At puberty the uterus may be slightly more than 2 inches in length. After
menstruation has been established, it is approximately 3 inches long. Pregnancy, of
course, causes considerable enlargement with subsequent
involution, while the uterus of postmenopausal women becomes atrophic
and shrunken. In the mature woman the uterus is approximately 7.5 cm
long, 5 cm wide, and 2.5 cm thick and weighs 30 to 40 gm. This is more
easily remembered as 1 inch thick, 2 inches wide, and 3 inches long, the
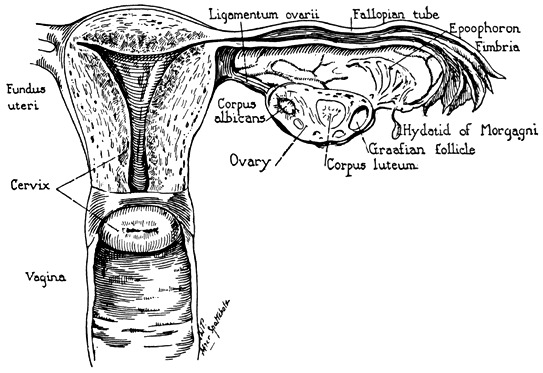
rule of “1–2-3.” The uterus is shaped like an inverted pear. The main pan is called the
body or corpus; the portion extending into the vagina is called the neck
or cervix. The upper part of the uterus above the insertion of the
tubes is the fundus. The narrow portion situated between corpus and cervix
is known as the isthmus and lies approximately at the level of the
course of the uterine artery and the internal os of the cervix. The cervix, most of which protrudes into the vagina, is 2 to 3 cm long. The
intravaginal portion of the cervix, known as the portio vaginalis, ordinarily
is covered with squamous epithelium with a number of mucus-secreting
glands (Fig. 2). The external os is shaped like the crater of a volcano. Above the external
os lies the fusiform endocervical canal, approximately 2 cm long
and lined with columnar epithelium and endocervical glands. At the upper
end of this canal at the junction with the uterine cavity is the
internal os. The endocervical canal in the nullipara is lined by mucosa
arranged in a series of folds. A vertical fold is present on the anterior
and posterior cervical walls; from these, oblique folds radiate. These
folds have been called the arbor vitae uteri or plicae palmatae. It
was formerly thought that tubular glands descend vertically from
the surface and divide into many branches forming compound racemose glands; however, secondary
changes caused by the intense growth activity
of the columnar cells result in the formation of tunnels, secondary clefts, and
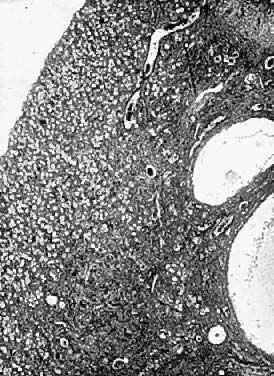
exophytic processes. 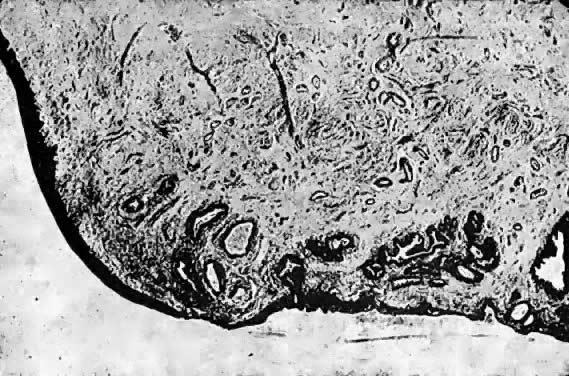 Fig. 2. Photomicrograph (low power) of the epithelial lining at the junction of
the cervix and vagina in the human. The glands of the cervix are definitely
evident. There are no glands underlying the squamous epithelium
of the vagina. (After R. Shroder.) Fig. 2. Photomicrograph (low power) of the epithelial lining at the junction of
the cervix and vagina in the human. The glands of the cervix are definitely
evident. There are no glands underlying the squamous epithelium
of the vagina. (After R. Shroder.)
