Techniques of Abdominal Myomectomy The general principles of sound surgical techniques are as much a part
of myomectomy as for any operation. Adequate exposure can be achieved
through a Pfannenstiel incision or through a vertical midline incision. However, when
the uterus is greater than 16 weeks' size and cannot be
delivered through the horizontal incision, a vertical midline incision
may be more appropriate. The surgeon must also take into account the
location of the myomas before deciding on the incision. For instance, a
broad ligament myoma may require dissection in the pelvic sidewall
with subsequent unroofing of the ureter; this is done more easily through
a vertical midline incision. Good clinical judgment rather than controlled
clinical studies provides better guidance in these situations. A
mild Trendelenburg position helps ensure exposure. Gloves must be
washed to remove free talc particles and laparotomy pads placed in plastic
bags to limit the exposure of the peritoneal surfaces to adhesion-promoting
substances. Gentle handling of the tissue is thought to reduce
the likelihood of adhesions. Although there is little that may be
gentle about myomectomy, the surgeon can operate in such a manner as
to minimize tissue damage. After the field is clear and exposure is adequate, the surgeon must plan
the uterine incision. This decision is based on the number and location
of the myomas. The most preferred incision is a vertical incision
on the anterior surface of the uterus. This minimizes blood loss and keeps
the ovaries from adhering to the posterior wall of the uterus postoperatively. Occasionally, the surgeon must perform “transcavity
enucleation” as described by Bonney,8 to avoid a posterior incision. In this manner, posterior fibroids can
be removed through an anterior uterine incision, which avoids the hazards
of a posterior incision. The caveat with this method is that the surgeon
must affirm that the posterior defect is adequately repaired, that
the cavity must not be compromised by suture, and that subsequently
delivery be performed by cesarean section. The surgeon often can remove
multiple myomas from a single incision, whereas at other times, multiple
incisions are required. Although there is no proof, it is felt
that minimizing the amount of damaged peritoneal surface relates linearly
to the extent of adhesion formation. Minimizing the length of the
uterine incision and the number of uterine incisions is a general strategy
to be employed. Another method for approaching the posterior myoma has been described by
Bonney and is called the Bonney hood (Fig. 2). This approach uses a transverse posterior fundal incision and subsequent
enucleation of the myoma. After interrupted sutures in layers are
used to close the dead space, the extra serosa is sutured with fine suture
to the anterior surface of the uterus, creating a functional anterior
incision. This allows a posterior approach but avoids a posterior
defect.  Fig. 2. The Bonny hood is demonstrated. A fundal posterior incision is made to
enucleate the myomas. After reapproximating the uterine muscle, the excess
serosa is draped over the fundus and fixed to the anterior surface
of the uterus with fine suture. This creates a functional anterior uterine
incision out of a posterior incision. Fig. 2. The Bonny hood is demonstrated. A fundal posterior incision is made to
enucleate the myomas. After reapproximating the uterine muscle, the excess
serosa is draped over the fundus and fixed to the anterior surface
of the uterus with fine suture. This creates a functional anterior uterine
incision out of a posterior incision.
|
The general strategy employed for removing a myoma regardless of location
involves incising through the pseudocapsule of the myoma. This maneuver
exposes the tissue planes and allows isolation of the capsule with
Allis clamps. Blunt and sharp dissection is used to peel the myoma out
of the capsule. The surgeon can use a knife, Mayo scissors, electrocoagulation, laser, or
blunt dissection to accomplish this. At some point, the
blood supply to the myoma is encountered and is best handled
by clamping or ligating before incising. This results in the excision
of the myoma, leaving an oozing cavitary defect in the uterus. If more
myomas are resectable through this incision, they are removed at this
point; otherwise, the wound is closed. With this method, the surgeon
mindful of vital adjacent structures (e.g. ureters, uterine vessels, cornua) to avoid pelvic injuries. Closing the resultant uterine defect involves much individualization of
approach. The goal is to restore normal anatomy and to ensure adequate
hemostasis, which requires attention to filling in the dead space. Despite
meticulous attempts to achieve this, much area often remains open
