As illustrated in Table 1,1,2,3,4,5,6,7,8 all honest clinical series reporting repair of pelvic organ prolapse show (1) some outright failures and (2) deterioration of initially good outcomes over time. Such clinical observations have encouraged surgeons to enhance their outcomes by supplementing their repairs with exogenous materials.
TABLE 1. Objective Success Rates of Pelvic Organ Prolapse Repair Procedures
Using Endogenous Tissues
Author, Year | Procedure | No. Patients | Follow-Up | Outcome (Objective) |
Tulikangas,1 2001 | Enterocele repair | 54 | 6–29 mo | 61% no prolapse |
Weber,2 2001 | Anterior colporrhaphy | 33 | Median 23 mo | 30% no prolapse |
Shull,3 2000 | Uterosacral ligament suspension | 298 | Mean 1 yr | 87% no support defects |
Comiter,4 1999 | Transvaginal culdosuspension | 100 | 6–35 mo | 96% no recurrence of prolapse |
Porter,5 1999 | Rectocele repair | 89 | >6 mo | 82% no prolapse |
Cundiff,6 1998 | Rectocele repair | 69 | 12 mo | 88% no prolapse |
Paraiso,7 1996 | Sacrospinous ligament suspension | 243 | Mean 74 mo | 58% no support defects |
Shull,8 1992 | Sacrospinous ligament suspension | 81 | 2–5 yr | 65% no prolapse |
The logical reconstructive materials to consider are the patient’s own tissues. For many years, gynecologic surgeons have relied on a variety of endogenous materials, including uterosacral ligaments, pelvic fascia, skin, muscle, and locally available connective tissue. These tissues may be used in their current location (e.g., uterosacral ligament suspension of vaginal apex) or moved to a new location for an alternative use (rectus fascia for suburethral sling). These materials are biocompatible and easy to use. The scientific evidence for their long-term durability varies. There are no randomized clinical trials comparing endogenous sources with other materials for reconstructive gynecologic surgery.
The uterosacral ligaments are probably the most used endogenous material for vaginal suspension. Every hysterectomy and many posthysterectomy vaginal support techniques rely on these native tissues. Several authors have studied the histologic components of this tissue, which is found to be composed of elastic, collagen, and smooth muscle fibers with scattered blood vessels.9 The term uterosacral ligament is a misnomer because these are not “ligaments” in any traditional sense given their composition of irregular connective tissue and abundant smooth muscle with its attendant autonomic nerve supply. Observant surgeons recognize how fleeting their form is when they are cut at the time of surgery. Their ligamentous appearance is secondary only to the anatomic tension of their environment. Surgeons selectively acknowledge the innervation of the uterosacral ligaments. Most commonly, these structures are transected and sewn without thought to the neural consequences. Certain surgical procedures use the opposite approach. During procedures to ablate midline pelvic pain, the uterosacral ligaments are transected or destroyed without regard to long-term vaginal support.
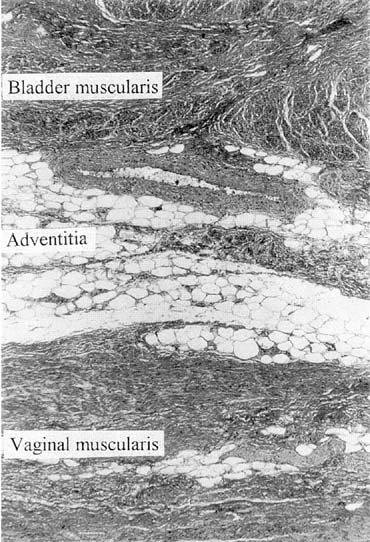
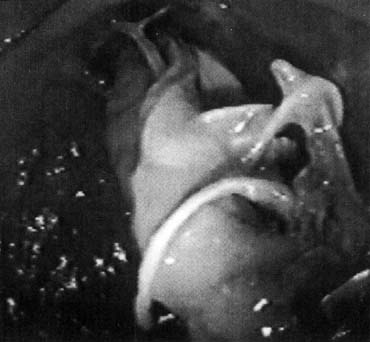
Vaginal fascia has been discussed in gynecologic operating rooms for more than 100 years, typically during anterior colporrhaphy. The histologic components of this material have been studied by Weber and Walters,10 who examined full-thickness sections of the bladder and vagina from autopsy specimens. Histologic examination confirmed that there is no vaginal “fascia”; rather the tissue between the vaginal mucosa and the bladder muscularis consists only of vaginal lamina propria and vaginal muscularis (Fig. 1). This layer is plicated during traditional anterior colporrhaphy. The histologic components of this tissue may help explain the relatively low anatomic success rates of anterior colporrhaphy. A layer that contains bladder adventitia and muscularis does not have the optimal biomechanical properties for long-term, durable anterior vaginal wall support. At the very least, this material is different from traditional fascia (rectus or fascia lata) and should not be considered to have similar biomechanical properties.
