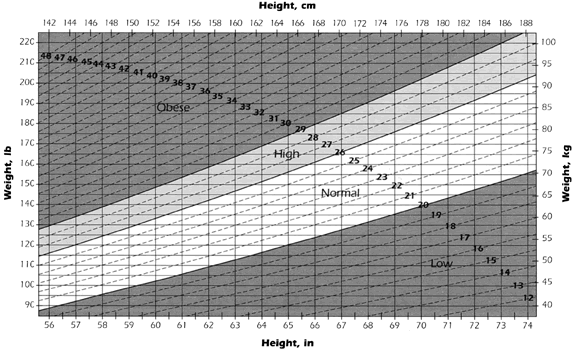
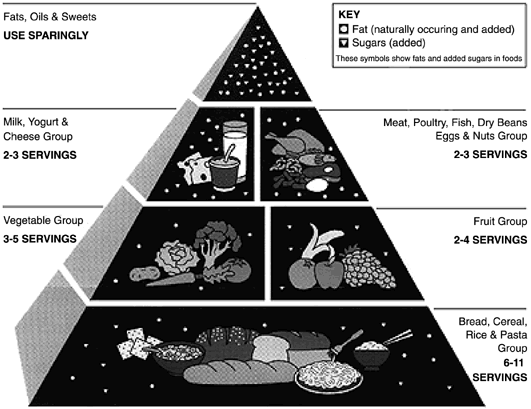
Calories The calorie cost of pregnancy is said to be approximately 80,000 calories
for the course of pregnancy.18 Calorie requirements should be determined for each individual based on
prepregnancy weight, diet history, height, activity level, time of gestation, and
weight gain goals. The 1989 RDA for calories is an additional 300 calories
per day after the first trimester,19 based on the assessed calorie needs before pregnancy. Energy intake is
adequate when the rate of maternal weight gain meets the IOM guideline.5 Additional calories should be derived from foods that were deficient in
the pregnant woman's diet before pregnancy. For example, most pregnant
women do not meet their calcium requirements before pregnancy; however, an
additional 2 cups of low-fat milk will increase calorie intake
by 250 calories (or approximately the recommended increase) and
provide the additional calcium. Carbohydrates Carbohydrates should constitute approximately 50% of the diet for most
pregnant women. Carbohydrates are important for energy production, and
they spare protein from being used as energy. Whole grains, fruits, vegetables, milk, yogurt, beans, and peas contain carbohydrates. The carbohydrate
group provides much of the fiber necessary to help prevent
constipation and hemorrhoids. At present, the safety of a high-protein, low-carbohydrate
diet is unknown. Ketones are produced with low-carbohydrate
diets, which may be contraindicated in pregnancy. Regular carbohydrate
intake is recommended according to the Food Guide Pyramid. Protein Protein is required for the growth of maternal and fetal tissue. The effects
of a protein deficiency on the outcome of pregnancy are difficult
to assess. Most women who have low protein intakes also have a caloric
deficit. The RDA for protein is 60 g/day to meet pregnancy needs,19 and can be met using protein from animal and plant sources. The average
protein intake in the United States is approximately 75 to 110 g/day. Quality
as well as quantity of protein should be considered when assessing
the protein intake of the pregnant women. Adolescents and women
with multiple gestations have increased protein requirements. Fat Fat requirements are unchanged during pregnancy. The American Heart Association
guidelines for fat are 30% of total calories per day. The fat
intake of a 2000-calorie diet is 60 g/day. Saturated fats should be limited
and the majority of fat should be monounsaturated and polyunsaturated. One
serving of monounsaturated and polyunsaturated fats includes
foods such as 1 teaspoon of olive, peanut, canola, or corn oil, 2 whole
walnuts, 6 almonds, and 10 peanuts, 2 teaspoons of peanut butter, one
eighth of an avocado, and 10 olives. Some research has shown that
the long-chain polyunsaturated fat acids, in particular docosahexaenoic
acid (DHA) and arachidonic acid (AA), in the maternal diet play a role
in fetal growth and development.20 DHA and AA (n-3 fatty acids, found in fish, shellfish, flaxseed, walnuts, and
canola oil) have been associated with neonatal brain development
and visual acuity of infants. They may also have a role in improving
birth weights, decreasing the incidence of intrauterine growth retardation, and
preventing preeclampsia.21 The quantity, ratio of n-3 to n-6 fatty acids, and level of safety have
not yet been established. Until then, no specific recommendations can
be made for pregnant women. Vitamin A Vitamin A is required for its role in vision, cellular growth and differentiation, and
immune function. Vitamin A deficiency in HIV-positive
pregnant women has been shown to increase maternal-fetal transmission
in parts of Africa. Further studies are underway to identify the role
of vitamin A in this population.22 Vitamin A is readily available in the food supply of the United States. The
best food sources of vitamin A include leafy green and orange vegetables, such
as spinach, dark green lettuce, carrots, sweet potato, acorn
and butternut squash, cantaloupe, and apricots. Vitamin A deficiency
is rare in industrialized countries, but quite common in other parts
of the world. In rat studies, vitamin A deficiency showed impaired
lung development23 similar to that seen in premature babies with respiratory distress syndrome. These
babies are often found to be vitamin A deficient. The vitamin
A content of breast milk is associated with the vitamin A status
of the mother during the third trimester of pregnancy. Breast milk of
the mildly undernourished woman can be vitamin A deficient after the first
few weeks of lactation, putting the infant at risk for health problems
related to inadequate vitamin A.24,25 A greater concern regarding vitamin A is the teratogenic effect in animal
and human studies of excess intake of preformed vitamin A (retinol).4,26,27,28 Beta-carotene, a precursor of vitamin A, does not seem to be teratogenic. In
humans it has been found that isoretinoin, a vitamin A analog, has
