Preexisting Diabetes MATERNAL GLUCOSE CONTROL. As outlined above, maternal metabolic control to near-euglycemic levels, at
least in the latter half of pregnancy, can reduce the perinatal
mortality rate and may prevent various manifestations of perinatal morbidity
by preventing or reducing fetal overproduction of insulin. Before
pregnancy and during early pregnancy, improved metabolic control has
the potential to prevent congenital malformations. Indeed, a program
of preconception counseling and care has been demonstrated to be effective
in reducing the likelihood of birth defects in the offspring138,139 and also has been shown to be cost-effective.140,141 Unfortunately, only about one third of persons with preexisting diabetes
currently receive preconception care according to a multicenter study.142 It is critical for obstetricians and other primary care providers, such
as internists, family physicians, and pediatricians, to provide effective
counseling to all diabetic women in or approaching the reproductive
age. The importance of family planning, the evaluation of co-morbid
conditions, and the importance of good metabolic control before conception
are all part of preconception care. The question is still unsettled
as to precisely how close to normal maternal circulating glucose
values must be in order to maximize the likelihood of a favorable outcome. Various
opinions exist, and the suggestions outlined below must be
considered tentative; they are based on the practices at our institution, and
they have neither been proved nor universally accepted. Nevertheless, virtually
all investigators in the field agree that the best
outcomes are associated with “good” control. The ideal approach to management of diabetic pregnancy is the “team
approach,” in which the high-risk pregnancy obstetrician and
the diabetologist collaborate as part of a multidisciplinary team, which
also includes specialized nurses, dietitians, social workers, and other
health-care providers. Not every component of this team is expected
to be available in every center. In addition, even if the diabetes
is being managed by a diabetologist, it is important for the obstetrician
to understand what is being done, and why. Therefore, the foregoing
discussion of medical management is included in this obstetrics textbook. Modern management of diabetes in pregnancy relies on the use of self-monitoring
of glucose levels. Test strips, impregnated with glucose oxidase
and an indicator, are covered with a drop of blood, and the reaction
is allowed to proceed for a predetermined period of time. The blood
is then washed or wiped off, depending on the type of strip, and inserted
into a reflectance meter that interprets the blood glucose. These
machines, originally quite complicated to use and expensive to purchase, have
come down in purchase price and are all relatively simple to
work with. They are battery powered and portable. Various schemes exist
as to the timing and frequency of self-testing of blood. In some centers, the
patient is asked to measure glucose just before meals and snacks, three
to four times per day. In other centers, glucose levels are
checked at fasting and at 1 or 2 hours after each meal. In yet other
centers, the time of glucose measurement after meals is determined by
testing initially every 15 minutes in order to choose the interval of
peak glucose levels. Relatively little in the way of comparative data
are available. Diabetologists tend to rely more heavily on fasting than
on postprandial hyperglycemia in managing non-pregnant adults with
diabetes, but existing studies suggest that in pregnancy postprandial
glucose values are better predictors of fetal macrosomia than are fasting
values.143,144,145 When 1-hour postprandial values exceed 130 mg/dL145 or average glucose values exceed 130 mg/dL,146 macrosomia has been found to be significantly more likely. A 1995 randomized trial compared daily preprandial glucose measurements
with postprandial testing. When insulin dosage was adjusted according
to postprandial values, macrosomia, neonatal hypoglycemia and cesarean
section rates were significantly lower compared with the group using
preprandial measurements.147 The single most important issue may well be whether patients are frequently
self-monitoring their glucose levels! In our own center we ask patients
to measure glucose a minimum of four times per day. This includes
fasting and 2 hours after each meal. Additional times are chosen if
they seem clinically necessary: for example, a 2 AM measurement if there
is a question of morning rebound hyperglycemia (the Somogyi effect) or
early morning insulin depletion (the “dawn phenomenon”). An
outline of our approach is depicted in Table 6. TABLE 6. Daily Self-Monitoring of Glucose in Diabetic Pregnancy
Times of Testing | Goals (mg/dL) |
Fasting | 60–100 |
2 h post-breakfast | 70–120 |
2 h post-lunch | 70–120 |
2 h post-dinner | 70–120 | There are also a number of different approaches to insulin administration
in diabetic pregnancy.148 It is rare for satisfactory metabolic control to be attained in a pregnant
woman with preexisting diabetes by a once daily insulin regimen. We
have found that a minimum of two injections of mixtures of short- and
intermediate-acting insulins are generally necessary, and that many
persons require three or four injections daily. To begin insulin therapy
in a previously uncontrolled patient, one should use a formula that
was developed on the basis of the normal insulin release pattern of
nondiabetic pregnant women.149 Insulin is injected before breakfast and before dinner. The morning total
dose is twice the evening total dose. The ratio of intermediate- to
short-acting insulin in the morning is 2:1; in the evening it is 1:1. For
example, a patient may receive 40 U of NPH insulin and 20 U of regular
insulin, mixed in the same syringe, before breakfast each day. The
total morning dose is thus 60 U. Before dinner she would receive one
half that dose, or 30 U, and it would be composed of a 1:1 ratio, or 15 U
each of NPH and regular insulins. This formula should be considered
a starting point. Subsequent adjustments are based on self-monitoring
results at particular times of day. For example, if the 2-hour post-breakfast
glucose exceeds our goals, we would increase the dose of