and fills in with blood. The resultant tamponade eventually aids in
hemostasis, but these pockets of blood provide a rich culture medium
for infection. Although some advocate the use of continuous suture in
this area, interrupted sutures afford greater chance at tissue approximation, minimizing
dead space. Layered interrupted sutures are time consuming
but provide the best opportunity at tissue coaptation. After the
myometrial layers have been adequately reapproximated, excess serosa
may be trimmed and the serosal defect repaired with a fine polyglycolic
suture in a running “baseball” fashion. This allows a
minimum of exposed suture material and decreases adhesion formation (Fig. 3). 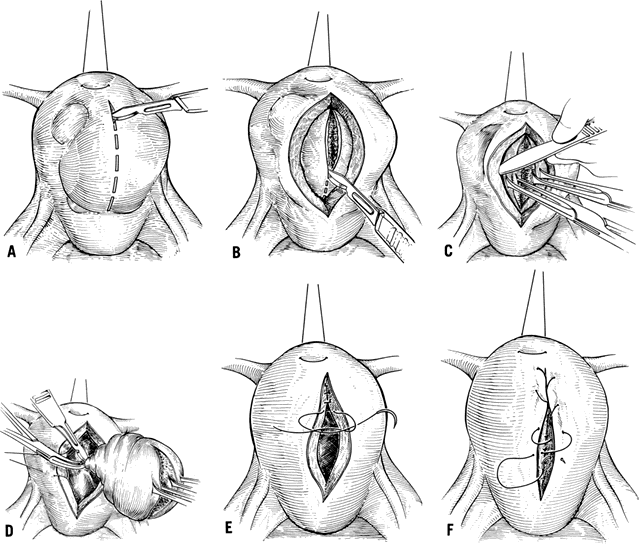 Fig. 3. Myomectomy technique. A. An anterior vertical uterine incision is made when possible. B. The pseudocapsule is incised, exposing the tissue planes. C. Blunt and sharp dissection of the pseudocapsule exposes the stalk of the
myoma. D. The remaining attachment of the myoma to the uterus is ligated and incised. If
possible, myomas are removed from this incision, avoiding a second
incision. E. The uterine muscle is reapproximated using layered interrupted delayed-absorbable
suture. F. The uterine serosa is sutured in a running “baseball” fashion, exposing
a minimum of suture and minimizing adhesion formation. Fig. 3. Myomectomy technique. A. An anterior vertical uterine incision is made when possible. B. The pseudocapsule is incised, exposing the tissue planes. C. Blunt and sharp dissection of the pseudocapsule exposes the stalk of the
myoma. D. The remaining attachment of the myoma to the uterus is ligated and incised. If
possible, myomas are removed from this incision, avoiding a second
incision. E. The uterine muscle is reapproximated using layered interrupted delayed-absorbable
suture. F. The uterine serosa is sutured in a running “baseball” fashion, exposing
a minimum of suture and minimizing adhesion formation.
|
Techniques to Reduce Blood Loss Myomectomy is perhaps one of the bloodiest gynecologic operations performed, and
the most significant morbidity associated with it includes blood
loss. A variety of methods have been described that are aimed at
minimizing blood loss. The two main approaches, medical and mechanical, diminish
uterine blood flow to the myoma. The blood supply to a myoma
is highly variable, and it is difficult to predict the blood vessel
location when dissecting the myoma. The anatomy can be significantly distorted
by the myomas, such that even the major blood supply to the uterus
may be altered. The optimal uterine incision to minimize blood loss
is an anterior, vertical incision. This cuts across a minimum of collateral
channels and a minimum number of blood vessels. One popular medical approach to decreasing blood loss in myomectomy uses 8-L-arginine
vasopressin (Pitressin). Dilute solutions of this are injected
directly into the myoma, raising a circumferential wheal and causing
vasoconstriction. Other agents such as Pitocin and epinephrine
have also been used. Most investigators report using pitressin diluted
from 0.2 to 1 unit per milliliter of diluent, using a maximum of 20 units
total. Some feel that this is unnecessary and that it only delays
bleeding. It does blanch the tissue and provide better visualization
of the tissue planes. Although the drug is not approved by the U.S. Food
and Drug Administration for this use, many have used it safely for
a number of years. Physicians must be cognizant of the potential side
effects of this medication, as for any medication. Its use is contraindicated
in patients with epilepsy, migraine, asthma, heart failure, and
nephritis. In patients with documented coronary disease, angina, and
even myocardial infarction has been described. Myomectomy is rarely performed
in such patients. Pitressin may produce water intoxication, the
early signs of which must be recognized. Headache, drowsiness, and
listlessness always precedes convulsions and coma, which can result from
severe overdosage of this medication. The most direct method to minimize blood loss during myomectomy is to use
mechanical methods that decrease uterine blood flow. Many methods have
been described using a variety of clamps and tourniquets, but all
apply the same general principle. Bonney8 first described the use of an atraumatic clamp that compresses the uterine
vessels and decreases uterine blood flow. The Bonney clamp is applied
from the pubic end of the abdominal wound; it must contain the round
ligament in its grip, or it slips below the uterine vessels. Blood
flow from the infundibulopelvic ligament is compressed using a ring forceps; the
uterine blood flow is essentially stopped. The location of