|
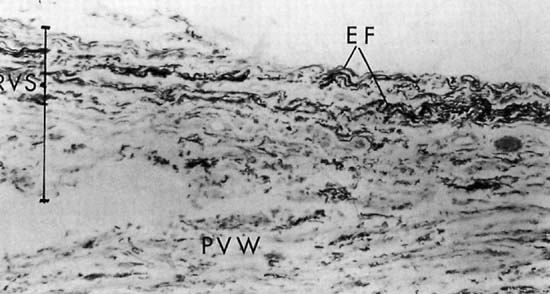
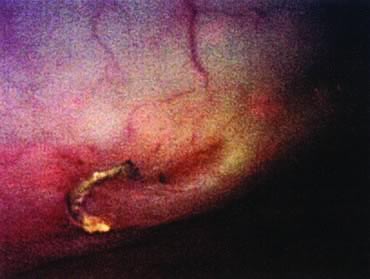
Repair of the posterior vaginal wall is a commonly performed gynecologic procedure. The most common material for this repair is the native rectovaginal fascia. There are no large case series that report the sufficiency of this tissue for its intended purpose. Experts commonly discuss the untoward side effects of posterior vaginal surgery without focus on anatomic recurrence of poor support. The exact histologic nature of the rectovaginal fascia has been the subject of some debate. One study found that the rectovaginal fascia consisted mainly of collagenous fibers11 and did not contain muscle cells (Fig. 2). In contrast, Milley and Nichols12 mentioned the presence of smooth muscle cells within the fascia. It may be that those muscle fibers originate from the external longitudinal muscle layer of the rectum.13 All authors agree that nerves of the hypogastric plexus run in the ventrolateral junction of the fascia with rectum. Surgical techniques that rely on this layer for posterior wall support conceivably could disable the delicate neuromusclar function of the rectovaginal axis. Techniques that document good efficacy with less dissection or plication5,6,14 may be preferable to traditional full-length colporrhaphy techniques.15,16
The vaginal wall is also known to be a pliable, readily available surgical tissue. This tissue has been used for patch slings and vaginal repairs. It is unsuitable for these purposes because it does not have the biomechanical properties to resist stretching.17 This fact is easily appreciated by anyone who has witnessed vaginal birth during which the vaginal skin widely dilates to accommodate the emerging fetus. Similarly, skin throughout the body has phenomenal properties of stretch, which makes it unsuitable for long-term support.
|
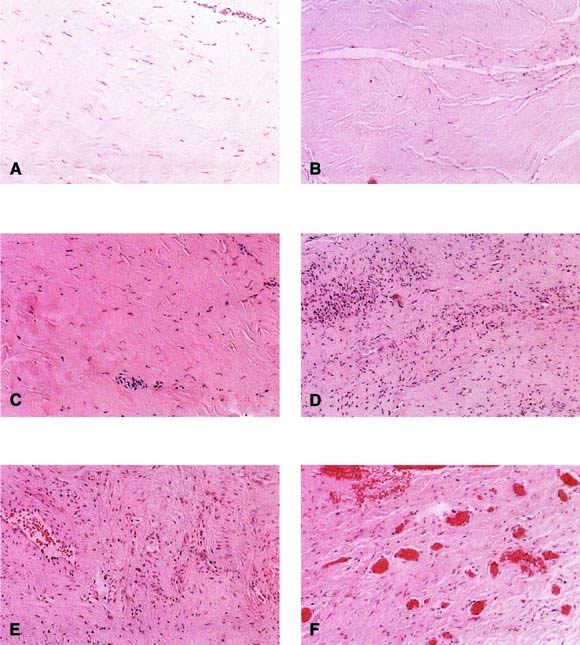
Autologous rectus fascia seems to be an ideal material for fascial reinforcement. This tissue is used commonly for suburethral sling and occasionally is used for sacrocolpopexy. Rectus fascia is biocompatible, strong, and appropriately pliable. Also it is readily available, although the amount harvested is directly related to the risk of incisional hernia. Despite its widespread use in suburethral slings and sacrocolpopexy, the rate of failure of this material is not well known. The surgeon would want this material to remodel to gain strength and appropriate vascularity. FitzGerald and coworkers18 reported on the histologic appearance of rectus fascia used for suburethral slings and found that after implantation there was fibroblast proliferation, neovascularization, and remodeling of the fascia graft. Some linear orientation of connective tissue and fibroblasts occurred, probably along the lines of force on the graft (Fig. 3). A randomized trial comparing materials in this area is needed.
|
Another source of endogenous fascia is fascia lata, typically harvested through a lateral incision in the thigh. This material is strong, durable, and readily available. One report of long-term problems after fascia lata harvest suggests caution, especially with increasing age. Walters and associates19 found that among 55 patients 2 years after fascia lata harvest, 25 (46%) had subjective complaints, including discomfort, weakness, lateral thigh bulge, or unacceptable incisional cosmesis. The histologic fate of fascia lata after implantation has not been reported.
There are additional, more experimental native tissues being considered for surgical use. Bioengineering and cell culture techniques have used novel techniques to harvest material from the patient, expand and enhance the tissue, and replace it in a clinically useful manner. Such tissue techniques include culture of muscle cells,20 ear chondroblasts,21 and entire bladder wall.22 Although these are not currently ready for routine clinical use, this area of investigation is expanding rapidly.