short-acting insulin given the following morning. If the post-lunch glucose
level is high, an adjustment in the morning dose of intermediate-acting
insulin would be made. The fasting glucose reflects the pre-dinner
intermediate dose, and so on. Few patients remain on the formula
for very long: individual dose adjustments are the rule. The following
tricks of the trade may be useful: - Many patients use Lente insulin as their intermediate-acting insulin. This
was historically preferable because Lente insulin was formerly available
as a pure pork formulation, whereas NPH insulin was a mixture from
beef and pork. Because pork insulin is immunologically closer to human
insulin than is beef, Lente was preferred in order to reduce immunologic
complications. Currently, both Lente and NPH insulins are available
as recombinant DNA preparations of human insulin, and thus the issue
of antigenicity is moot. It has been found, however, that when Lente
and regular insulin are mixed together in the same syringe and injected, an
absorption peak results that is intermediate between the absorption
peaks of the two formulations if injected separately. NPH and
regular insulin, when mixed in the same syringe, provide absorption peaks
that are distinct and resemble those resulting from separate injections.150,151 Because the goal of the mixed, split-insulin regimen outlined above is
to provide an insulin absorption peak to cover each of the meals, it
generally is advantageous to use NPH rather than Lente as the intermediate-acting
insulin. However, if a particular patient is doing well with
Lente and her metabolism is in good control, it is not appropriate
to change to NPH merely for the sake of a principle.
- If a patient manifests consistently high fasting glucose levels despite
increases in her pre-dinner intermediate insulin dose, particularly if
her 2-hour post-dinner glucose value is normal or low, her intermediate
insulin may be peaking too early in the morning and wearing off by
the time she arises (the dawn phenomenon). She should measure her blood
glucose at 2 AM. If it is normal or low, she should move the pre-dinner
intermediate-acting insulin dose to bedtime in order to get a longer
effect. If the 2 AM glucose is quite high, it is reasonable for her
simply to increase the pre-dinner intermediate-acting insulin.
- Some patients will manifest very high glucose levels after breakfast and
then become relatively hypoglycemic before lunch. This is often due
to morning insulin absorption lagging behind the absorption of the food
eaten at breakfast. A useful solution is to increase the interval between
the morning insulin injection and breakfast. We have occasionally
had to prescribe the morning insulin as much as 1 hour before breakfast
was consumed.
To approximate physiologic insulin release more closely, one approach is
to use the continuous subcutaneous insulin infusion pump. With the insulin
pump, a continuous basal insulin infusion is provided, and bolus
doses are administered before each meal. Advantages of the pump are
greater flexibility with regard to meal timing. The disadvantages are
that it is expensive, that only short-acting insulin is infused, and that
interruption of infusion can more rapidly lead to DKA. A prospective
randomized trial of pump versus intensive conventional insulin therapy
in pregnant women with diabetes failed to reveal any difference in
metabolic control or perinatal outcomes,152 so the pump must be viewed as elective for most patients. The occasional
highly motivated and intelligent subject with diabetes who is very
difficult to control with conventional therapy may indeed benefit from
a trial of pump therapy. Although relatively strict goals for metabolic control in the second half
of pregnancy are outlined in Table 6, it is likely that somewhat more liberal goals can be used in the periconceptional
period. As pointed out earlier, there is a lack of universal
agreement as to exactly how close to normal maternal glucose levels
need to be during the period of organogenesis in order to minimize the
risk of birth defects. In our center, if we are fortunate enough to
make initial contact with a diabetic woman before conception, we attempt
to maintain glucose levels less than 150 mg/dL, with glycosylated
hemoglobin levels in the near-normal range (depending on the method). Once
conception has been established, we aim for the glucose levels outlined
in Table 6. When a patient is first seen during the first 8 weeks of pregnancy, we
often hospitalize her to improve control as rapidly as possible. This
is not the ideal situation, as rapid normalization of metabolic control
may be associated with deterioration of retinopathy, at least transiently. Therefore, ophthalmologic consultation is appropriate for these
patients, as it is for all pregnant women with overt diabetes. Insulin requirements can be expected to increase markedly as pregnancy
progresses,153 and this should not be cause for alarm. Brittleness, defined as marked
instability of diabetic control seen in some persons who are apparently
following their regimen assiduously (see above), appears to decrease
during the second half of pregnancy,44 and this phenomenon may be quite helpful in the management of these difficult
cases. Although there has been a persistent adherence to the clinical “pearl” that states that decreasing insulin requirement
is an ominous sign for perinatal well-being in diabetic pregnancy, proof
of this assertion is lacking. When a significant decrease in
insulin requirement occurs in the third trimester, it is reasonable to
step up the pace of fetal surveillance, but there is little or no evidence
that early delivery should be based on this finding in the absence
of signs of fetal compromise. In addition to daily self-monitoring of glucose, frequent contact with
a member of the health-care team is important in diabetes management. In
our center, a diabetes nurse educator is in telephone contact with
each patient initially on a daily basis, with longer intervals instituted
later as deemed appropriate. Such a practice is invaluable not only
in helping maintain good metabolic control, but also in reassuring the
patient that help and counseling are always readily available, and
in preventing problems such as mild upper respiratory infections from