been associated with fetal anomalies involving the central nervous
system, and craniofacial, cardiovascular, and thymus malformations.29 Due to the possibility of its teratogenic effects, the World Health Organization (WHO) recommends that vitamin A intake in supplements used
before or during pregnancy should not exceed 10,000 IU/day (3000 μg).30 The Teratology Society of the United States recommends total vitamin A
intake from food or supplements should not exceed 8000 IU/day (2400 μg).31 In 2001, the Food and Nutrition Board of the National Academy of Sciences
recommended a Dietary Reference Intake (DRI) of 770 μg (2500 IU) of
vitamin A for pregnant women and an upper tolerable limit of 3000 μg (10,000 IU) per
day.32 The IOM recommends avoiding vitamin A supplements, especially in the first
trimester.5 Preformed vitamin A is found only in animal products, such as meat, milk, eggs, fish, and
chicken. Beef liver has more than three times the
amount of preformed vitamin A than is currently recommended. Provitamin
A beta-carotene sources includes dark green leafy and orange vegetables
and fruits. When vitamin A supplements are prescribed, it is important
to check the source of the vitamin A. Some prenatal vitamins do not
identify the source of vitamin A as either preformed vitamin A or beta-carotene. Avoid
vitamins that do not list the specific type of vitamin
A. Women with a low serum retinol concentration and poor intake of
vitamin A would require supplementation. Folic Acid Inadequate intake of folic acid periconceptually is associated with an
increased risk of neural tube defect. An additional 400 μg of folic
acid used 3 months before and for the first 12 weeks of pregnancy has
been shown to decrease the occurrence of neural tube defect by 70%.2,3 In addition, the Centers for Disease Control and Prevention recommend
that to prevent recurrence of neural tube defect, a folic acid supplement
of 4 to 5 mg/day should be consumed.33 Folic acid is necessary as a coenzyme in single-carbon transfers from one
compound to another. This is important, especially in pregnancy, in
the synthesis of several amino acids involved in nucleic acid synthesis. A
deficiency of folic acid in pregnancy may impair cell growth and
replication and has been associated with poor pregnancy outcome.34 Three sources of folate are currently available. The first source is folate
naturally occurring in food. The bioavailability of dietary folate
is approximately 50% of synthetic folate.35 In 1998, the Food and Drug Administration (FDA) required that enriched
grains be fortified with folic acid 1.4 μg/g of product.36 These fortified foods are the second source of folic acid. A recent report
suggests the incidence of neural tube defects in the United States
has decreased by 19% since the introduction of folate-fortified foods.37 The third source of folic acid is synthetic folate from vitamin supplements. Folic
acid in fortified foods and those provided by supplements
are absorbed at a rate of 85% to 100%.38 The recommended DRI for folic acid in pregnancy set by the Food and Nutrition
Board of the National Academy of Sciences is 600 μg/day with
an upper tolerable intake level of 1000 μg/day.39 A level of 600 μg/day was found to maintain normal folate status. This
level can be achieved with a diet high in naturally occurring folate
and a supplement of 400 μg/day. Dietary sources of folate include
leafy green vegetables, orange juice, strawberries, dried peas, beans, and
foods enriched with folate such as whole-grain breads, pasta, rice, and
cereals. Women at risk for folic acid deficiency includes those
with low dietary intake, and those who use medications that are folate
antagonists such as trimethoprim, triamterene, carbamazepine, phenytoin, phenobarbital, and
primidone. Most pregnancies are unplanned, and even those that are planned often do
not meet the recommendation for folate. At their gynecology visits, women
of childbearing age should be advised to take a folic acid supplement
and include good food sources of folic acid in their diets. Iron Iron-deficiency anemia is the most common form of nutritional deficiency. The
rate of iron-deficiency anemia is high among low-income pregnant
women. No improvement in anemia has been seen in this group since the 1970s. Iron-deficiency
anemia is associated with a higher incidence
of low-birth-weight infants and a shorter length of gestation. Trials
have shown that when iron supplementation was provided, the incidence
of low-birth-weight infants was decreased.41 Iron absorption is increased during pregnancy and depends on maternal iron
stores. Heme iron (iron from animal sources) is two to three times
more absorbable than non-heme iron, plant-based foods, and iron-fortified
foods. Non-heme iron is better absorbed in the presence of heme iron
and vitamin C. Absorption is impaired when consumed with phytate (e.g., bran, whole
grains), calcium (especially calcium in supplements), tannin
