The D&C is a surgical procedure during which the physician explores a cavity beyond his or her view. It is therefore an art dependent on the surgeon's sense of palpation and knowledge of probable findings. Variations in the shape, size, and consistency of the uterus can be determined by history and physical examination, and there are hazards of misdiagnosis of these factors. Before beginning a D&C, these variations should be ascertained and anticipated. The technique must be adjusted to new variations as they are perceived by the surgeon, sensitive to the messages transmitted through the instruments to his or her hands. Unless one is continually thinking of possible variations and complications, it is easy to perforate the uterus.
Endocrine and Anatomic Variations
EXTERNAL OS.
The external os is the only visible structure in the procedure. It may be patulous or stenosed, depending on the patient's reproductive and surgical history (e.g., prior cervical conization). These conditions are easily seen and dealt with and are independent of the condition of the internal os or uterus.
CERVICAL CANAL.
The cervical canal is a fairly voluminous hollow structure lined with secretory glands folded in many rugae. The canal usually offers no resistance to instrumentation until after menopause, when it may become more atrophic and rigid.
INTERNAL OS.
The internal os is an active “sphincter” of the lower uterine cavity. It is relaxed in the presence of estrogen (the first 2 weeks of the cycle, or in unopposed prolonged estrogen stimulation) and is constricted under progesterone influence (in the secondary phase or in early pregnancy). In postmenopausal women, the internal os may be very tightly closed and may resist probing and dilation to the point of possible laceration or perforation. Patients with a history of diethylstilbestrol exposure in utero may have a stricture at this point. Difficulty encountered in the office may require a hospital D&C under general anesthesia. In contrast, the internal os is open and soft in pregnancy-associated problems such as incomplete abortion or bleeding in the postpartum period.
MYOMETRIUM.
The uterine musculature responds to hormones in a manner opposite to the internal os: contracted and firm under estrogen influence, relaxed and easily perforated under progesterone influence. It is also thinner and more fibrous after menopause.
UTERUS.
The uterus is most commonly found in the anteverted, partially anteflexed position. Traction on the cervix will correct the anteversion and partially correct the anteflexion as well. In about one third of normal women, the uterus is retroverted. In a much smaller number, it is extremely retroflexed. These conditions can also be corrected with traction. It has been estimated in hysteroscopy that about 15% of patients have extreme anteflexion or retroflexion, making visualization of the entire uterus difficult. During a D&C, this exaggerated flexion can cause perforation.
In certain disease states, such as fibroids or endometrial carcinoma, the uterus may be distorted and difficult to outline by palpation with the sound or curet. As a result, significant pathology can be missed by blind curettage alone. Uterine anomalies such as unicornuate uterus or septate uterus may yield confusing findings during the initial palpation; these anomalies must be kept in mind as explanations for these findings. Undiagnosed, such anomalies may prevent a thorough exploration of the endometrial cavity.
Risks of the Technique
Because of the variations in anatomy and endocrinology, there are a number of known risks that the physician should keep in mind while doing the procedure. These are arranged in order of frequency.
- Laceration of the cervix. As a result of resistance to dilation at the internal os, the tenaculum
holding the cervix may tear through and cause bleeding. This can be
minimized by using less force over a longer time during the dilation effort.
- Tears of the internal os. It is quite easy to tear this structure rather than dilate it. Occasionally
such tears result in severe hemorrhage from damaged uterine vascular
branches or misdiagnosis of the tear as a submucous fibroid.1
- Fundal perforation. Under the influence of progesterone, the myometrium may be surprisingly
soft and easily perforated, such as in the management of an incomplete
first-trimester abortion.
- Perforation due to flexion. If anteflexion or retroflexion of the uterus is not appreciated and corrected
by traction, dilators or curets may go through the anterior or
posterior wall beyond the internal os and reach the peritoneal cavity. Intrauterine
devices were found lying in the anterior or posterior wall
in as many as 2% of women in some early series, illustrating how easily
such perforations can occur. These transmigrations of intrauterine
devices happened without excessive pain or bleeding at the time of
insertion and were otherwise asymptomatic.
- False passage at the internal os. If the internal os is tight or atrophic, a false passage leading from
the cervical canal just before the internal os into the peritoneal cavity
can occur. This would lead to an absence of curettings at best and
possible damage to adnexal vessels, bladder, or bowel at worst.