the ureter must be known before applying any clamps. Other variations
of this method use bulldog clamps, rubber-shod clamps, or tourniquets. The
disadvantage of using a tourniquet is that a small incision is often
made in an avascular area or the broad ligament (although this does
not have to be done). The tourniquet is then applied around the cardinal ligaments, obstructing
uterine vessel blood flow. A tourniquet can also be applied around
the infundibulopelvic ligament in a similar fashion. The defect in the
broad ligament must be repaired with a fine suture and therefore becomes
another site for possible adhesion formation. The unknown aspect of
these mechanical methods is the duration that the blood supply to the
uterus can be occluded before irreversible ischemic damage occurs. Some
investigators recommend that the clamps or tourniquets be released
every 15 minutes to prevent this phenomenon. Ranney and Frederick1 described a histamine-like substance that accumulates in the uterus that
has its blood supply obstructed. They suggest that this may lead to
postoperative shock. There are no reported cases of postoperative thrombosis
of the uterine vessels, necrosis of the uterus, and damage to
the tubes and ovaries, but the physicians must be aware of the potential
hypoxic injury to the uterus with this method. An additional strategy for minimizing the risk during myomectomy involves
the use of autologous blood. The acquired immunodeficiency syndrome (AIDS) crisis
has resulted in many hospitals offering patients undergoing
elective surgery the option of donating their own blood preoperatively. This
alleviates the AIDS risk and protects against hepatitis, which
is a much more common problem. Although this is not always practical
for the patient with menorrhagia, it does allow many the option. In
the patient with large fibroids, gonadotropin-releasing hormone (GnRH) agonists
can be used concomitantly to shrink the myomas. The GnRH agonists
allow time for the patient to bank autologous blood, and they
decrease the size of the myomas and thereby make myomectomy technically
easier. Patients can store up to 1 unit every 2 weeks, and with the
ability to freeze autologous blood, a large quantity of blood can be obtained. These
patients should be given supplemental iron during this
time. The cell saver has also been used, but there are not many reports
regarding its safety in this type of procedure. Techniques to Prevent Adhesion Formation Perhaps the biggest threat that myomectomy poses to fertility is that of
adhesion formation. Gehlbach and colleagues demonstrated that adhesions, even
at the time of myomectomy, significantly reduces the likelihood
for future conception.3 Second-look laparoscopy has demonstrated the formation of a significant
degree of pelvic adhesions as early as 8 days after abdominal myomectomy.9 Higher degrees of adhesion have been demonstrated with posterior wall
incisions, uterine size larger than 13 weeks' gestation, and with myomectomies
involving intramural myomas. Multiple strategies have been described
for minimizing this potential complication. The vertical, anterior
uterine incision can minimize blood loss and prevent the formation
of adhesions that involve the tubes, ovaries, or bowel. This incision
should be employed if feasible. Other strategies engage a variety of
uterine suspension techniques; medical approaches using dextran 70, promethazine, and
corticosteroids; copious irrigation; and meticulous hemostasis. The
uterus can be suspended in the classic manner described
by Gilliam, and the round ligaments are sutured to the fascia with silk
sutures. Mattingly and Thompson10 describe a modified Gilliam suspension using delayed absorbable suture. Olshausen11 describes a procedure that fixes the uterus to the anterior abdominal
wall and in some respects is similar to a Gilliam suspension. However, Mattingly
and Thompson suggest that the anchored uterus is a source of
pain during pregnancy.10 Another strategy for reducing postoperative adhesion formation was described
by Horne and associates.12 They demonstrated that treatment with intravenous Decadron (20 mg) and
intramuscular Phenergan (25 mg) every 4 hours for 48 hours postoperatively
can significantly reduce adhesions. DiZerega and Hodgen13 showed that instillation of 100 to 200 ml of 10% dextran 70 into the pelvic
cavity at time of closure also reduces adhesion formation by a flotation
effect. Diamond and others14 are actively pursuing the potential for Interceed (TC-7) and HAL-F (Seprafilm) to
prevent adhesion formation. Other means aimed at reducing adhesions involve gentle handling of tissue, placing
laparotomy pads in plastic bags to prevent lint particles
from escaping, removing particles from gloves, meticulous hemostasis, and