developing into DKA. Indeed, one of the most common misconceptions among
our patients is that insulin dosage must be reduced if food intake
is decreased because of illness. Of course, the opposite is true. Illness
often causes insulin resistance, and hepatic glucose production increases
in the face of illness despite decreased caloric intake. Therefore
insulin dosage should be increased or should remain the same during
illness, depending on circulating glucose levels and the presence or
absence of ketonuria. PRENATAL SCREENING AND DIAGNOSIS. Because congenital anomalies occur with increased frequency among diabetic
pregnancies, it is important that available methods of prenatal diagnosis
be applied, where appropriate. Serum alpha-fetoprotein (AFP) testing, either
alone or in combination with measurement of human chorionic
gonadotropin and estriol, is commonly offered to pregnant women
throughout the United States in order to detect open neural tube defects, ventral
wall defects, and a number of other potential problems including
chromosomal trisomies. Women with diabetes should likewise be offered
these tests; however, it is important to note that maternal serum
alpha-fetoprotein values have been demonstrated to be lower in diabetic
pregnancies than in other pregnancies,154,155,156 while diabetic pregnancies have been demonstrated to be at increased risk
for neural tube defects.157 In one study,155 maternal serum unconjugated estriol levels were also significantly lower
than those of controls. Evidence now exists that the decrease in AFP
values may be related to poor metabolic control,158,159 suggesting that the patients whose glucose levels place their babies at
the greatest risk for congenital anomalies are also the ones most likely
to have lower AFP levels, which might thus be misleading. Unfortunately, it
is not clear at present what the temporal relationship is between
poor control, organogenesis, and low AFP levels. Insufficient data
exist to determine whether fetuses of diabetic mothers, in whom neural
tube defects are present, manifest low enough AFP levels to cause
their diagnosis to be missed. Structural anomalies, known to be more prevalent among infants of diabetic
mothers, may sometimes be diagnosed prenatally with targeted (level
II) ultrasound examinations.160 Because virtually all types of anomalies are more prevalent among these
pregnancies, it is difficult to target the ultra-sound examination. In
addition, diabetic women whose glucose metabolism is well controlled
during the time of organogenesis may have birth defect risks that are
only slightly elevated, if at all. Level II ultrasound facilities and
expertise are not universally available, and existing centers do not
have limitless resources. The success of ultrasonographers in identifying
birth defects is greatest if the particular patient has a high a
priori risk of a particular anomaly; in such cases, the sonographer will
be highly motivated to search for the presumed anatomic abnormality
until he or she has either found it or determined that it does not exist. An ultrasound examination performed to “rule out birth defects” in
a low-risk pregnancy, however, could miss a subtle abnormality. Therefore
we perform level II ultrasound examination and echocardiography
when there is evidence that metabolic control during organogenesis
was poor, usually on the basis of a glycosylated hemoglobin level that
is markedly elevated. A specific cutoff level cannot be supplied because
this varies with the method of measurement used in a particular
laboratory. In fact, in one series of diabetic pregnancies, the critical
threshold of glycohemoglobin for cardiac defects was the upper limit
of normal (6.1% in the laboratory reporting these results).161 The authors of this report recommended fetal echocardiography for every
diabetic pregnancy unless glycohemoglobin levels were within the normal
range. Ultrasound also is used to monitor fetal growth and amniotic
fluid volume at intervals throughout the third trimester. Umbilical
artery blood flow velocity measured by Doppler velocimetry has been found
to be similar between diabetic and non-diabetic pregnancies.162 Such measurements are not a standard method of fetal evaluation at the
present time, and their value is uncertain. Other screening tests that we have found to be helpful include renal function
testing in early pregnancy, including blood urea nitrogen, serum
creatinine, uric acid, 24-hour urinary protein, and 24-hour urinary
creatinine clearance determinations. These help to evaluate early nephropathy, and
serve as a baseline for later comparison, because diabetic
persons are more likely to develop hypertensive disorders of pregnancy. Ophthalmologic
examination should be carried out, preferably by an
ophthalmologist. To diagnose asymptomatic bacteriuria before pyelonephritis
can develop, urine cultures are obtained initially and at 1- to 2-month
intervals. Electrocardiography should be obtained in diabetic
women with vascular disease or longstanding diabetes. TIMING OF DELIVERY. Although at one time maternal diabetes meant automatic early delivery
because of the high risk of fetal death near term, the advances already
described have made it possible for most diabetic pregnant women managed
with contemporary approaches to deliver at or near term. The achievement
of near-euglycemia in diabetic pregnancy is the major preventive
measure that has allowed this dramatic change in management. Elective
delivery generally should not be undertaken if fetal lung maturity
is in doubt. To document lung maturity, we usually perform amniocentesis
and test the fluid for the presence of phosphatidylglycerol. We have
found a 20% prevalence of absent phosphatidylglycerol as late as 39 weeks
in pregnancies complicated by preexisting diabetes.163 Current approaches emphasize term vaginal delivery after spontaneous labor
has begun if no undue risk to mother or fetus is engendered, but
situations still may arise where intervention is necessary. The general
approach used in our center is as follows164: - Deliver even without documented fetal lung maturity if maternal or fetal
compromise puts the life of either at risk. Examples of this situation
would be maternal eclampsia or severe preeclampsia even in a very preterm
diabetic pregnancy, or proven severe fetal compromise.