|
The uterine cavity lies above the internal cervical os. It is roughly triangular
in shape and measures approximately 3.5 cm in length. Ordinarily, the
anterior and posterior walls of the uterus lie in apposition
so that little if any actual cavity is present. At each cornu or horn
of the uterus, its cavity is continuous with the lumen of a fallopian
tube. In a considerable number of women the uterus is retroverted or retroflexed, a
condition rarely of clinical significance. Particularly in parous
women, the uterus may adopt a variety of positions, all of which must
be considered normal. Lying upon the uterus are coils of intestines
which, in the absence of adhesions, are continually changing their position. The “positions” of the uterus are of considerable interest
but of much less importance in gynecologic practice than 50 years ago. The
normal position of the uterus is a nulligravid female is in moderate
anteflexion or bent slightly anteriorly, and the uterus as a whole
is inclined toward the symphysis in ante version against the bladder, adapting
its position as the latter organ distends or empties (Fig. 3 and Fig. 4). In a variable number of women, perhaps a third, the uterus is retroverted
or inclined posteriorly or retroflexed toward the sacrum. Quite
a few disabilities were attributed to these “malpositions,” including
dysmenorrhea, functional uterine bleeding, backache, dyspareunia, and
leukorrhea. Many suspension operations were carried out, but
these procedures are rarely employed at the present time. It should
be mentioned that many normal uteri are in mid position, with the axis
of uterus almost parallel to the spine. Prolapse of the uterus, by contrast, may
be a real problem. If the cervix almost reaches the introitus, the
prolapse is classified as first degree. A cervix visible at
the introitus is considered a second-degree prolapse, while the protrusion
of cervix and some of the corpus is called third-degree prolapse
or procidentia. Before total hysterectomy became the usual procedure, a
variety of operative procedures to support the uterus were employed; today, however, support
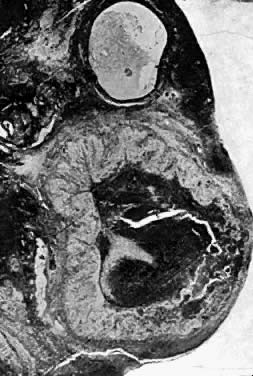
operations without hysterectomy are unusual. 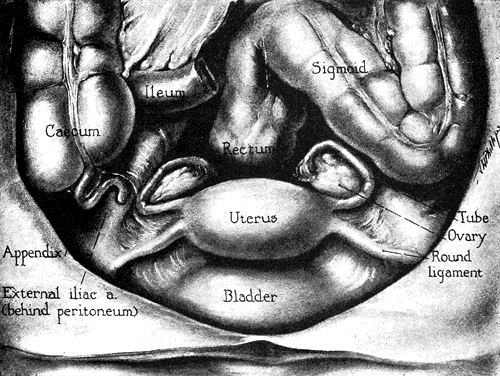 Fig. 3. Dissection showing the cephalic aspect of the female genitalia and their
relationships. Fig. 3. Dissection showing the cephalic aspect of the female genitalia and their
relationships.
|
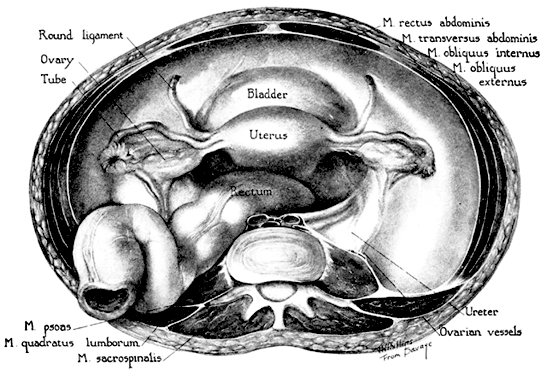 Fig. 4. Transverse section of the abdomen above the crests of the ilia. This section
is 1 inch above the pubis and extends through the disk between the
sacrum and the last lumbar vertebra. Fig. 4. Transverse section of the abdomen above the crests of the ilia. This section
is 1 inch above the pubis and extends through the disk between the
sacrum and the last lumbar vertebra.