copious irrigation with heparinized Ringer's lactate (1000 units/1000 ml). Although
none of these methods has been proved to prevent
adhesions, most investigators make mention of them. Vaginal Myomectomy Rarely, a myoma manifests as a mass protruding through the cervical os. Invariably, these
tumors are pedunculated submucous myomas, which may
make them amenable to removal through the vaginal approach. This form
of myomectomy has the risk of increased infection, and occasionally hemostasis
cannot be achieved, forcing the surgeon to proceed to abdominal
hysterectomy. Despite these hazards, this approach has the advantages
of not entering the peritoneal cavity with subsequent adhesion formation, producing
a much faster recovery, causing generally lower blood
loss, and being a less deforming procedure. Goldrath15 described the use of laminaria tents preoperatively to dilate the cervix
and aid exposure of the pedicle. The surgeon can double clamp the pedicle
and incise the stalk with cautery or a knife and then place Heaney-type
sutures of 0-Vicryl. When this is feasible, a relatively fast
operation can be done. A myomas with a thicker stalk does not always
permit this approach, and the surgeon can incise the pedicle with cautery. The
pedicle usually then recedes into the uterine cavity, and the
physician observes for bleeding. If hemostasis cannot be achieved by
conservative management, hysteroscopy can be performed to attempt hemostasis
using directed cautery. If this fails, the surgeon can attempt
to pack the uterus and vagina and carefully observe the patient. The last
resort is to perform an abdominal hysterectomy. The physician should always use prophylactic broad-spectrum antibiotics
for this operation, because the risk of infection is especially high. These
patients should be counseled preoperatively about these risks and
the possibility of abdominal hysterectomy. Despite the hazards, this
operation usually results in a good outcome. Goldrath reports that 83 of 92 patients
had successful vaginal myomectomies.15 Hysteroscopic Resection of Myomas The patient with symptomatic submucous myomas presents the clinician with
a therapeutic challenge. When the uterus is small, an abdominal myomectomy
has distinct disadvantages. Dilation and curettage (D&C) does
not often resolve the menorrhagia, and multiple D&Cs frequently
must be performed. Hormonal therapy is often ineffective. The patient
who presents with menorrhagia needs a diagnosis, and the most direct
approach is to perform hysteroscopy in addition to endometrial sampling. This
approach distinguishes between submucous myomas, dysfunctional
uterine bleeding, endometrial polyps, and neoplastic causes. The patient who has submucous myomas as the cause of menorrhagia has many
options for treatment. For the patient who does not desire fertility, vaginal
or abdominal hysterectomy is appropriate management. The patient
who desires fertility has two options: hysteroscopic resection and
abdominal myomectomy. Not having to enter peritoneal cavity reduces
the likelihood of adhesion formation and clearly favors hysteroscopic
resection. Hysteroscopic resection is rapidly becoming the method of
choice for the surgical treatment of submucous myomas. Data suggest that
hysteroscopic resection provides fertility results that are comparable
or perhaps superior to abdominal myomectomy while providing the added
benefit of lower incidence of postoperative morbidity and shorter
length of hospital stay.16 Neuwirth17 described 28 patients who underwent hysteroscopic resection of submucous
myomas and had long-term follow-up. The patients were between 25 and 48 years
of age at the time of surgery; therefore, it is difficult to
make firm conclusions about postoperative fecundity. However, 17 of
the 28 patients had normal menses postoperatively, 7 underwent hysterectomy, 2 were
lost to follow-up, and 2 had repeat resections. Of the 17 patients, 5 became
pregnant with a total of 8 pregnancies. These numbers
suggest that this method is a viable treatment option for the infertile
patient with symptomatic submucous myomas. However, hysteroscopy
is not without its risks. Although rare, uterine perforation, distention, medium
hazards, infection, and hemorrhage are serious complications. The
patient must be counseled about the possibility of altered menses
postoperatively and the unknown potential for Asherman's syndrome. The technique of hysteroscopic resection sometimes requires laparoscopic
guidance. This prevents the possibility of damage to intraabdominal
structures during the procedure. The instruments required are a urologic
resectoscope that is designed to pass through the hysteroscope. The
uterus is distended with 32% dextran 70 delivered by pump or by hand-held
