An abscess is a circumscribed collection of pus formed by liquefaction necrosis within tissue. Fibrous tissue is deposited around these pus collections if they are not drained. This tends to isolate the purulent collection further, serving to localize microbial enzymes or toxins injurious to the host, thus making it more difficult for antimicrobial agents to penetrate the capsule and sterilize the contents. In addition, local exhaustion of complement and enzymatic degradation of immunoglobulins occur, favoring the persistence of the bacterial infection.1
An abscess can result from a number of obstetric or gynecologic infections (Table 1).2 The clinical diagnosis is generally based on the findings of fever and a palpable adnexal or pelvic mass. This mass may be distinctly palpable and fluctuant or less distinct and characterized by a “fullness” noted during bimanual pelvic examination. Diagnosis of a pelvic abscess commonly occurs after failure of an initial antibiotic treatment for an obstetric or gynecologic soft-tissue infection. In other cases, particularly pelvic inflammatory disease, a pelvic abscess is diagnosed at the initial evaluation. A concurrent leukocytosis and elevated erythrocyte sedimentation rate or C-reactive protein are commonly noted laboratory abnormalities.
Etiology | % |
Pelvic inflammatory disease | 3–16 |
Postcesarean endometritis | 2 |
Postoperative hysterectomy | 3 |
Imaging techniques are helpful in characterizing the size of the abscess and in determining whether the collection of purulent material is unilocular or multilocular. Inflamed pelvic tissues and bowel often adhere to one another without associated pus collections. These masses have been referred to as tubo-ovarian complexes. Patients with tubo-ovarian complexes are more likely to respond to antibiotic treatment alone. Patients with a true localized purulent collection (“bag of pus”) will be more likely to require surgical drainage.
Ultrasound is the first method of choice to evaluate a pelvic mass thought to be an abscess. It easily differentiates between fluid-containing and solid lesions. Pus collections have fine internal echoes of differing sizes. Transvaginal sonography can augment the findings noted during abdominal scanning.
Computed tomography is a cross-sectional imaging method that generates information similar to that noted on transverse sections on ultrasound. The examination is optimally performed after the patient is administered oral contrast to opacify the bowel loops and intravenous contrast to identify vascularity and to opacify the urinary tract. Like ultrasound, computed tomography can locate inflammatory masses and abnormal fluid collections, but is used more often than ultrasound to search for a suspected abscess during the postoperative patient examination that is limited by open surgical wounds and abundant bowel gas. An abscess has a low-density center if liquefaction has occurred. A thick wall may be demonstrated, depending on the age of the abscess.
Magnetic resonance imaging is recommended to clarify or supplement ultrasound findings. If the origin of the pelvic mass is unclear, the fluid content is in question, or the extent of the disease is not determined by ultrasound, magnetic resonance imaging is useful. Radioisotope scanning with gallium-67 citrate or indium-III-labeled leukocytes can be used to locate abscesses. Scanning is not hampered by surgical dressing, distended bowel, surgical clips, open wounds, or ventilators. Isotope scanning is most useful in patients with no localizing signs because the whole body is imaged. If a focal area of increased tracer activity is found, ultrasound or computed tomographic scanning can be used to clarify the nature of the area of activity.3
The microbiology of pelvic abscesses is predominately anaerobic. The intra-abdominal abscess rat model of Weinstein and colleagues4 is an excellent description of the phases of pathogenesis of mixed aerobic-anaerobic infections of the abdomen and pelvis:
Phase I: Initial stage of peritonitis, sepsis, and an associated high mortality
rate of nearly 40% appeared to be due to facultative gram-negative
bacteria, particularly Escherichia coli.
Phase II: Abscesses developed in the surviving rats during the secondary
phase of infection due to anaerobic bacteria, particularly Bacteroides fragilis.
A similar biphasic disease process occurs in many clinical entities that are encountered in obstetrics and gynecology. Pelvic inflammatory disease, pelvic cellulitis, and endomyometritis are analogous to the initial phase of peritonitis in this model,4 but if untreated or inadequately treated, these infections will progress to an abscess phase characterized by pelvic abscess formation.2
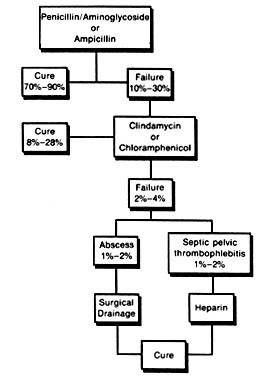
The traditional approach to the treatment of mixed anaerobic-aerobic soft-tissue pelvic infections sheds additional light on the pathophysiology of abscess formation and on the importance of using antimicrobials with activity against penicillin-resistant anaerobes as part of initial therapy (Fig. 1).5 Initial treatment with penicillin and gentamicin or with ampicillin alone has a relatively high failure rate. Most initially resistant infections respond to the addition of an antibiotic with extended anaerobic activity (e.g., clindamycin, metronidazole). Patients who do not improve with broad-spectrum antimicrobial therapy should be evaluated for the presence of an abscess, septic pelvic thrombophlebitis, or drug fever.
Broad-spectrum antibiotics are given as the initial treatment for patients with a diagnosis of pelvic abscess. The gold standard of antimicrobial regimens for the treatment of pelvic abscess is combination therapy with either clindamycin or metronidazole in conjunction with an aminoglycoside, third-generation cephalosporin, or aztreonam. Other agents with therapeutic utility include single-agent treatment with an extended-spectrum cephalosporin (e.g., cefoxitin, cefotetan, cefotaxime, ceftizoxime), an extended-spectrum penicillin (e.g., mezlocillin, piperacillin), carbapenems (imipenem), and β-lactamase inhibitors plus a β-lactam (e.g., ampicillin/sulbactam, ticarcillin/clavulanate, piperacillin/tazobactam; see ahead to Table 3).
When antimicrobial treatment is started, a decision on the need for surgical intervention is required. Pelvic abscesses will occasionally respond to antimicrobial treatment alone. This is particularly true in patients with pelvic inflammatory disease that is complicated by a tubo-ovarian abscess. Treatment with broad-spectrum antimicrobials results in a satisfactory response to therapy without the need for surgery in 75% of cases.6 Patients with pelvic abscesses that complicate postoperative infections, such as postcesarean endomyometritis or posthysterectomy cuff cellulitis, usually require surgical intervention. Most abscesses complicating posthysterectomy infections are cuff abscesses and can be easily drained by dilating the vaginal cuff. Patients with postoperative adnexal abscesses (tubo-ovarian abscesses) are less likely to respond to antibiotic treatment alone, but trial use of antibiotic therapy is warranted before surgical drainage. Because of the high rate of persistence of infection after vaginal drainage,7 an adnexal abscess that fails to respond to antibiotics should be treated by abdominal exploration.
Surgical options for the drainage of pelvic abscesses have increased in the past several years. Most surgeons prefer laparotomy and drainage, with or without extirpation of the infected tissues, as the treatment of choice. An endoscopic approach has been used increasingly to drain or excise infected tissue. A percutaneous computed tomography-directed drain can be placed in some cases. The choice of options is based on the skills and facilities available at each hospital.
A ruptured pelvic abscess remains a surgical emergency. Such patients generally present with persistent fever, increasing leukocytosis, and a rigid abdomen. Immediate surgery after the initiation of antimicrobial therapy and fluid resuscitation is necessary. Standard treatment consists of drainage of the pelvic abscess and copious irrigation of the abdominal cavity.