Much of the beauty of soft tissue pelvic architecture derives from the
abilities of the organs of the three primary systems in this area—urinary, reproductive, and rectal (gastrointestinal)—to function
independently of one another. Each is capable of the limits of its
normal range of function without permanent alteration of the anatomy or
function of its neighbors, the organs are capable of independent expansion
and contraction. There are connective tissue spaces between these organs that permit this
relatively independent function.25 These spaces are divided by connective tissue septa that not only afford
mechanical support but also provide the physical routes of blood vessels, lymphatics, and
nerve tissues to and from the pelvic organs. These
structures are contained within the septa along reasonably constant
routes and do not trespass on the connective tissue spaces. Although
their location is quite regular within the septa, individual variations
as to the site of origin and their relative size are occasionally seen. The
anatomic ligaments form natural barriers to the spread of infection, cancer, and
hematomas. The septa, on the other hand, through their
blood vessels and lymphatics, form natural routes for the transmission
of infection and malignancy arising from the pelvic organs. A detailed
knowledge of the anatomy of these spaces and partitioning septa
is essential to the understanding of their actual and potential functional
importance in both health and disease. From accurate knowledge and
experience, the surgeon can know not only where to find major vessels
and so avoid unnecessary blood loss but also how to avoid unnecessary
surgical penetration of adjacent organs. To the oncologic surgeon, this
anatomic knowledge helps to demarcate the likely limits and routes
of direct spread of malignant disease and to determine the extent of
necessary extirpation. To the surgeon concerned with pelvic reconstruction, the
implications are obvious in the need to reestablish original
relationships between the organs. The connective tissue capsules or adventitia of the bladder, birth canal, and
rectum are attached to the pelvis, and at certain points to one
another, by condensation of connective tissue that contain the principal
blood vessels and lymphatics to and from these organs. Although these
septa vary in strength and thickness from person to person, their
relation and position are constant. Potential spaces exist between these septa, and the spaces are filled with
fat and loose alveolar tissue but are essentially free of blood vessels
and lymphatics (Fig. 12, Fig. 13, Fig. 14). These areas become actual spaces only by dissection, but this is easily
accomplished bloodlessly and bluntly once access to the space has
been gained by surgical penetration through a septum.
|