- Deliver as soon as fetal lung maturity can be documented when there is
a significant maternal or fetal problem that does not pose an immediate
risk to the life of either. Examples of this situation would be poor
or undocumented maternal metabolic control, hypertensive disorders of
pregnancy or chronic hypertension, previous classic cesarean section, intrauterine
growth retardation of the fetus, suspected fetal macrosomia, or
equivocal antepartum fetal testing without definite fetal compromise.
- If none of the above conditions are present, consider elective delivery
at 38 weeks' gestation if the patient expresses a high level of anxiety
because of the physical, emotional, and monetary expense of having
diabetes and going through pregnancy.
For elective delivery, the cervix should be favorable and lung maturity
should be present. We consider the presence of oligohydramnios at or
after 38 weeks as reason to forgo amniocentesis and to proceed with delivery. It
should be noted that in some centers amniocentesis for fetal
lung maturity is not considered necessary, even in diabetic pregnancy, if
a gestational age of 39 weeks or more has been attained and dates
are well documented. In our study of lung maturity,163 patients in whom phosphatidylglycerol was not present were not delivered, and
thus it is impossible to state how many infants, if any, would
have developed respiratory distress syndrome. A repeat cesarean section, if desired by the patient, is scheduled for 38 weeks' gestation
after lung maturity has been documented; however, vaginal
birth after cesarean is encouraged. We have allowed patients with
diabetes to proceed past 40 weeks when the cervix is unfavorable for
induction, metabolic control is excellent, and the pregnancy is otherwise
uncomplicated. However, the recent availability of various means
to induce cervical ripening hold promise in such cases, and we have
frequently had success using prostaglandin gel or laminaria, or both. FETAL-PLACENTAL FUNCTION TESTING. A number of different techniques for assessing fetal well-being in diabetic
pregnancy are available. These include fetal movement determinations (“kick
counting”), nonstress testing, contraction stress
testing, and the biophysical profile. Urine or serum estriol determinations, once
the mainstay of antepartum testing in diabetic pregnancy, have
been largely abandoned because of their poor positive predictive
accuracy and the expense and inconvenience associated with their use.165 Most current approaches utilize either the biophysical profile166 or the nonstress test167 as the primary testing modality. When the nonstress test is used, either
contraction stress testing or the biophysical profile is used as a
backup procedure when the nonstress test is nonreactive. Some investigators
advocate twice-weekly nonstress testing in diabetic pregnancies.167,168 It should be pointed out that no antepartum test is predictive of fetal
compromise that may result from an acute event, such as maternal DKA
or umbilical cord accident. Poor metabolic control, even in the absence
of DKA, has been associated with pathologic antepartum and intrapartum
fetal heart rate tracings.169 The potential effects of maternal hypoglycemia on fetal heart rate tracings
are controversial. Increased fetal activity has been noted when
blood glucose levels less than 60 mg/dL were induced in one study.170 In another report, no decrease in reactivity was present when gravidas
with diabetes underwent insulin clamp studies to reduce their glucose
levels to approximately 40 mg/dL.171 In our own center, we perform nonstress testing weekly in otherwise uncomplicated
diabetic pregnancies. More frequent testing, ranging from
twice weekly to daily, is used when complications such as hypertension, intrauterine
growth retardation, vascular disease, or suboptimal metabolic
control are encountered. The contraction stress test or biophysical profile is used as a secondary
test if the nonstress test is nonreactive. In patients at particularly
high risk, such as those with vascular disease or hypertensive disorders
plus intrauterine growth retardation, or both, the contraction
stress test or biophysical profile is substituted for the nonstress test
at least once weekly, even when all nonstress tests are reactive. The
gestational age when antepartum testing is to be initiated varies according
to each patient's circumstances. There is no point initiating
antepartum testing at a gestational age when delivery is not a viable
alternative (i.e., before 24 to 25 weeks). Even after this gestational age, patients with
a low likelihood of fetal compromise, if tested positive, are likely
to have a false-positive rather than a true-positive result, and delivery
may be more likely to harm the pregnancy than to benefit it. Therefore
patients with a very high likelihood of fetal compromise, such as
those with evident intrauterine growth retardation and vascular disease, may
be tested as early as 24 to 25 weeks.172,173 The majority of diabetic pregnant women begin testing at 28 to 34 weeks, depending
on the adequacy of metabolic control and the presence or
absence of vascular complications. MODE OF DELIVERY. Maternal diabetes is not an indication for cesarean section, but its complications
may be. Fetal macrosomia occurs with increased frequency
in diabetic pregnancies, as described earlier in this chapter. Presumably
because the fetal head size is normal but the trunk large,102 shoulder dystocia may be a significant problem in such cases. If shoulder
dystocia could be anticipated, it would be best to deliver the macrosomic
