There are several ways to perform cesarean hysterectomy successfully. Any competent gynecologic surgeon should be able to accomplish the task with dispatch and should respect, but not fear, the special problems posed by the operation. When cesarean hysterectomy is planned in advance, the surgeon has the luxury of a complete preoperative evaluation of the patient and her hematologic and coagulation status. The surgeon can prepare to fill the bladder to test its integrity, should that become necessary. The surgeon can choose experienced assistants and discuss procedures, necessary instruments, and sutures before the operation. In emergent cases, such luxuries do not exist, and the scene often becomes chaotic.16,17
Cesarean hysterectomy is really two operations: cesarean section and hysterectomy, one following the other in an orderly fashion. Haste is required only briefly at the beginning of each operative segment. Efforts at speed that replace careful surgical technique court disaster. The surgeon should strive for steady, deliberate progress through the successive steps of the operation.
Cesarean hysterectomy can be accomplished through any commonly used abdominal wall incision. A vertical incision provides excellent exposure; however, many patients and surgeons are not satisfied with the cosmetic effects. A transverse incision may be desirable, especially if a previous transverse scar is present. This incision can provide adequate exposure, and, if needed, it can be converted into a Cherney incision by transecting the rectus muscle at its insertion. An alternative approach used specifically in patients with previous transverse incisions is division of the rectus muscle converting a standard Pfannenstiel to a modified Maylard incision. The following description of cesarean hysterectomy technique is that used by the authors for emergent procedures.
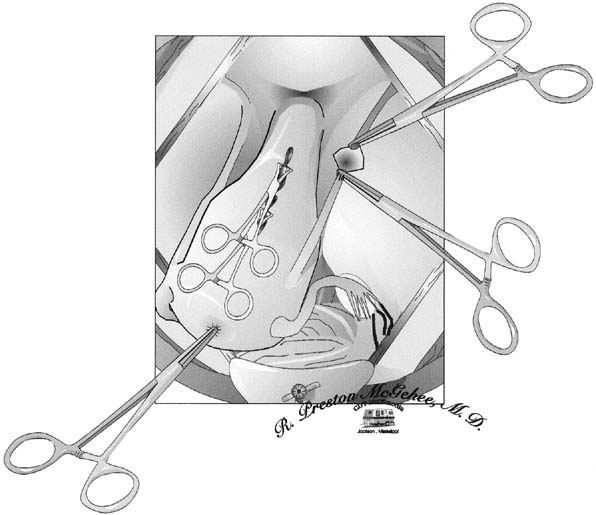
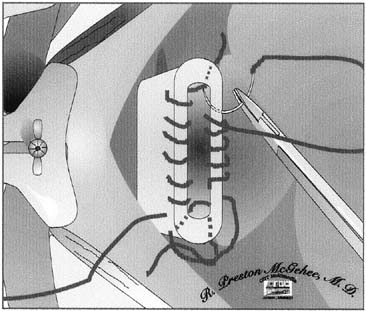
The type of uterine incision used is guided by obstetric indications; however, a low vertical incision is less likely to extend and lacerate the uterine vasculature. After the infant is delivered and the decision is made to proceed with cesarean hysterectomy, the placenta is expressed unless there is a placenta accreta. The fundus is grasped, and the uterus is removed from the pelvis and placed on the abdominal wall. Using the uterine incision for traction is not advisable, because it may lead to extension of the incision. From this point forward, the uterus should be kept under upward traction to constrict the uterine vasculature and diminish blood loss. The hysterotomy incision is then closed with a running suture or towel clips (Fig. 1). Reapproximating the myometrium optimizes uterine contractility and decreases the occurrence of uterine atony. If there is considerable bleeding from the placental site, the uterus can be packed with one or two laparotomy pads above the site before uterine closure; this may serve to tamponade the bleeding.
|
The round ligaments are divided between Kocher clamps and ligated (see Fig. 1). Care should be taken to place the tips of the clamps in the avascular portion of the broad ligament to avoid lacerating Sampson's artery. The broad ligament is then incised anteriorly to the point where the vesicouterine peritoneum was dissected off the lower uterine segment to create the bladder flap. Posteriorly, the broad ligament is incised laterally and parallel to the infundibulopelvic ligament to expose the retroperitoneum. The loose areolar tissue encountered in this space, which is more pronounced than usual because of the enlarged uterus, can be carefully dissected parallel to the course of the ureter. This allows visualization of the retroperitoneal space and the ureter throughout its course.