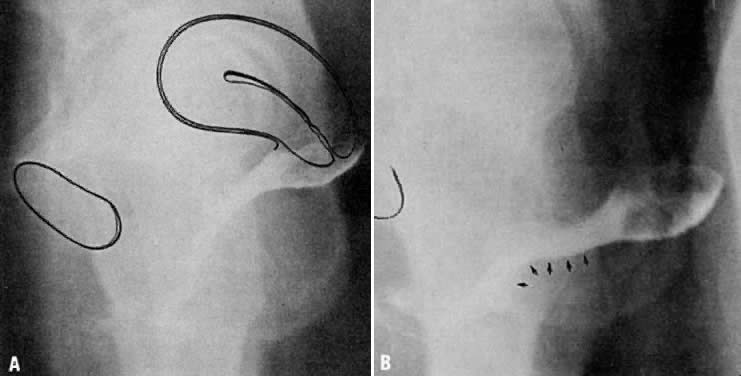
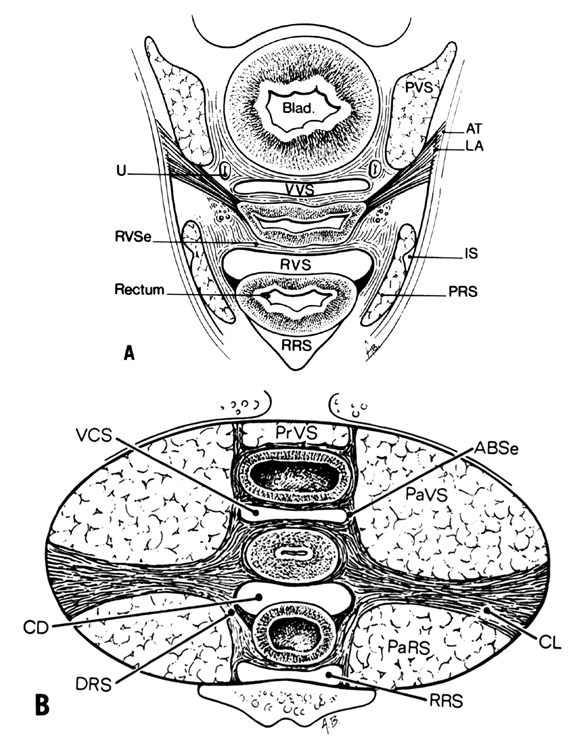 Fig. 12. A. Connective tissue planes and
spaces of the female pelvis. Frontal section through female pelvis
near upper third of vagina. The paravesical (PVS) is shown
lateral to the bladder (Blad.). The vesicovaginal space (VVS)
is seen between the bladder and vagina, and the rectovaginal space
(RVS) is shown between the vagina and the rectum. The paired
pararectal spaces (PRS) are seen lateral to the rectum. Note
that the ischial spines (IS) are found in the lateral wall
of the pararectal spaces. The cardinal ligaments of the vagina (horizontal
connective tissue ground bundle) are shown extending from the sides
of the vagina to the pelvic wall. The tissue fuses laterally to
the connective tissue capsule of the levator ani (LA), which
itself takes origin from the fascia of the obturator internus muscle
along a white line identified as the arcus tendineous (AT).
The rectovaginal septum (RVSe) is noted between the vagina
and the rectovaginal space. The ureters (U) can be seen in
the tissue between the paravesical space and the vesicovaginal space.
Note the retrorectal space (RRS). B. Diagrammatic
cross section of the female pelvis through the cervix. The prevesical
space (PrVS) is seen anterior to the bladder. It is separated
from the paravesical space (PaVS) by the ascending bladder
septum (ABSe). The latter also separates the paravesical
space from the vesicocervical space (VCS). The descending
rectal septum (DRS) separates the retrorectal space (RRS)
from the pararectal space (PaRS). Note the posterior cul-de-sac
(CD) and cardinal ligament (CL). (Adapted
from von Peham H, Amreich J: Operative Gynecology. LK Ferguson (trans):
Philadelphia, JB Lippincott, 1934)
Fig. 12. A. Connective tissue planes and
spaces of the female pelvis. Frontal section through female pelvis
near upper third of vagina. The paravesical (PVS) is shown
lateral to the bladder (Blad.). The vesicovaginal space (VVS)
is seen between the bladder and vagina, and the rectovaginal space
(RVS) is shown between the vagina and the rectum. The paired
pararectal spaces (PRS) are seen lateral to the rectum. Note
that the ischial spines (IS) are found in the lateral wall
of the pararectal spaces. The cardinal ligaments of the vagina (horizontal
connective tissue ground bundle) are shown extending from the sides
of the vagina to the pelvic wall. The tissue fuses laterally to
the connective tissue capsule of the levator ani (LA), which
itself takes origin from the fascia of the obturator internus muscle
along a white line identified as the arcus tendineous (AT).
The rectovaginal septum (RVSe) is noted between the vagina
and the rectovaginal space. The ureters (U) can be seen in
the tissue between the paravesical space and the vesicovaginal space.