fetus by cesarean section. However, current approaches to estimating
fetal weight are imprecise at best, and no prospectively tested
approach to the prenatal diagnosis of macrosomia, or potential shoulder
dystocia, is clearly effective. Therefore a compromise must be reached
between an inappropriately aggressive approach toward cesarean section
and an increased likelihood of shoulder dystocia with attempts at
vaginal delivery. In our center, most pregnancies with a fetus estimated to weigh 4500 g
or more are delivered by cesarean section without a trial of labor. When
the estimated fetal weight is between 4000 and 4499 g, other factors
are taken into consideration, including the mother's past obstetric
history, clinical assessment of pelvic architecture, and the progress
of labor. What used to be called midforceps deliveries, and under
current nomenclature are called low-forceps deliveries, are generally
inadvisable when fetal macrosomia is believed to be present and the second
stage of labor is prolonged.174 A previous history of shoulder dystocia is also a relative contraindication
to vaginal delivery unless the circumstances are clearly favorable (i.e., a much smaller fetus). In the absence of macrosomia, the decision to perform
a cesarean section should be based on the usual obstetric indications. Although
cesarean section is more likely to be necessary when
labor is induced in the presence of an unfavorable cervix, there is no
compelling reason to proceed with operative delivery without an adequate
trial of labor, just as in a nondiabetic pregnancy. Similarly, diabetes
is not a contraindication to attempted vaginal birth after cesarean. METABOLIC MANAGEMENT FOR LABOR AND DELIVERY. During labor, attention must be paid to the maintenance of maternal euglycemia
in order to lessen the likelihood of neonatal hypoglycemia, and
in extreme cases, to avoid maternal DKA. Studies utilizing animal models
suggest that maternal hyperglycemia may predispose the fetus to
lactic acidemia and hypoxemia.175,176 The combination of hypoxia and hyperglycemia in primates has been associated
with central nervous system damage.177 Studies of pregnant women have linked the infusion of large amounts of
glucose-containing solutions to fetal acidosis.76,77 The need for meticulous control of glucose metabolism during labor has
led to the application of constant intravenous glucose and insulin infusions
in this setting. Glucose is needed to prevent starvation ketosis; studies
utilizing an artificial pancreas178,179 during labor have demonstrated that many women with type I diabetes require
no insulin during the first stage, despite glucose infusion rates
of 6 to 10 g/hour. Monitoring of circulating glucose levels throughout
labor is thus critical, so that insulin can be supplemented if hyperglycemia
is present. To avoid inadequate circulating insulin levels and
resultant ketonemia, some centers routinely use constant insulin infusions
during labor for persons with type I diabetes. When labor is to be induced, it is reasonable to begin early in the morning, after
the usual insulin and snack the night before. If a patient's
diabetes has been well controlled, it can be anticipated that
her fasting glucose levels will be within the target range. To provide
basic caloric requirements, an intravenous line is established, and 5% dextrose
in one-half normal saline is infused with a mechanical device
at a constant rate of 100 to 125 mL/hour. Blood glucose is measured
every 1 to 2 hours; reflectance meters are very useful in this situation. The
target for glucose is 60 to 120 mg/dL. If this level is exceeded, an
insulin infusion is begun, usually at a rate of 1 to 1.25 U/hour. This
can be accomplished either through a “piggyback” intravenous
line using a separate mechanical infusion device, or by adding
insulin to the 5% dextrose solution already running at a constant
rate, at a concentration of 10 U/L. Adjustments in the insulin infusion
rate can be made if glucose levels either fall below or exceed the
target range. This is achieved by an adjustment of either the infusion rate of the separate insulin drip or the concentration of insulin in the glucose-and-insulin solution. Patients entering the hospital in spontaneous labor may require a modification
of this adjustment approach, depending on when their last insulin
injection occurred. If induction is carried out for more than 1 day, and
the patient is to be allowed to rest for the night, care should
be taken to avoid disturbed glucose metabolism. One useful approach is
to discontinue induction just before dinner time, and to give the patient
her usual subcutaneous pre-dinner (and bedtime) insulin and her
usual evening meal and snack. The procedure is begun the next morning
as before. If the evening insulin dose is omitted, it is possible for
DKA to ensue during the night, so close attention is warranted. Patients admitted for elective cesarean section are best scheduled for
induction in the early morning. The usual meal, snack and insulin are
administered the night before so that the morning blood glucose will be
within the target range. An intravenous line is initiated, and normal
saline or some other non-glucose-containing solution is infused. Once
delivery has been accomplished, glucose can be infused in order to maintain
the circulating glucose value within a reasonable range. During the immediate postpartum period, insulin requirements often decrease
precipitously to levels below the prepregnancy dose. This is particularly
true in women who have undergone cesarean section because they
are not allowed to eat during the first day or two after the operation. Fortunately, the