Before approaching the uterine arteries, the bladder must be dissected free and displaced below the operative field. Because most patients undergoing cesarean hysterectomy have had previous surgery, significant adhesive disease is frequently encountered; as a result, sharp dissection is the technique of choice. It is extremely important to avoid lateral dissection into the highly vascular bladder pillars. It is also wise not to extend the dissection farther than is necessary to safely ligate the uterine arteries, because excessive dissection can cause additional bleeding and waste time.
|
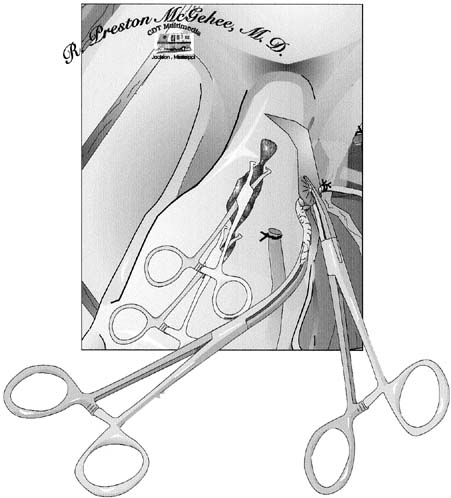
Attention is then directed toward the uterine arteries. The uterine vessels are significantly enlarged in pregnancy, and care must be taken in isolating or skeletonizing them. A Heaney clamp is placed with the distal portion perpendicular to the vessels at the level of the internal cervical os (Fig. 2). Once the same procedure is repeated on the contralateral side, the uterine arteries are secure, and blood loss should rapidly diminish. The use of a second clamp on the specimen side reduces back-bleeding from the uterus, which can obstruct the operative field. Additionally, until the utero-ovarian ligament is ligated, a substantial blood supply to the uterus persists. We suture ligate the uterine arteries using an 0 or 00 polyglycolic suture using a Heaney ligature; others advocate passing a suture beyond the tip of the clamp and tying behind the clamp. Using a Heaney ligature divides the pedicle in half and may cause less chance of the vessels retracting. Another technique to prevent retraction of the vessels is to incorporate the leaves of the broad ligament into each pedicle. Care must be taken not to place lateral or downward traction on these clamps, which might tear friable tissues and cause bleeding that cannot be easily controlled. These clamps should be supported and not manipulated.
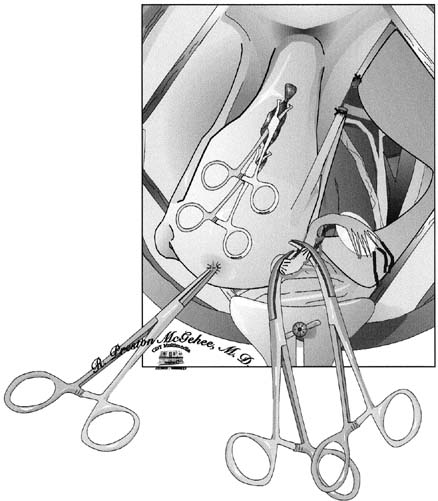
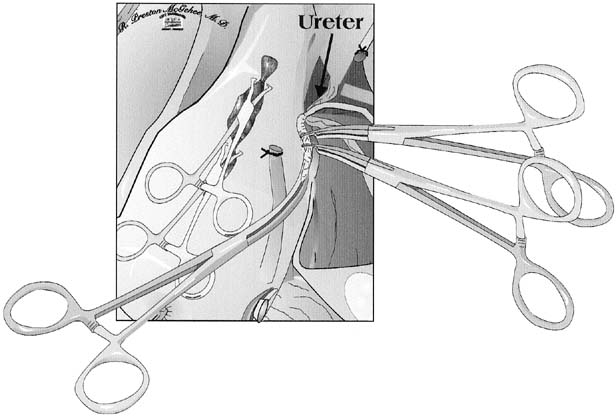
A second Heaney clamp is placed medial to the first clamp. Careful placement of clamps medially, moving down the parametrial tissue and hugging the cervix, will allow ligation of any branches of the uterine artery not ligated with the first clamp and ensure the safety of the ureter. After the uterine arteries are ligated, the utero-ovarian ligament can be approached. An avascular window in the peritoneum is created, thus allowing the pedicle to be isolated. Heaney clamps are placed close to the uterus to ensure that the ovary is not damaged (Fig. 3). Once divided, a free tie is placed on the specimen side, the utero-ovarian ligament is suture ligated. Because most patients in our series required oophorectomies because of infundibulopelvic ligament hematomas, we place a free tie proximal to the suture ligated pedicle.