Note the retrorectal space (RRS). B. Diagrammatic
cross section of the female pelvis through the cervix. The prevesical
space (PrVS) is seen anterior to the bladder. It is separated
from the paravesical space (PaVS) by the ascending bladder
septum (ABSe). The latter also separates the paravesical
space from the vesicocervical space (VCS). The descending
rectal septum (DRS) separates the retrorectal space (RRS)
from the pararectal space (PaRS). Note the posterior cul-de-sac
(CD) and cardinal ligament (CL). (Adapted
from von Peham H, Amreich J: Operative Gynecology. LK Ferguson (trans):
Philadelphia, JB Lippincott, 1934)
|
|
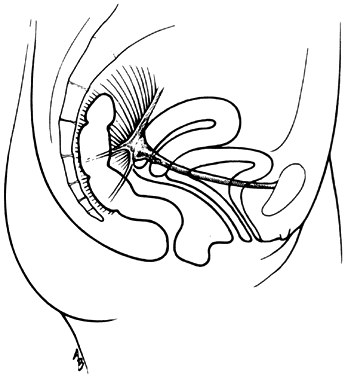
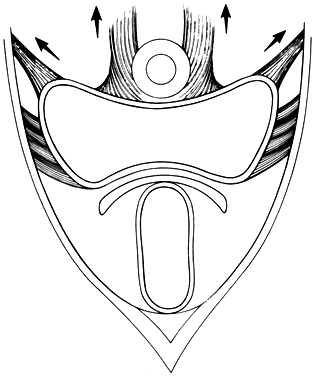
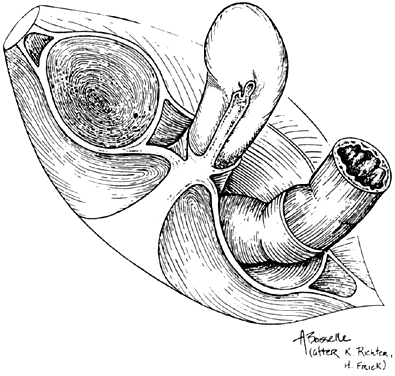 Fig. 13. Stereograph showing the connective tissue
septa and paravaginal spaces in relation to the bladder, uterus,
and rectum. The spaces permit these three organs to function independently
of one another.(Nichols, DH, Randall CL:
Vaginal Surgery, 3rd ed, p 42. Baltimore, Williams & Wilkins,
1989, with permission)
Fig. 13. Stereograph showing the connective tissue
septa and paravaginal spaces in relation to the bladder, uterus,
and rectum. The spaces permit these three organs to function independently
of one another.(Nichols, DH, Randall CL:
Vaginal Surgery, 3rd ed, p 42. Baltimore, Williams & Wilkins,
1989, with permission)
|
|
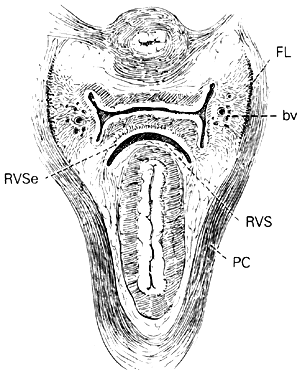
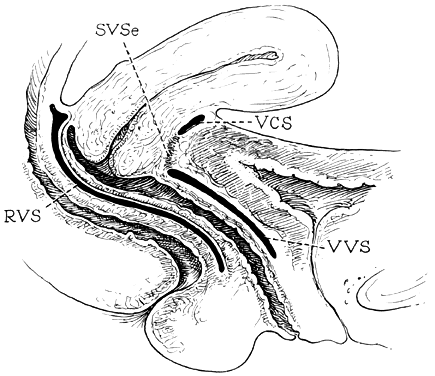 Fig. 14. Median sagittal section through the
female pelvis showing the midline connective tissue spaces between
bladder, vagina, and rectum. The vesicocervical space (VCS)
is separated from the vesicovaginal space (VVS) by fusion
between the adventitia of the cervix and bladder, called the supravaginal
septum (SVSe). The rectovaginal space (RVS) is shown between
the rectum and the vagina, extending from the perineal body to the
bottom of the cul-de-sac of Douglas. The rectovaginal septum is
a condensation of tissue attached to the posterior vaginal wall
along the full length of the rectovaginal space.