|
The peritoneum covers the uterus and is separated from the uterine musculature
by a thin layer of periuterine fascia, which is a continuation
and extension of the transversalis fascia. This mobile fascial layer
is areolar tissue and is easily separated except :for a midline seam or
raphe between the uterus and bladder anteriorly and between uterus and
peritoneum posteriorly at the level of the isthmus. Posteriorly it
sweeps down over the posterior vaginal wall and the cul-de-sac. The musculature of the uterus is in several layers. There is an outer longitudinal
layer (stratum supra-vasculare) continuing into the tubes
and round ligaments. The vascular layer (stratum vasculare) consists of
many interlacing spiral groups of smooth muscles and contains many blood
vessels. An inner layer consists of muscle fibers arranged both longitudinally
and obliquely. The blood supply of the uterus is derived chiefly from the uterine arteries (Fig. 5). These arise from the hypogastric artery and swing toward the uterus, which
they reach at approximately the level of the internal os (Fig. 6 and Fig. 7). Here the uterine arteries divide, the descending limb coursing downward
along the cervix and lateral wall of the vagina. The ascending limb
passes upward alongside the uterus and continues below the fallopian
tube. Frequent anterior and posterior branches go to vagina, cervix, and
uterus. 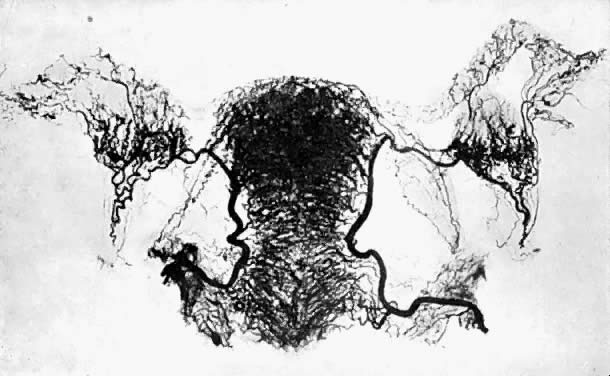 Fig. 5. Arterial blood supply of the normal tube, ovary, and uterus. (Courtesy
of Dr John A. Sampson.) (From Norris: Gonorrhoea in Women. Philadelphia: Saunders.) Fig. 5. Arterial blood supply of the normal tube, ovary, and uterus. (Courtesy
of Dr John A. Sampson.) (From Norris: Gonorrhoea in Women. Philadelphia: Saunders.)
|
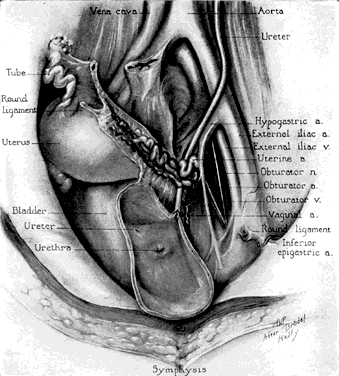 Fig. 6. Ventral view of a deep dissection of the urinary bladder and the blood
supply to the left side of the internal genitalia, showing the relation
of the uterine vessels to the ureter. Fig. 6. Ventral view of a deep dissection of the urinary bladder and the blood
supply to the left side of the internal genitalia, showing the relation
of the uterine vessels to the ureter.
|
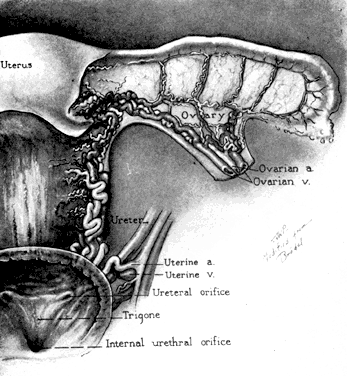 Fig. 7. Blood supply of the internal organs of generation with relation to the
ureter and trigone of the urinary bladder. Fig. 7. Blood supply of the internal organs of generation with relation to the
ureter and trigone of the urinary bladder.
|
The ovarian artery, which ordinarily arises from the aorta, passes along
the ovary, dividing into a number of branches. At several places in
the broad ligament there are anastomotic connections between the tubal
branch of the uterine artery and the ovarian artery. A branch of the
uterine artery nourishes the round ligament. The veins in general accompany
the arteries. Using injection and microradiographic and histologic techniques, Farrer-Brown
et al1 restudied the vascular anatomy of the uterus. Their studies showed that the uterine arteries run a tortuous course between the two layers of the
broad ligament along the lateral side of the uterus .... and turn laterally
at the junction of the uterus and fallopian tube, run toward the
hilum of lhe ovary, and terminate by joining the ovarian arteries. In
the broad ligament each uterine artery supplies lateral branches that
immediately enter the uterus and give off tortuous anterior and posterior
arcuate divisions, which run circumferentially in the myometrium
approximately at the junction of its outer and middle thirds. In the
midline the terminal branches of both arcuate arteries anastomose with
those of the contralateral side.