|
With the entire blood supply to the uterus now secured, a critical decision must be made in performing an emergent cesarean hysterectomy. If the patient is unstable or if the amount of bleeding has been excessive, the surgeon should consider to a subtotal hysterectomy, which shortens operating time while still accomplishing the primary goal of hemostasis. Although most surgeons prefer to remove the cervix, subtotal hysterectomy should not be dismissed, because it can be life-saving. If subtotal hysterectomy is performed, the uterus is amputated with a scalpel; if possible, this is achieved by cutting superiorly to the ligated uterine arteries while angling the scalpel blade medially and downward. This technique allows removal of an inverted cone of the cervix, which will facilitate approximation of the edges of the cervical stump regardless of the degree of dilation. Once amputated, the cervical stump can be approximated in an anterior-to-posterior fashion using interrupted figure-of-eight ligatures. Special care should be taken to avoid the bladder. If the patient is stable and the cervix can be removed, the task can prove to be difficult. As the cardinal ligament dissection proceeds downward, dissection of the bladder must also advance. Sharp dissection with Metzenbaum scissors (with tips pointing downward) ensures that no harm comes to the friable posterior bladder wall. Bladder dissection need never progress more than 1 cm distal to the current cardinal ligament dissection.
The next step is the identification of the lowest extent of the dissection: the junction of the cervix and vagina. This junction can usually be felt between the thumb and forefinger by palpating the upper vaginal walls and encountering the thickened cervix. When the cervix is dilated, it can be difficult to discern its lower end. The best approach to this problem is to place each clamp of the cardinal ligament complex medial to the preceding pedicle and roll it off the cervix to lie exactly against the lateral cervical wall. Each pedicle should be no longer than the distal third of the clamp used to grasp it. This allows the surgeon to progress down the cervix while each pedicle falls laterally, and the safety of the ureter is further ensured. In some cases, the uterosacral ligaments are quite prominent and may need to be individually clamped to completely ligate them. As the surgeon approaches the external cervical os, the towel clamps or suture line approximating the uterine incision can be released, and the cervix can be palpated from within the uterus (Fig. 4). Once the cardinal ligament dissection reaches the lower limit of the cervix, the vagina is entered, usually at the last pedicle. Curved scissors are used to amputate the cervix. The inside blade of the scissors is placed just beneath the cervix in the vaginal fornix and circumscribes the upper vagina to complete the dissection under direct vision. Allis or Kocher clamps maintain traction on the anterior, posterior, and lateral angles of the vaginal wall. The vaginal epithelium is very friable and must be handled gently.
|
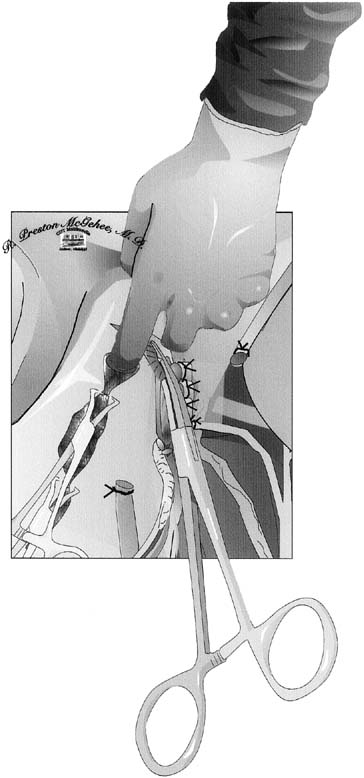
Surgical management of the vaginal cuff begins with supporting angle sutures that incorporate the lateral vaginal angles, the lower cardinal ligament pedicles, and the uterosacral pedicles. We usually hold this suture as a stay suture for subsequent identification of the vaginal angles. The surgeon may now choose to close the vaginal cuff with continuous or interrupted sutures or to leave the cuff open and to secure hemostasis of the anterior and posterior cuff edges with a continuous nonlocking suture (Fig. 5). This will allow for drainage until spontaneous reperitonealization occurs.
If the surgeon is suspicious that the integrity of the bladder wall has been breached at any time during the operation, the bladder may be filled with an opaque solution and the operating field inspected for extravasation of the solution. A second choice would be to administer intravenous indigo carmine and examine the area of the bladder for extravasation. If there has been an inadvertent cystotomy, the area is dissected so that two rows of sutures can be placed without tension. The bladder is closed with two continuous layers of 4.0 polyglycolic sutures, with the second layer imbricating the first. Permanent suture material should never be chosen for bladder closure. The bladder is then refilled to ensure its integrity. When bladder repair is necessary, postoperative antibiotic coverage and Foley catheter drainage of the bladder should be continued for 7 to 10 days. A Jackson-Pratt drain is placed in the retroperitoneal space near the bladder repair and led out through a separate stab wound in the lower abdomen.
All pedicles are individually reinspected for hemostatic security. The pelvis is copiously washed with warm saline and water. Sponges and retractors are removed, and instrument, needle, and sponge counts are checked. The abdominal incision is closed in the routine fashion.