Fig. 14. Median sagittal section through the
female pelvis showing the midline connective tissue spaces between
bladder, vagina, and rectum. The vesicocervical space (VCS)
is separated from the vesicovaginal space (VVS) by fusion
between the adventitia of the cervix and bladder, called the supravaginal
septum (SVSe). The rectovaginal space (RVS) is shown between
the rectum and the vagina, extending from the perineal body to the
bottom of the cul-de-sac of Douglas. The rectovaginal septum is
a condensation of tissue attached to the posterior vaginal wall
along the full length of the rectovaginal space.
|
Safe extirpative or reconstructive surgery for benign pelvic disease requires
identification, penetration, and invasion of the midline anterior
and posterior spaces, but the oncologic surgeon requires penetration
and dissection of the lateral spaces as well. Vesicovaginal Space The vesicovaginal space lies in the midline and is bounded anteriorly by
the bladder adventitia, laterally by the bladder septa, or pillars, and
posteriorly by the adventitia of the vagina. Superiorly it ends at
the point of fusion between the adventitia of the bladder and vagina. This
point of fusion is called the supravaginal septum or vesicocervical
ligament.27 From our dissections we have found that this point of fusion occasionally
contains multiple fasciculi, oriented in the same general direction
but occurring at slightly different levels (Fig. 15). Inferiorly, the vesicovaginal space is limited by the fusion of the
urethral and vaginal adventitia.
|
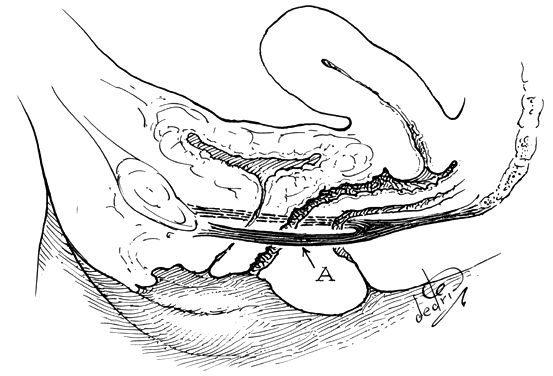
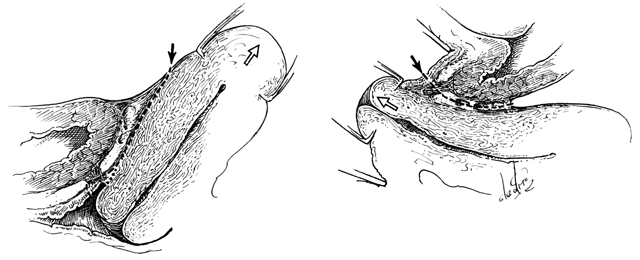 Fig. 15. Left. Site and direction of the
anterior peritoneal incision often used in the so-called endofascial
type of abdominal hysterectomy is shown by solid arrow in
drawing. This dissection following the route of the broken line
is often beneath the connective tissue capsule of the uterus and
must cut across the lower part of the supravaginal septum to reach
the vagina, or may enter the vagina behind most of the supravaginal
septum, as shown by dotted line. The open arrow shows
direction of removal of the uterus. Right. A desirable route
of incision and dissection with vaginal hysterectomy is shown by
solid arrow. The supravaginal septum may be incised immediately
after opening the vagina, and the dissection may be carried superiorly
between the connective tissue capsules of the uterus and bladder
(the so-called vesicocervical space) until the anterior peritoneal
plication is reached. Should the operator's dissection be beneath
the connective tissue capsule of the uterus, he will find himself
tunneling interior to (and failing to recognize) the peritoneum
on the anterior surface of the uterus well above the anterior peritoneal
fold. The open arrow shows direction of removal of the uterus.
Fig. 15. Left. Site and direction of the
anterior peritoneal incision often used in the so-called endofascial
type of abdominal hysterectomy is shown by solid arrow in
drawing. This dissection following the route of the broken line
is often beneath the connective tissue capsule of the uterus and
must cut across the lower part of the supravaginal septum to reach
the vagina, or may enter the vagina behind most of the supravaginal
septum, as shown by dotted line. The open arrow shows
direction of removal of the uterus. Right. A desirable route
of incision and dissection with vaginal hysterectomy is shown by
solid arrow. The supravaginal septum may be incised immediately
after opening the vagina, and the dissection may be carried superiorly
between the connective tissue capsules of the uterus and bladder
(the so-called vesicocervical space) until the anterior peritoneal
plication is reached. Should the operator's dissection be beneath
the connective tissue capsule of the uterus, he will find himself
tunneling interior to (and failing to recognize) the peritoneum
on the anterior surface of the uterus well above the anterior peritoneal
fold. The open arrow shows direction of removal of the uterus.