Techniques to Minimize Risks
The physician should evaluate these variations and keep the risks in mind. A D&C should then be performed in a systematic manner, following the “ritual” outlined in 1958.2
- Catheterization. The patient should be catheterized before bimanual pelvic examination. A
preoperative enema or laxative to clear the pelvis of feces is also
of significant benefit. Before an office procedure, the patient should
void spontaneously just before getting on the examining table, to eliminate
the need for catheterization.
- Bimanual pelvic examination. The pelvis is examined bimanually to evaluate the size, shape, mobility, and
position of the uterus and to detect the presence of extrauterine
masses. A rectovaginal examination, especially in retroversion, is
very useful at this point.
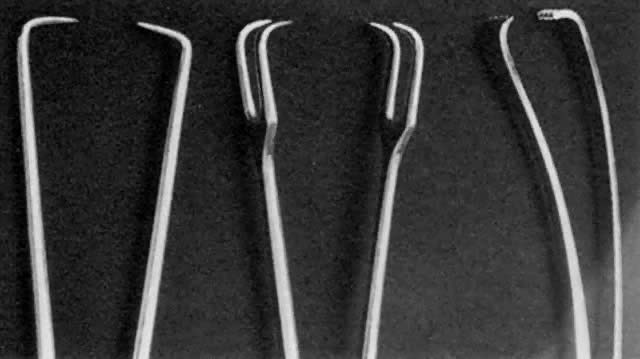
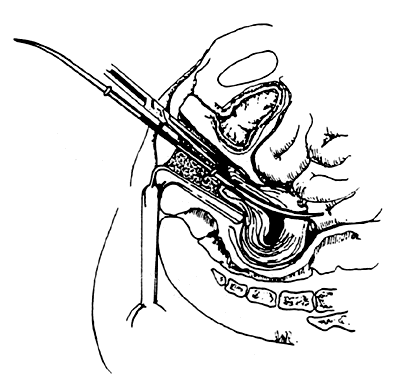
- Grasping the cervix. To visualize the cervix, the blade of a weighted speculum (Auvard) is
inserted into the vagina to retract the posterior wall and assist in the
exposure of the cervix. The single-toothed uterine vulsellum, the less
traumatic Littlewood forceps, or the double-toothed Jacobs tenaculum
can be used (Fig. 1). I prefer to put one tenaculum deep into the cervical canal, ensuring
a large grasp of the anterior cervix. Other techniques include one tenaculum
at the 3 o'clock position and one at the 9 o'clock position, or
one tenaculum sideways over the external os to avoid the canal. A retroverted
uterus may be corrected more completely by placing the tenaculum
on the posterior cervix. With a shallow, superficial grasp, there
is a greater risk of tearing during any resistance to dilation at the
internal os. The middle finger of the hand holding the tenaculum should
be placed between it and the patient's pubis to absorb the resistance
to dilation and avoid trauma to the delicate clitoris and urethra.
- Uterine sound. The uterine sound is held like a pen, not a skewer. The sound should be
used as a probe to gently and delicately determine the location and
direction of the internal os and then the location and direction of the
uterine cavity. It is helpful for the operator's hand to be on
the patient's buttocks to steady the arm and increase the tactile
sensitivity of the fingers holding the sound. Traction on the cervix
by the tenaculum will help correct uterine flexion. The curve of the malleable
sound should be adjusted and rotated to determine the exact direction
and location of the internal os and uterine cavity. This finding
will determine the direction for inserting the dilators and curets.
The sound should be passed until it meets the resistance of the fundus of the uterus, such that further pressure on the sound will cause the tenaculum holding the cervix to move. At this point, the depth of penetration by the sound should be marked with an index finger and the size of the uterus measured. The adult multiparous normal uterine cavity can be 7 to 9 cm long, and the hypoplastic or postmenopausal cavity can be as small as 5 cm or less.
I cannot stress enough that to avoid perforation, gentle technique is required in performing this procedure on the thin or atrophic uterus. Sounding should be avoided in the postpartum or pregnant uterus. Difficulty in passing the standard 3-mm-diameter sound may require using the smallest-diameter dilators in the internal os until the sound can again be used.
