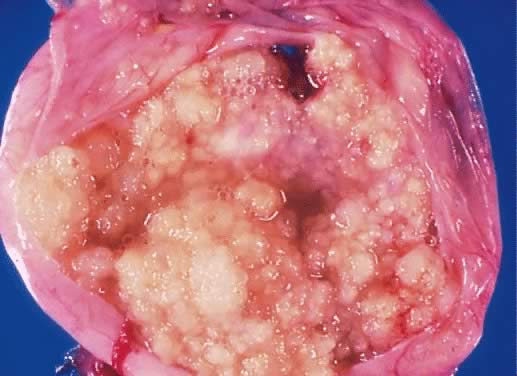
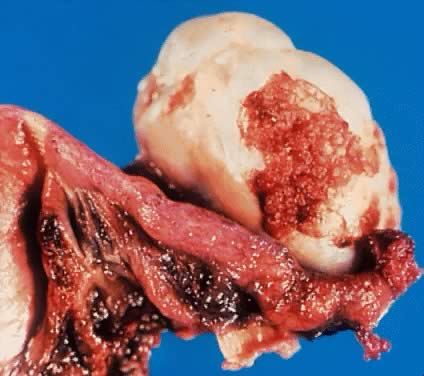
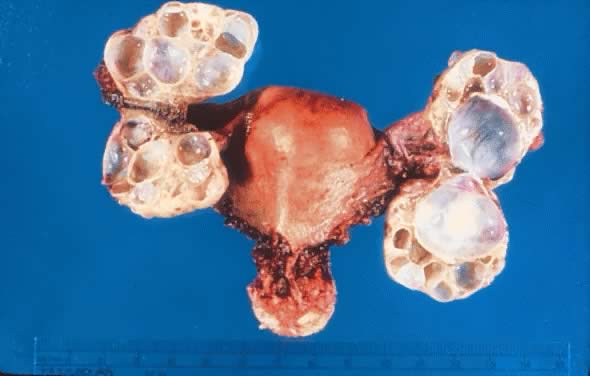
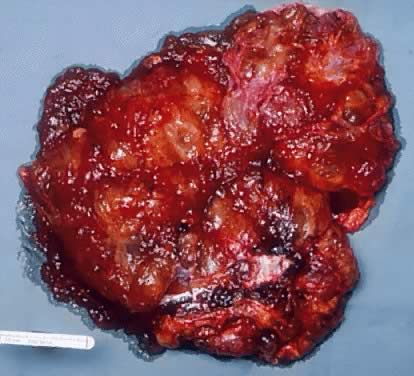
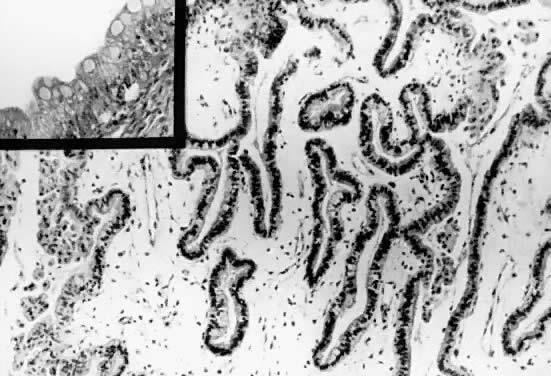
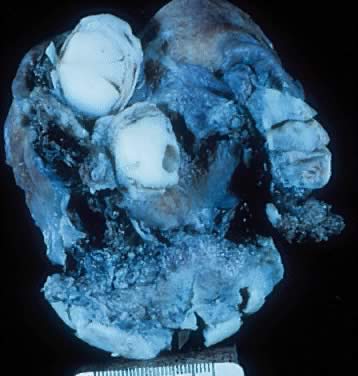
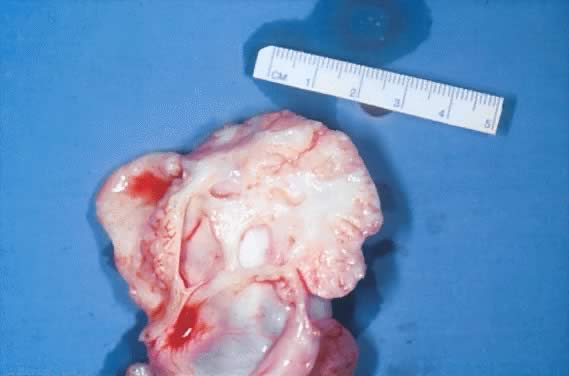
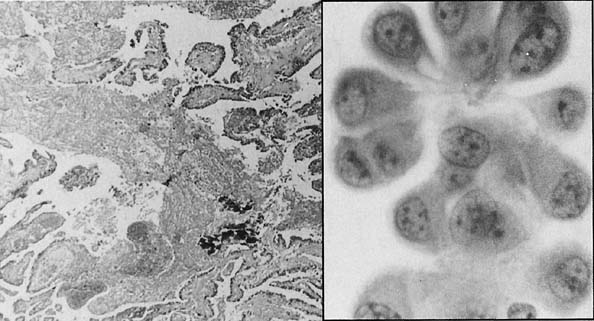