|
Supravaginal Septum Anterior entry between the vagina and the peritoneal cavity is often through
anatomic areas somewhat different, depending on whether the approach
is from the vaginal or from the abdominal side. This structural difference
may help explain why the surgeon who customarily operates by
the abdominal route may experience unexpected difficulty in separating
bladder from cervix when he approaches a hysterectomy vaginally; similarly, the
surgeon who is more comfortable with performing a hysterectomy
through the vagina may wonder why unfamiliar difficulty may arise
during the course of abdominal hysterectomy. This anatomic difference may be explained in Figure 15. A customary route of dissection is identified by the arrows. The vaginal
operator may incise directly through the point of fusion between the
bladder and the vagina, providing ready access to the anterior vesicouterine
perineal fold. When this is not promptly evident, the physician
may well have carried this dissection beneath the connective tissue
capsule of the uterus, well above the anterior peritoneal reflection, and
succeeded in peeling the peritoneum along with this uterine connective
tissue capsule from the anterior surface of the uterus. The abdominal
operator, on the other hand, will incise first directly into the
anterior peritoneum, continuing the dissection beneath the connective
tissue capsule of the uterus beneath or through the so-called supravaginal
septum to the vagina. The former is the essence of the so-called
endofascial hysterectomy. Recognizing these differences and becoming
comfortable with both techniques will provide valuable surgical experience
and enable one to find the anterior vesicouterine peritoneal fold
when operating through the vagina, as well as finding the longitudinal
muscle layer of the vagina more safely when operating for benign disease
through a transabdominal approach. That the connective tissue capsule of the vagina is continuous with that
of the bladder is seen in the accompanying photomicrograph (Fig. 16) in which the sagittal section had been obtained postmortem through the
vagina, cervix, and bladder of an aging patient with procidentia. Notice
the continuity of this layer which must be traversed, as well as
the looseness of the areolar tissues filling the potential vesicovaginal
and vesicocervical spaces.
|
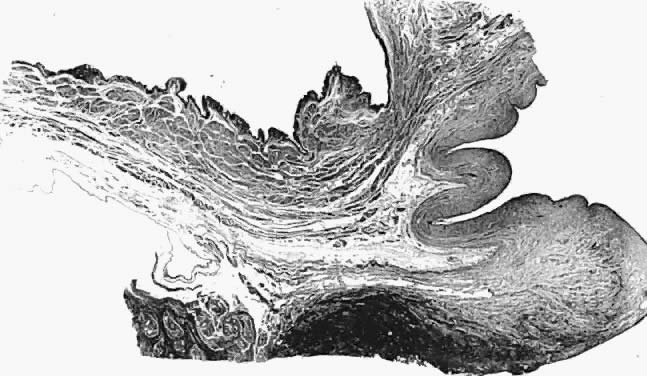 Fig. 16. Photomicrograph of sagittal section
through bladder (top left), vagina (top right), and
cervix (lower right) of autopsy specimen of elderly patient
with untreated genital prolapse. Descent of the cervix has drawn
it and the vagina away from the bladder. An attachment (supravaginal
septum) of fibromuscular connective tissue capsule of the bladder
to that of the vagina is shown.
Fig. 16. Photomicrograph of sagittal section
through bladder (top left), vagina (top right), and
cervix (lower right) of autopsy specimen of elderly patient
with untreated genital prolapse. Descent of the cervix has drawn
it and the vagina away from the bladder. An attachment (supravaginal
septum) of fibromuscular connective tissue capsule of the bladder
to that of the vagina is shown.