kind of meticulous metabolic control that is the
cornerstone of management of diabetic pregnancy is no longer necessary
after delivery, and glucose levels may be allowed to increase up to 150 or 200 mg/dL
without major short-term risk. The subcutaneous insulin
dose can then be slowly increased as the patient's dietary restrictions
are lifted, and fine-tuning of control can be accomplished at
home after discharge. Gestational Diabetes The principles guiding management of the pregnancy complicated by gestational
diabetes are the same as for preexisting diabetes: maintenance
of relative euglycemia to prevent perinatal mortality and morbidity. A
panel of circulating glucose measurements should be performed a minimum
of once weekly. Many centers now recommend daily self-monitoring for
persons with gestational diabetes, similar to the recommendations outlined
for preexisting diabetes. In our center glucose values at fasting, 2 hours
post-breakfast, and 2 hours post-lunch are monitored at least
once weekly. If the fasting value exceeds 100 mg/dL or either of the
other two values exceed 120 mg/dL, the patient is considered to require
more intensive monitoring or therapy. Because the patient knows in
advance the day on which glucose measurements are to be made, we consider
it most likely that her blood glucose levels are no better on days
when metabolic monitoring is not carried out. Because gestational diabetes
usually is diagnosed in the third trimester and thus presents
a limited time in which there is an opportunity to reduce perinatal morbidity
and mortality, we believe that an immediate response to maternal
hyperglycemia is preferable to waiting 1 week to see if the elevated
glucose level is consistent. Therefore, we prescribe daily self-monitoring
of glucose immediately, bring the patient back within 1 to 2 days
for repeat testing, or start insulin therapy. Insulin therapy initiated in response to elevated maternal glucose levels (described
above) is aimed at reducing the perinatal mortality risk; in
our experience, approximately 28% of women with gestational diabetes
require such treatment.180 It generally is true that the more abnormal the GTT results are, the more
likely is the patient to require insulin; however, 26% of women with
gestational diabetes whose GTT results were ranked in the lower third
manifested hyperglycemia to the extent that insulin was needed.180 Therefore GTT results should not be the sole determinant of the mode of
therapy to be used. The above discussion of insulin therapy used to reduce the perinatal mortality
rate does not include insulin used to reduce the rate of morbidity, particularly
macrosomia. Prophylactic insulin, given without regard to the level of fasting or postprandial glucose, has
been suggested as effective in preventing macrosomia.113 Other apparently effective approaches include daily self-monitoring of
glucose with institution of insulin if relatively strict thresholds, such
as 90 mg/dL fasting and 100 mg/dL postprandially, are exceeded.113 Buchanan and colleagues181 studied gestational diabetic pregnant women whose fasting serum glucose
was less than 105 mg/dL and who underwent ultrasound evaluation at 29 to 33 weeks' gestation. The 98 persons whose fetal abdominal circumference
measurement met or exceeded the 75th percentile for gestational
age were randomized to diet only (N := 29) or diet plus twice daily insulin (N = 30). The
prevalence of large-for-gestational-age newborns
was reduced from 45% in the diet group to 13% in the insulin-treated group. The
authors concluded that ultrasound in the early third trimester
can identify candidates who would benefit from insulin treatment. Although
none of these approaches should be considered the standard of
care at the present time, it is reasonable to apprise patients of such
options, considering that the prevention of macrosomia could help some
of them avoid a cesarean section or difficult vaginal delivery. Antepartum testing of fetal and placental well-being is recommended in
gestational diabetes, but there is a lack of universal agreement as to
the optimum time for initiation of testing or the most appropriate test
to be used. Landon and Gabbe182 suggest daily maternal assessment of fetal movements beginning at 28 weeks' gestation, with
weekly nonstress testing beginning at 40 weeks' gestation, in
otherwise uncomplicated gestational diabetic pregnancies
without identifiable risk factors. Earlier initiation of nonstress testing
is recommended if risk factors (i.e., hypertensive disorders, previous stillbirth, poor or undocumented metabolic
control) are present. In our center, we initiate weekly nonstress
testing at approximately 36 weeks' gestation in otherwise uncomplicated
gestational diabetic pregnancies in order to avoid inadvertently
omitting testing for patients with prospectively or retrospectively identified
risk factors. Decisions concerning the timing and mode of delivery in gestational diabetic
pregnancy should be made on the basis of the same considerations
described for those with preexisting diabetes. A randomized trial of
induction of labor at 38 weeks versus expectant management included 200 women
who required insulin during pregnancy, 187 of whom had gestational
diabetes and 13 of whom had preexisting diabetes.183 Cesarean section rates were not significantly different between the induction
group (25%) and the expectant management group (31%). Although 23% of
newborns in the expectant management group were equal to or greater
than 90th percentile, only 10% of those in the induction group were