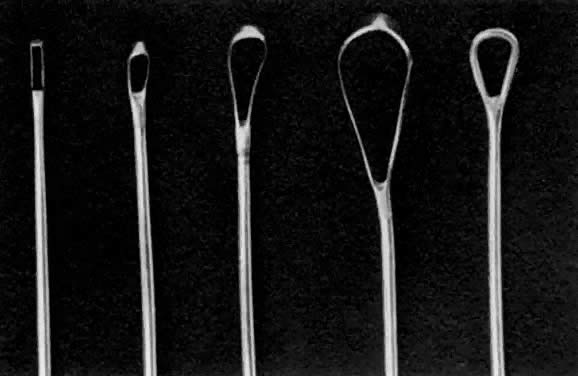
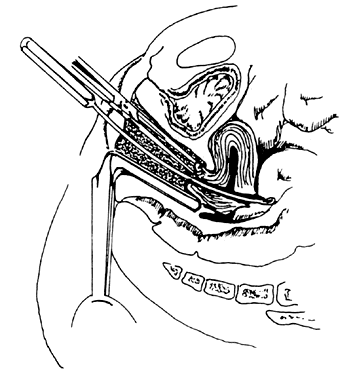
Once the sound is in the uterine cavity, it should be gently moved from side to side to determine the size, symmetry, and presence of distorting lesions of the endometrial cavity. Variations such as uterine septa, leiomyomata, synechiae, and the bicornuate uterus should be kept in mind so they can be diagnosed at this time. - Endocervical curettage. In postmenopausal bleeding or suspected malignancy, the endocervical canal
should be curetted before cervical dilation. A sharp, narrow-tipped
curet of the Kevorkian-Young or Duncan type is preferred (Fig. 2). The specimen should be collected on a small piece of Teflon-coated gauze
that has been inserted onto the weighted speculum blade and tucked
under the external os. All endocervical tissue can thus be obtained
and separately labeled.
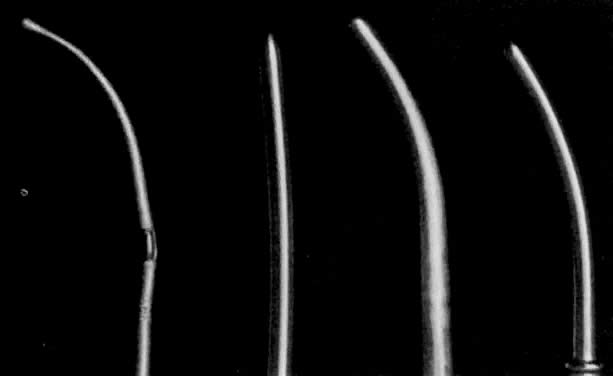
- Dilation of the internal os. Hagar dilators are round-tipped and short and are useful in the small
or atrophic uterus. Pratt dilators have a long, tapered tip that makes
dilation easier and less traumatic but may perforate the smaller uterus. Hanks
dilators have a stop at 8 cm, the depth of most adult uteri, to
minimize the risk of perforation (Fig. 3). For ordinary diagnostic curettage, dilation of 8 to 10 cm is all that
is necessary. Dilators are measured in millimeters of diameter (Hagar) or “French” circumference in millimeters (Pratt). To convert
French markings to diameter, divide the French value by pi, or roughly
by three. Thus, a 31 French Pratt will dilate the cervix to about 10 mm.
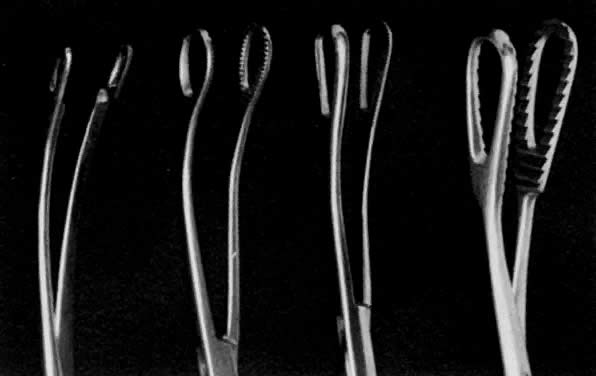
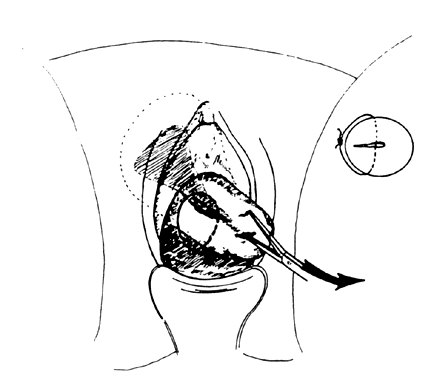
- Exploration with forceps. Especially for menometrorrhagia, the uterus should be explored at this
point with a small Randall kidney forceps or a small Ring forceps (Fig. 4) to look for polyps, fragments of placenta, or submucous fibroids that
may be better felt by two-point palpation. These large instruments also
further define the cavity and thus minimize the risk of perforation
by the smaller curet. The forceps should be widely opened inside the
cavity, closed, twisted 180°, and then withdrawn to permit avulsion
and removal of endometrial polyps or placental fragments.