|
Vesicocervical Space The vesicocervical space is the continuation of the vesicovaginal space
superiorly above the supravaginal septum. The posterior border becomes
the connective tissue adventitia of the cervix, with which the adventitia
of the vagina is continuous. The superior border is the peritoneum
lining the vesicouterine peritoneal pouch. Cutting the supravaginal
septum establishes communcation between the vesicovaginal space and the
vesicocervical space. Ascending Bladder Septa Although the ascending bladder septa are weak cephalad, they become the
stronger bladder pillars (which contain efferent veins from the vesical
plexus and ureter) by the addition of the lateral strong connective
tissue portions of the cardinal ligament. Medially, they are loose in
texture and contain fat and ureter. These septa contain the lateral inferior
extensions of the bladder and connect it to the upper surface
of the cardinal ligament, lateral to the cervix. Prevesical Space of Retzius The prevesical space of Retzius is in the form of a triangle extending
from the umbilicus laterally to the lateral umbilical ligament (obliterated
hypogastric artery). Anteriorly, the transversalis fascia extends
from the umbilicus to the pubis; it extends inferiorly to the cardinal
ligament and the supravaginal septum. It is separated from the paravesical
spaces by the ascending bladder septa. The prevesical space thus
includes the area between the pubis and the anterior vesical wall roofed
by the fascia between the medial umbilical ligaments. The ascending bladder septum above the ureter contains many blood vessels
including the inferior vesical artery and large veins of the vesical
plexus. Below the ureter, however, blood vessels are scant and the tissues
between bladder and vagina can be easily separated here without
hemorrhage. Paravesical Spaces The paired paravesical spaces, right and left, are natural, fat-filled, preformed
spaces that lie above the cardinal ligament and its prolongation (horizontal
connective tissue ground bundle); they are bounded medially
by the bladder pillars and laterally by the pelvic walls, the
internal obturator muscle, and the levator ani. The roof is formed by
the lateral umbilical ligament (vesicohypogastric fascia). Descending Rectal Septa The descending rectal septa run alongside the vagina from the undersurface
of the cardinal ligament and its vaginal prolongation to the lateral
surface of the rectum and thence to the sacrum. Retrorectal Space The retrorectal space lies in the midline between the sacrum and the adventitia
of the rectum, between the posterior portion of the rectal pillars. This
space communicates with the pararectal spaces above the uterosacral
ligaments. Pararectal Spaces The paired pararectal spaces are only potential and are not preformed. They
lie below the cardinal ligament and its vaginal prolongation. The
medial border is formed by the rectal pillar, the lateral by the levator
ani. The posterior portion extends backward above the ischial spine
but under the cardinal ligament to the anterior surface of the lateral
part of the sacrum. Behind the cardinal ligament the independent caudal
portion of each side becomes continuous with the cranial portion
of the opposite side. The upper rectum is surrounded by a single circular pararectal space. The
boundaries of this space, formed by communication of two pararectal
spaces and the retrorectal space, are formed laterally and below by the
cranial surface of the levator, above and medially by the rectum, descending
rectal septa, and the cardinal ligament. It is made L-shaped
by the horizontal part below the cardinal ligament and the cranial and
ascending portion behind the cardinal ligament. The cranial portion
of the space is bounded anteriorly by the cardinal ligament and posteriorly
by the lateral part of the sacrum. The sheaths of the great vessels
of the pelvic wall form the lateral border; the pararectal space is
bordered medially by the rectal septa and ureteric sheath. The inferior
or horizontal division is bounded below by the levator ani, above
by the cardinal ligament, and medially by the rectal septum. The two pararectal
spaces communicate with each other posterior to the rectum, where
there is no limiting membrane. Rectovaginal Septum and Space Centered in a relatively avascular rectovaginal space, the posterior vaginal
wall and anterior rectal wall have functional independence of one
another. This space permits the two organs to glide over one another
with considerable mobility. The anterior wall of this space is formed
by a specialized connective tissue layer of fused peritoneum, the rectovaginal
septum.28,29 As seen in sagittal section, this septum appears as a distinct, strong
connective tissue layer between the vagina and rectum, oriented in a curved
sagittal plane following the curvature of the pelvis. It is attached
cranially to the caudal end of the peritoneum of the rectouterine
pouch and extends inferiorly to the caudal attachment to the pelvic floor
in the area of the perineal body. Anteriorly, the rectovaginal septum
is always intimately attached to the posterior aspect of the vaginal
connective tissue adventitia. Transversely, the septum curves posterolaterally, paralleling
the course of the paracolpium (Fig. 17). Histologically, it consists of a fibromuscular elastic layer of dense
collagen, abundant muscle, and coarse elastic fibers; the latter are
best demonstrated with specialized orcein staining.