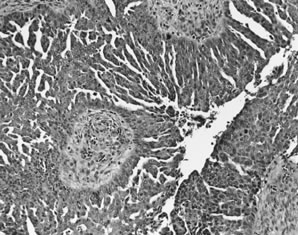
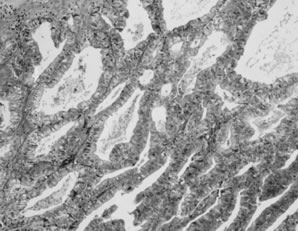
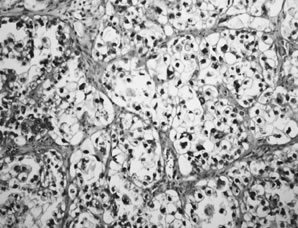
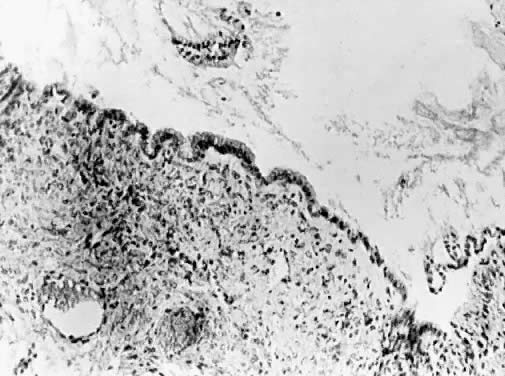 Fig. 5.
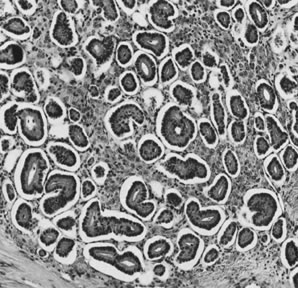
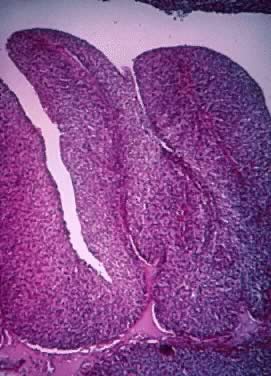
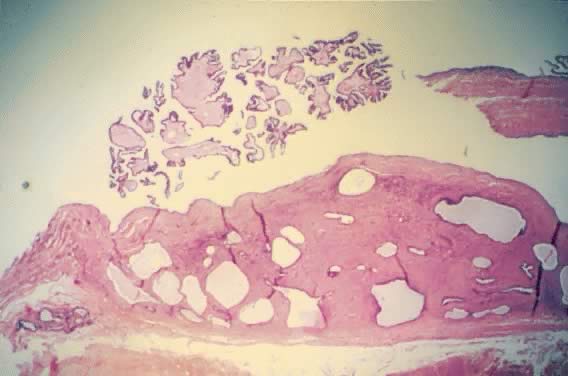
Fig. 5.