large for gestational age. These findings suggest that induction of
labor at 38 weeks is not associated with increased risk of cesarean
section in this group of patients. Insulin is usually not required during
labor, but circulating glucose levels should be monitored in order
to identify those patients who would benefit from this form of therapy (i.e., those with glucose levels equal to or greater than 120 mg/dL). Because gestational diabetes is a relatively potent predictor of the later
development of diabetes, with approximately 40% of such persons developing
this disorder within 20 years of their index pregnancies,8 it is appropriate to test women with previous gestational diabetes annually9 for disorders in glucose tolerance. The first such test usually is recommended
to be given during the postpartum visit: The patient already
must be seen for other reasons, and therefore she is likely to find this
to be a convenient time to undergo the test. Diagnostic criteria for
the 75-g, 2-hour oral GTT, recommended for nonpregnant patients, are
outlined in Table 7. TABLE 7. Diagnostic Criteria forth 75-g, 2-hour Oral Glucose Tolerance
Test in the Nonpregnant State
Diabetes mellitus is diagnosed if: - Fasting plasma glucose is
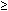 140 mg/dL on at least two occasions, or 140 mg/dL on at least two occasions, or
- On a 75-g, 2-hour oral glucose tolerance test the 2-hour value is
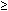 200 mg/dL and at least one other value (30, 60, or 90 minutes) is 200 mg/dL and at least one other value (30, 60, or 90 minutes) is 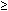 200 mg/dL. 200 mg/dL.
Impaired glucose tolerance is diagnosed if:- Fasting plasma glucose is <140 mg/dL, and
- On a 75-g, 2-hour oral glucose tolerance test the 2-hour value is 140–200 mg/dL and at least one value (30, 60, or 90 minutes) is
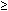 200 mg/dL. 200 mg/dL.
(Adapted from National Diabetes Data Group: Classification and diagnosis
of diabetes mellitus and other categories of glucose intolerance. Diabetes 28:1039, 1979)Diagnosis of gestational diabetes early in pregnancy has been found to
be significantly predictive of persistent glucose abnormality at the time
of postpartum testing.184,185 It should be remembered, however, that early testing usually is performed
because the patient has some indication or risk factor, and in neither
of these studies were all gravidas tested early. Nevertheless, it makes physiologic sense that persons
whose glucose abnormality is present early in pregnancy are at
higher likelihood of having had abnormal glucose tolerance before pregnancy. Apparently
independent risk factors for subsequent diabetes include
the following: Elevated fasting glucose in the oral GTT performed during pregnancy 184,185,186,187
Obesity184,187,188
Elapsed time since the index pregnancy187
Degree of abnormality of the postpartum GTT186
The likelihood of persistent diabetes after pregnancy, and of subsequent
development of diabetes, may vary with the particular population being
studied. For example, in the Danish population studied by Damm and
associates,186 nearly one third of the subsequent diabetes reported was type I diabetes. This
is a possible explanation for the lack of correlation between
maternal obesity and subsequent diabetes in this study, because obesity
is not a feature of type I diabetes. In fact, the data from this study
suggest that body mass index equal to or greater than 25 kg/m2 was present in 30% of those with subsequent normal glucose tolerance, in 46% of
those with subsequent impaired glucose tolerance, and in 58% of
those with type II diabetes, but in only 11% of those with subsequent
type I diabetes. In contrast, the study of Kjos and co-workers184 encompassed a Mexican-American population with a high prevalence of type
II diabetes (9% had diabetes during the first 2 months postpartum and
an additional 10% had impaired glucose tolerance). Because the average
body mass index in these patients was approximately 30 kg/m2, it was not surprising that obesity was not discernible as an independent
risk factor. None of these studies of risk factors for future diabetes
allow the exclusion of certain subsets of former gestational diabetic
persons from further testing. Indeed, in the Kjos study184 even the lowest risk group, who had maintained all fasting glucose levels
during pregnancy below 105 mg/dL, manifested a 10% prevalence of impaired
glucose tolerance or diabetes within 2 months postpartum. Contraception Pregnancy in any woman with preexisting diabetes ought to be planned, not
unintentional. Unless the person is in excellent metabolic control
throughout her reproductive years, unplanned pregnancies are likely to
be associated with a greater risk of major congenital malformations than
are those in which prepregnancy counseling and planning have taken
place. Family planning, including a discussion of contraception, is appropriate-in
fact mandatory--for any adolescent or adult woman with diabetes
who has reached menarche. Virtually the full range of contraceptive
options is open to women with diabetes mellitus; however, specific
risks may be different for such women than for the general population. Because
the health risks associated with pregnancy are greater for
a woman with diabetes than for women in general, the benefits of contraception
may also be considered to be greater.189 If a diabetic woman and her husband have completed their family, permanent
surgical sterilization may be the method of choice. The method risks
are all taken at the time of surgery, and no further attention need