They found that “each arcuate artery throughout its course gives
off numerous branches running both centrifugally towards the serosa and
centripetally towards the endometrium.” The arteries to the serosa
at first were directed radially and then frequently became more circumferential. There
is a plexus of small arterial radicals with a radial
distribution located immediately below the serosa. The inner two
thirds of the myometrium is supplied by tortuous radial branches of the
arcuate arteries. They provide numerous branches terminating in a capillary
network which surrounds groups of muscle fibers. An abrupt change
in the density of the arterial pattern occurs at the junction of the
basal layer of the endometrium with the subjacent myometrium. The endometrial
vessels are relatively sparse in comparison with those of the
myometrium at all stages of the menstrual cycle. The uterus is partially supported by three pairs of ligaments. The most
important are the paired cardinal (Mackenrodt's) or transverse cervical
ligaments. Arising from the anterior and posterior marginal walls
of the cervix, these ligaments fan out laterally to insert into the
fascia overlying the obturator muscles and the levator ani muscles. The
cardinal ligament forms the base of the broad ligament. Its muscles
and connective tissue surround the uterine blood vessels as well as
the accompanying nerves and lymphatics. The paired round ligaments extend from the anterosuperior surface of the
uterus through the internal inguinal rings and through the inguinal
canals to end in the labia majors. They are composed of muscle fibers, connective
tissue, blood vessels, nerves, and lymphatics. The round ligaments
stretch with relative ease, particularly in pregnancy. The uterosacral ligaments are condensations of fascia that arise from the
posterior wall of the uterus at the level of the internal cervical
os. They insert in the sacral fascia over the second and third sacral
vertebrae. In addition to muscle and connective tissue, blood vessels, lymphatics, and
parasympathetic nerve fibers are present. The broad ligament is formed by folds of peritoneum covering the tubes, the
infundibulopelvic vessels, and the hilus of the ovary. It contains
a number of structures: tube, round ligament, ovarian ligament, uterine
and ovarian blood vessels, nerves, lymphatics, and mesonephric remnants. Below
the infundibulopelvic structures, the anterior and posterior
leaves of peritoneum lie in apposition, leaving a clear space below
the tube with its tubal branch of the uterine artery. This avascular
area is useful to the surgeon in isolating the adnexal structures and
in avoiding blood vessels while performing tubal ligations. The endometrium varies greatly, depending on the phase of the menstrual
cycle. In the proliferative phase immediately after menstruation, the
surface of the mucosa is relatively smooth and lined with columnar epithelial
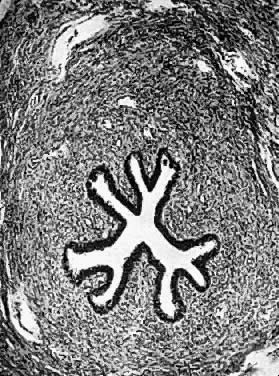
cells, some of which may have cilia (Fig. 8). Uterine glands are straight or slightly curved and run an oblique course. The
stroma is a reticular connective tissue with many spindle-shaped
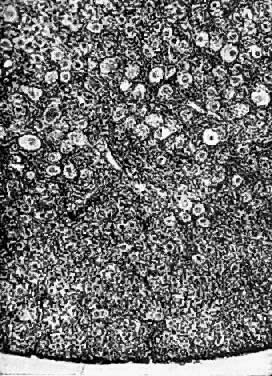
nuclei of mesenchymal cells in a reticulum of argyrophilic fibers. 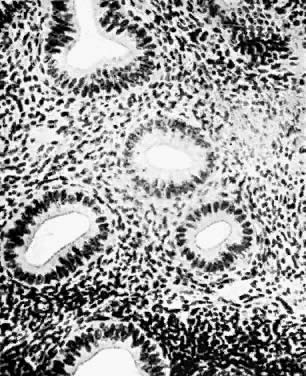 Fig. 8. Normal endometrial glands of uterus (resting stage). Note the character
of the epithelial cells of the glands; they are slightly higher than
the ciliated columnar cells of the surface epithelium. There is a distinct
basement membrane. Fig. 8. Normal endometrial glands of uterus (resting stage). Note the character
of the epithelial cells of the glands; they are slightly higher than
the ciliated columnar cells of the surface epithelium. There is a distinct
basement membrane.