in teas, and polyphenol in some vegetables. The Continuing Survey of Food Intakes by Individuals (1994 to 1996) (CSFII)42 suggests that one quarter of adolescent girls and women of childbearing
age meet the RDA for iron through diet. National Health and Nutrition
Examination Survey (NHANES) III data indicate 11% of non-pregnant women 16% to 49% had
iron deficiency and 3% to 5% had iron-deficiency anemia.43 The dietary reference intake for iron is 27 mg/day. The tolerable upper
level of intake is 45 mg/day.32 In 1998, the Centers for Disease Control and Prevention made recommendations
for preventing and treating iron-deficiency anemia during pregnancy. Primary
prevention includes recommending iron supplementation (30 mg) for
all pregnant women at the first prenatal visit, encouraging
dietary iron from foods with high iron content, and increasing foods that
enhance iron absorption. Iron supplementation may be beneficial to
prevent women from entering a subsequent pregnancy with low iron stores. For
secondary prevention, they recommend screening for anemia at the
first prenatal visit.44 The guidelines for anemia recommended by the IOM should be followed. The
cutoff values for anemia in pregnancy are as follows: first trimester, hemoglobin 11 and
hematocrit 33; second trimester, hemoglobin 10.5 and
hematocrit 32; and third trimester, hemoglobin 11 and hematocrit 33.5 Treat anemia with iron supplementation, 60 to 120 mg/day, and discuss
dietary changes to improve absorption and intake. When hemoglobin and
hematocrit become normal, decrease iron to 30 mg/day.44 Studies are being done to determine the best way of dosing iron, and have
looked at weekly, daily, and multiple daily dosing. Compliance with
iron supplementation is often poor because of associated gastrointestinal
side effects. Calcium Calcium is important for bone and tooth formation and maintenance, and
is required in nerve transmission and regulation of the heartbeat. During
pregnancy, calcium is transferred to the fetus at a rate of approximately 330 mg/day
by the third trimester.5 Calcium absorption and urinary excretion of calcium are greater than before
pregnancy. These changes are noticeable before fetal calcium demands
are actually increased.45 Multiple studies have measured the changes in bone density in pregnant
and lactating women. Bone mineral losses are seen by 3 months postpartum, and
by 5 months after menses begin the bone mineral content returns
to prepregnant status.46,47 Lactating adolescents are the one group at risk for permanent bone loss. One
study of lactating adolescents showed bone loss in the forearm
of those not supplemented with calcium, whereas those supplemented with
calcium had little to no bone loss.48 A study of Indian women with typical calcium-deficient diets showed that
neonates whose mothers received a calcium supplement had higher bone
density than those whose mothers did not receive a supplement.49 Low calcium intake has also been associated with an increased risk of pregnancy-induced
hypertension. In some studies, calcium supplements have
been shown to reduce blood pressure of pregnant women and their offspring. Bucher
conducted a metanalysis on 14 randomized controlled trials. In
most of the studies, the usual calcium intakes were below the
RDA for calcium. It was found that 375 to 2000 mg of supplemental calcium
had a blood pressure-lowering effect. The study showed calcium supplementation
reduced the risk of developing pregnancy-induced hypertension
and preeclampsia in women who were normotensive before pregnancy. When
a further study was done of women with hypertension, 1000 mg of
calcium significantly lowered their diastolic blood pressure.50 Another study done by the Calcium for Preeclampsia Prevention (CPEP) group
found no change in blood pressure in a group of women with usual
calcium intakes of 1100 mg/day who were given an additional 2000 mg/day
by calcium supplement.51 The differences in Bucher's metanalysis and the CPEP study may be
explained by the latter group having a normal calcium intake. When calcium
intakes are within the normal range, additional calcium may not
have a beneficial effect. The Food and Nutrition Board of the National Academy of Sciences set the
Adequate Intake (AI) for calcium in pregnancy at 1000 mg/day for women
age 19 to 50 years and 1300 mg/day for adolescents under 19 years. The
tolerable upper intake level is 2500 mg/day through diet and nutritional
supplement.52 Milk (8 oz), yogurt (8 oz), and cheese (1.5 oz) all provide approximately 300 mg
of calcium. Almonds, broccoli, sardines and salmon with bones, soy
milk, and calcium-fortified orange juice are also good sources
of calcium, although portions vary. Dietary calcium sources should be
encouraged. If calcium intake is below the RDA, a calcium supplement
should be considered. Calcium carbonate from Tums and other supplements
should be taken with a meal. Calcium citrate can be taken with or without
meals. |