- Uterine curettage. The standard Sims uterine curet comes in many sizes, but the small or
medium is adequate for most D&Cs (see Fig. 2). The Heaney curet has a serrated cup, which permits ridges of decidua
basalis to regenerate after vigorous curettage. The curet should have
a slight curve modeled after the final curve of the malleable sound. The
curet should be held by the entire hand for a firm grip during the “backstroke” coming out of the uterine cavity, but should
be inserted gently to allow palpation of the fundus of the uterus. It
should always be inserted in the direction of the flexion of the uterus. A
fresh 4″
 4″ gauze should be in between the vaginal retractor and the external
cervical canal to receive the endometrial curettings. A systematic
curettage should consist of directing the tip of the curet to one of
the uterine horns and pulling with a continuous stroke from this area
out of the uterine cavity to obtain a strip of endometrium to be placed
on the gauze. The curet is reinserted, the contralateral uterine horn
palpated, and another continuous stroke made down to the outside. This
should be repeated with insertion in the anterior flexed position, rotating
the curet inside the uterus to address the posterior wall of
the fundus, with two or three more strips attempted from the posterior
wall. After four or six strips of endometrium are attempted, the cavity
should be curetted in its entirety, with frequent withdrawals of the
curet to bring the specimen into the waiting sponge. To ensure thorough
curettage, the curet is passed from one cornu to the other over the
fundus of the uterus. A small, sharp curet might be useful to reach
the cornual regions. Only after the tissue is evacuated should the curet
be used as a scrub brush to denude the endometrium down to the decidual
layer and possibly to detect irregularities suggestive of submucous
fibroids hiding underneath the endometrium. The end point of this
scrubbing should be the detection of a scratching sensation or sound (the “uterine
cry”), which represents a sharp curet running
over myometrium. Too vigorous a pursuit of this end point may lead to
formation of synechiae (Asherman's syndrome) (see below).
4″ gauze should be in between the vaginal retractor and the external
cervical canal to receive the endometrial curettings. A systematic
curettage should consist of directing the tip of the curet to one of
the uterine horns and pulling with a continuous stroke from this area
out of the uterine cavity to obtain a strip of endometrium to be placed
on the gauze. The curet is reinserted, the contralateral uterine horn
palpated, and another continuous stroke made down to the outside. This
should be repeated with insertion in the anterior flexed position, rotating
the curet inside the uterus to address the posterior wall of
the fundus, with two or three more strips attempted from the posterior
wall. After four or six strips of endometrium are attempted, the cavity
should be curetted in its entirety, with frequent withdrawals of the
curet to bring the specimen into the waiting sponge. To ensure thorough
curettage, the curet is passed from one cornu to the other over the
fundus of the uterus. A small, sharp curet might be useful to reach
the cornual regions. Only after the tissue is evacuated should the curet
be used as a scrub brush to denude the endometrium down to the decidual
layer and possibly to detect irregularities suggestive of submucous
fibroids hiding underneath the endometrium. The end point of this
scrubbing should be the detection of a scratching sensation or sound (the “uterine
cry”), which represents a sharp curet running
over myometrium. Too vigorous a pursuit of this end point may lead to
formation of synechiae (Asherman's syndrome) (see below). - Repeat forceps exploration. After the curettage is complete, the Randall kidney clamp may reveal an
avulsed polyp or placental fragment that had escaped the efforts to
be withdrawn by a curet.
- Repeat uterine sound. As a final step, the uterus should be gently resounded to ensure that
perforation has not occurred.