|
The endometrium is considered to have three layers: the pars basalis, the
zona spongiosa, and the superficial zona compacta. The straight branches
of the radial arteries of the uterus terminate in capillaries in
the basal layer, while the spiral or coiled branches penetrate to the
surface epithelium, where they give rise to superficial capillaries. Sinus-like
dilatations of the capillaries in the superficial layer are
called “lakes.” These vascular lakes and capillaries are
drained by small veins. Proliferation of the endometrium occurs under the influence of estrogen; maturation
occurs under the influence of progesterone. After formation
of the corpus luteum, the endometrial glands grow, become tortuous, and
secrete. The tubules grow laterally to join adjacent tubules. The
spiral capillaries develop a terminal network of superficial capillaries. These
changes result in the formation of a predeciduum prepared for
the arrival of the trophoblast (Fig. 9). If there is no implantation of trophoblast, the levels of estrogen and
progesterone fall and spasm of the spiral arteries results in ischemia
and subsequent shedding of most of the endometrium, with associated
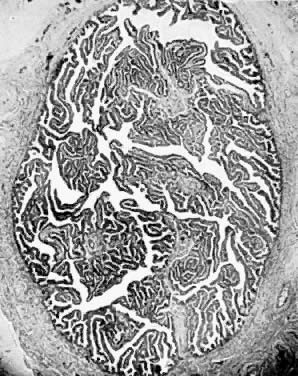
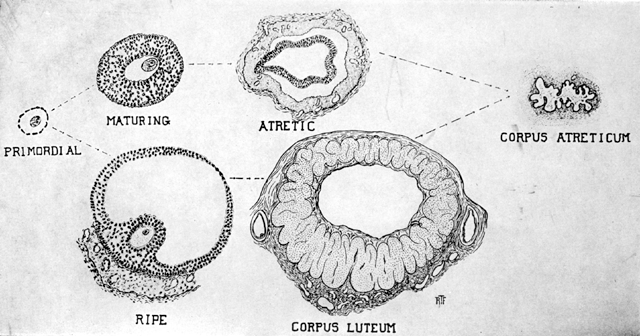
bleeding.  Fig. 9. Endometrium in the premenstrual stage. Note that the glands are apparently
increased in number and size. The tortuosity of the glands is clearly
seen. Fig. 9. Endometrium in the premenstrual stage. Note that the glands are apparently
increased in number and size. The tortuosity of the glands is clearly
seen.
|
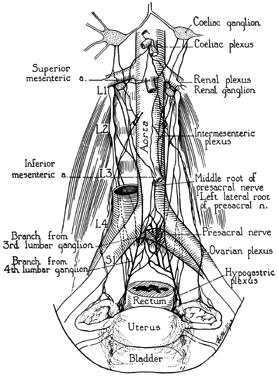
Data on the lymphatic vessels of the uterus have been coordinated by Reynolds2. The entire uterus has a rich capillary bed as extensive as the blood
capillary system. The lymphatic capillary bed is arranged in four zones: 1) the
lower uterine segment with its rich supply of fine capillaries, 2) the
subserosa of the corpus with a few lymphatics, 3) a deep subserosal
network, and 4) a plentiful supply in the muscularis proper. These
vessels increase greatly in number and size during pregnancy. The
collecting system of the uterine lymphatics is formed from anastomoses
of a lateral-uterine descending network of lymph vessels which unites
with collecting vessels from the utero-ovarian pedicle and the external
lilac area. |