|
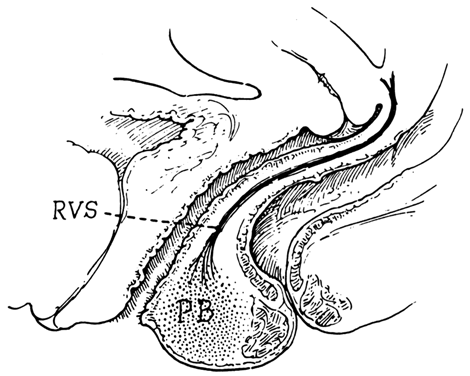
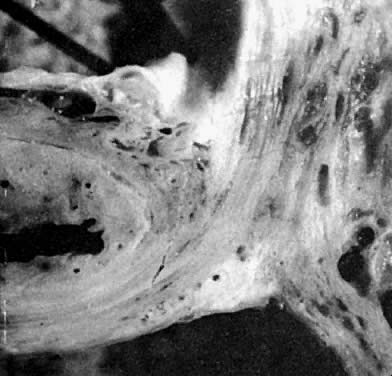
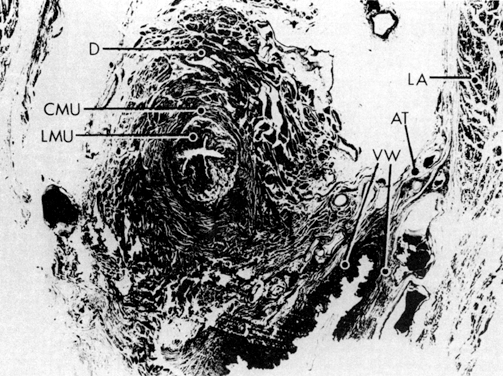
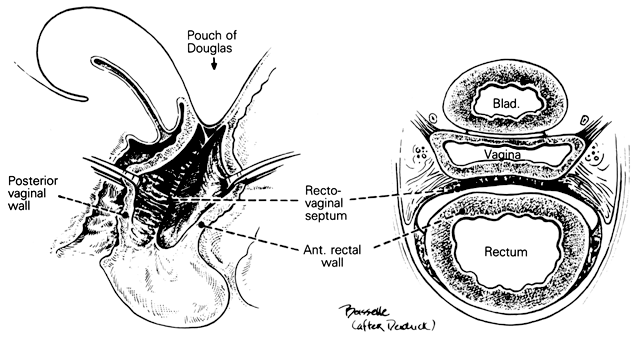 Fig. 17. The rectovaginal septum. Sections showing
the partly dissected rectovaginal septum. It extends from the pouch
of Douglas to the perineal body and forms the anterior surface of
the rectovaginal space. Its adherence to the posterior vaginal wall
is illustrated along with its posterolateral curve.(Nichols
DH, Milley PS: Surgical significance of the rectovaginal septum.
Am J Obstet Gynecol 108:215, 1970)
Fig. 17. The rectovaginal septum. Sections showing
the partly dissected rectovaginal septum. It extends from the pouch
of Douglas to the perineal body and forms the anterior surface of
the rectovaginal space. Its adherence to the posterior vaginal wall
is illustrated along with its posterolateral curve.(Nichols
DH, Milley PS: Surgical significance of the rectovaginal septum.
Am J Obstet Gynecol 108:215, 1970)
|
The rectovaginal space is in the midline between the rectovaginal septum, which
is attached to the posterior vaginal wall, and the fat-covered
rectal adventitia. The lateral walls are separated from the pararectal
spaces by a descending rectal septum (rectouterine) on each side. The
roof is the peritoneum and rectouterine peritoneal pouch (cul-de-sac
of Douglas), and the inferior margin of this space is the perineal body. |