be paid by the couple to contraception, other than an awareness that
no method is 100% effective. Oral contraceptive preparations are second in effectiveness to permanent
sterilization. Potential risks associated with oral contraceptive use
include cardiovascular, thromboembolic, and lipoprotein disorders. Because
many of these risks are also increased in persons with diabetes, there
has been longstanding concern about the use of this method of
contraception in diabetic women. Data suggesting a multiplicative effect
are lacking, however, and current very-low-dose formulations appear
to have minimal demonstrable risk,190 even with respect to renal and retinal complications191 specific to diabetes. Another concern with oral contraceptives is the
potential worsening of glucose metabolism in persons who are unable to
compensate fully with an augmented insulin response. Theoretically type
I diabetic women can simply increase their insulin dose; however, type
II diabetic women who do not receive insulin therapy might need to
do so if their metabolism were to worsen with the use of oral contraceptives. For
this reason it is necessary to monitor glucose metabolism
closely in women with diabetes and in those with former disturbances
in carbohydrate metabolism (e.g., gestational diabetes) when they are taking oral contraceptives. It makes
sense to use formulations with the lowest dose of estrogens and progestins
when prescribing oral contraceptives to such women. At least two
studies have failed to demonstrate an adverse effect of modern low-dose
oral contraceptives on carbohydrate metabolism in persons with previous
gestational diabetes.192,193 Currently, subcutaneous levonorgestrel implants (Norplant System), have
gained popularity in the United States. There are no available long-term
studies specific to diabetes, but mild alterations in carbohydrate
metabolism have been reported with their use in nondiabetic women.194 This form of contraception should not be considered contraindicated in
women with diabetes or previous gestational diabetes, but patients should
be apprised of the lack of data. The intrauterine device, slightly less effective than combined oral contraceptives, has
the advantage of requiring no active effort by the user
once it has been inserted. The known risk of infection and subsequent
infertility is a disadvantage of this method, although there is currently
no evidence to suggest that this risk is higher among diabetic
persons. Although some studies have reported a high failure rate of this
method in persons with diabetes,195 others196,197,198 have found no such problem. Barrier methods of contraception appear to have virtually no risk of medical
complications, but they require faithful adherence by the user and
partner and have a relatively higher failure rate compared with the
above approaches. The condom also offers an advantage in protection against
sexually transmitted diseases. |
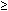 300 mg/day) in the absence of other renal disease,”45 complicates both type I and type II diabetes. However, because type II
diabetes tends to have a later age at onset than type I diabetes, and
because the prevalence of nephropathy is associated with the duration
of diabetes, pregnant women with type I diabetes are considerably more
likely to have nephropathy than are those with type II diabetes. In
one series from a tertiary-care institution 23% of pregnant women with
preexisting diabetes manifested nephropathy, which was defined as a urinary
protein excretion equal to or greater than 300 mg/ day before the
third trimester.46 Nephropathy appears to develop in five stages.45 The first two stages are manifested by renal hypertrophy and hyperfunction, and then renal lesions without clinical signs. In the third stage, occurring
within 7 to 15 years in 25% to 40% of persons with type I diabetes, microalbuminuria becomes evident (only 30 to 300 mg/day). Once microalbuminuria is present
in a given person, it is highly likely that she will progress to clinically
evident diabetic nephropathy and ultimately end-stage renal disease; this progressive decrease in glomerular filtration rate usually occurs
at a rate of 10 mL/min/year. Hypertension and retinopathy usually are
present when nephropathy is detected.
300 mg/day) in the absence of other renal disease,”45 complicates both type I and type II diabetes. However, because type II
diabetes tends to have a later age at onset than type I diabetes, and
because the prevalence of nephropathy is associated with the duration
of diabetes, pregnant women with type I diabetes are considerably more
likely to have nephropathy than are those with type II diabetes. In
one series from a tertiary-care institution 23% of pregnant women with
preexisting diabetes manifested nephropathy, which was defined as a urinary
protein excretion equal to or greater than 300 mg/ day before the
third trimester.46 Nephropathy appears to develop in five stages.45 The first two stages are manifested by renal hypertrophy and hyperfunction, and then renal lesions without clinical signs. In the third stage, occurring
within 7 to 15 years in 25% to 40% of persons with type I diabetes, microalbuminuria becomes evident (only 30 to 300 mg/day). Once microalbuminuria is present
in a given person, it is highly likely that she will progress to clinically
evident diabetic nephropathy and ultimately end-stage renal disease; this progressive decrease in glomerular filtration rate usually occurs
at a rate of 10 mL/min/year. Hypertension and retinopathy usually are
present when nephropathy is detected.