syringe. A cutting electrosurgical current (30 watts) is delivered
by a cautery unit. The submucous myoma is shaved down to the surface
of the surrounding myometrium, and hemostasis is ensured (Fig. 4). A sterile 12- or 14-French Foley catheter may be placed in the uterine
cavity and distended to provide tamponade and further ensure hemostasis
when necessary. This is left in place overnight. Premarin (2.5 to 5 mg) is
given daily for 21 days to avoid Asherman's syndrome. The
patient's recovery is relatively quick. This procedure requires
an experienced surgeon with highly specialized training. Some investigators
have used preoperative GnRH analog to shrink the myoma, although
there are no controlled studies to document the benefit. 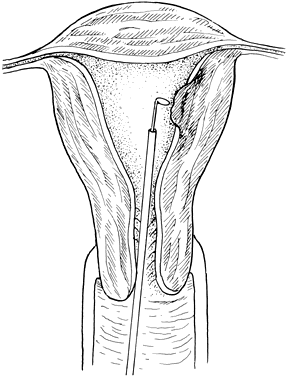 Fig. 4. Hysteroscopic resection of a submucous myoma. Under direct visualization
using laparoscopic guidance, a submucous myoma is shaved using the rectoscope. This
allows a vaginal approach to submucous myomas, avoiding
intraperitoneal adhesion formation. Fig. 4. Hysteroscopic resection of a submucous myoma. Under direct visualization
using laparoscopic guidance, a submucous myoma is shaved using the rectoscope. This
allows a vaginal approach to submucous myomas, avoiding
intraperitoneal adhesion formation.
|
Laser Myomectomy Many investigators have written about the use of laser in performing myomectomy. The
laser results in less adhesion formation, perhaps because
of less tissue damage. Some authorities believe that better hemostasis
can be achieved with the laser. Statements about improved reproductive
performance have also been made. Reyniak and Corenthal18 described a method of using a hand-held laser and microsurgical techniques
with a 70% pregnancy rate postoperatively in a small series. McLaughlin19 used a carbon dioxide (CO2) laser for metroplasty and myomectomy and claimed that there was less blood
loss and less tissue damage. Starks20 reported a 59% viable term pregnancy rate for 24 patients undergoing CO2 laser myomectomy. He states that the advantages of the CO2 laser for myoma surgery are decreased adhesion formation and improved
reproductive performance. Although these initial results are promising, it
is technically difficult to design a controlled study to document
these claims with greater certainty. It is safe to say that the laser
is at least equal to conventional myomectomy, but the data showing superiority
are lacking. Pelviscopic Myomectomy The laparoscopic approach to myomectomy is becoming increasingly popular. It
has been argued that, compared with the abdominal approach, this
technique offers a lower degree of blood loss, a shorter length of hospital
stay, and a lower overall complication rate.21 However, the procedure is not without its drawbacks. Laparoscopic resection
of multiple myomas is more time consuming, larger myomas are more
difficult to remove from the abdomen, and adequate repair of the myometrium
after removal of an intramural fibroid is often too difficult
to accomplish. Semm wrote about pelviscopic myomectomy using specialized pelviscopic instruments. After
numerous procedures, subsequent laparoscopy in a number
of patients revealed a minimum of adhesion formation. The surgeons
devised a number of methods to ensure hemostasis and to morcellate the
tissue to allow its removal laparoscopically. The procedure is limited
mainly to the removal of subserosal myomas, although it has been used
to remove intramural myomas. Blood loss is reportedly minimal, and
hemostasis is achieved with a minimum of complications. This operation
is limited to small myomas and should be performed only by the surgeon
skilled in operative pelviscopy. Based on their experience with 109 myomectomies, 70 of which were performed
laparascopically, Darai and colleagues recommended that laparoscopic
myomectomy be reserved for patients presenting with the maximum number
of four myomas, with none surpassing a diameter of 7 cm.22 Comparable spontaneous pregnancy rates have been reported after laparoscopic
and abdominal myomectomies in selected groups of patients.23 However, these results should be considered in the context of the criteria
chosen for patient selection. The outcome of the method employed
is closely associated with the size of the myoma, the number of myomas
present, and myoma location. Inadequate uterine repair after laparoscopic
myomectomy may result in grave obstetric consequences because of
uterine rupture.24,25 Women of childbearing age with symptomatic intramural fibroids should
undergo abdominal myomectomy or a modified laparoscopic procedure to ensure
proper closure of the myometrial defect.26It may be more prudent to reserve the complete laparoscopic approach for
the pedunculated and subserosal fibroids. |