Our evolving understanding of the immunology of the hepatitis B virus (HBV) has led to the development of safe and effective therapies for preexposure and postexposure virus-specific prophylaxis. Perinatal transmission of HBV from mothers with chronic infection to their at-risk neonates remains a significant route for the perpetuation of the HBV carrier state, with its concomitant health risks, worldwide. This section outlines the evidence supporting antenatal identification of HBV-carrier mothers and targeted HBV immunoprophylaxis in their newborn children. Widespread adoption of such approaches, combined with ongoing HBV vaccination protocols in high-risk populations, including medical personnel, will make significant inroads against the overall prevalence of HBV-related disease.
Biology and Serology
Although an exhaustive discussion of HBV biology is beyond the scope of this chapter, an understanding of the basic structures and serologic tests associated with the virus is essential to understanding the logistics of perinatal transmission and prevention.
HBV is a small (42-nm) DNA virus that contains partially double-stranded DNA within its core.1 Using its own DNA polymerase for replication, the virus is able to reproduce within a host's infected hepatocytes, drawing from the cell's pool of nucleotide precursors.
Much attention has been paid to the use of HBV-specific markers in serum to distinguish active from previous infection and to determine the relative infectiousness of a particular individual. Not surprisingly, these concerns are directly applicable to determining relative risks for vertical (maternal–fetal) transmission of the virus.
Hepatitis B surface antigen (HBsAg) is the HBV serum marker that has come to be used most commonly in clinical situations and screening protocols. Discovered by Blumberg and co-workers in 1965,2 it initially was not known to be a virus-associated marker. The antigen, first isolated in the serum of an Australian aborigine during a study of serum protein polymorphisms (hence its being labeled the “Australia antigen”),3 was found incidentally to cross-react with the serum of multiply transfused patients. Later found to be present in the serum of institutionalized patients, it was even believed to be possibly associated with Down syndrome.4 Subsequent work by Blumberg's group and others established a link between the newly identified antigen and acute hepatitis B, an association confirmed by electron microscopy identification of particles dense with the antigen in the serum of patients who were acutely ill with hepatitis.5 Those particles now are known to represent incomplete portions of the viral envelope, synthesized in great excess during the process of virus replication. In addition, intact viral particles bear the surface antigen on their outer envelope. The presence of HBsAg in serum indicates infectivity, although such presence alone cannot distinguish acute from chronic infection, an often confusing exercise that requires a more complete elaboration of HBV-related serologies.
Although HBsAg is the first antigen detectable in the course of HBV infections, predating even the appearance of symptoms in those patients who become clinically ill, it is the predictable appearance and disappearance of other HBV-associated antigens and antibodies over time that allows patterns compatible with either acute or chronic infection to be described. Currently, six distinct antigens and antibodies are assayable in clinical specimens through the use of commercially available test kits and machinery. The agar gel precipitation techniques first used by Blumberg's group to demonstrate HBsAg antigen–antibody complex formation has given way to fully automated, multisample readers that capitalize on advances in molecular biology to detect the presence or absence of the specific immunogens in question.
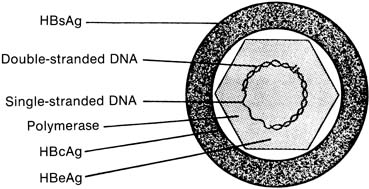
The complete hepatitis B viral particle, also known as the Dane particle, after Dane and co-workers who described it in 1970,6 consists of the viral core surrounded by its HBsAg-rich envelope (Fig. 1). If the envelope is removed by the use of detergents in vitro, a viral core antigen (HBcAg) can be identified. Unlike HBsAg, HBcAg does not circulate free in serum and is found in blood only as an integral component of the internal viral nucleocapsid. A third antigen, the e antigen (HBeAg), is serologically distinct from both HBsAg and HBcAg. HBeAg is associated primarily with the core antigen in the virus's internal structure, but unlike HBcAg, it can be found circulating in serum, frequently in complexes with immunoglobulin.7 All three serologically unique antigens stimulate the production of equally distinct antibodies (HBsAb, HBcAb, and HBeAb) in the course of nonchronic host infection.
Also located within the viral core are the viral DNA and DNA polymerase. The presence of HBeAg has been closely correlated with both the infectivity of a particular patient's serum and the microscopic detection in serum of the HBV virus itself8,9 and an increased risk for chronic liver disease.10,11 Seropositivity for HBeAg should be taken as a marker of active viral replication, the most infectious phase of the disease, either in acute or in chronic illness. Practically, however, HBsAg is used in screening protocols because of the high concentrations of this antigen produced in response to viral presence and replication. More vigorous HBV serologic testing is performed, along with liver function evaluation, in HBsAg-positive individuals, both symptomatic and symptom-free, to characterize the nature and extent of their disease. The appearance of HBsAb in the serum of patients occurs in the setting of resolution of acute infection; it is this antibody that appears to confer protective immunity. However, both HBcAb and HBeAb have been shown experimentally to be protective against reinfection.12,13,14,15 Still, the currently available HBV vaccine's efficacy is conferred by stimulating production of HBsAb by exposure to HBsAg; vaccine-related immunity can be distinguished from natural immunity in most cases by the absence of HBcAb in the serum of successfully vaccinated patients.16
Epidemiology, Transmission, and Prevention
Infection with HBV has been accepted as a health concern of worldwide importance, because 5% to 10% of those infected become chronic HBV carriers,17 and 25% to 30% of those carriers die as a result of long-term sequelae of HBV-related disease.18 Workers in an endemic area in Asia (Taiwan) found more than 15% of subjects screened in a general program to be HBsAg-positive; deaths in 54% of the carriers were attributable to primary hepatocellular carcinoma (PHC) and cirrhosis compared with 1.5% of deaths among noncarriers.19 Since that initial linkage, the virus has been shown to be the cause of approximately 80% of all cases of PHC globally.20
In areas endemic for HBV, up to 20% of the general population is chronically infected, with perinatal/neonatal and childhood infections existing as a primary route for expanding the reservoir of carriers (Table 1). This is especially significant because the risk of chronic HBV infection for a child infected in the newborn period in the absence of prophylactic therapy is 70% to 90%.21,22
Table 1. Global Distribution of Hepatitis B Virus (HBV) Infection
| Low Prevalence | Intermediate Prevalence | High Prevalence | |
| HBsAb-positive rate | 0.2%–0.5% | 2%–7% | 8%–20% |
| HBsAb-positive rate | 4%–6% | 20%–55% | 70–90% |
| Childhood infection | Uncommon | Common | Very common |
| Locations | North American, Western Europe, Australia | Eastern Europe, Middle East, Soviet Union, South America | China, southeast Asia, sub Saharan Africa, Pacific Islands |
HBsAg, hepatitis B surface antigen; HBsAb, hepatitis B antibody
(Adapted from Maynard JE, Kane MA, Hadler SC: Global control of hepatitis B through vaccination: Role of hepatitis B vaccine in the expanded programme on immunization. Rev Infect Dis [suppl 3] 11:574, 1989)
In areas of low endemicity for HBV carriage, however, such as the United States, screening programs for the general population have been targeted to decrease household, transfusion, sexual, and perinatal transmission risks among contacts of HBsAg-positive individuals. Population subsets have been identified that are at increased risk for HBV acquisition, and HBV vaccination is recommended for individuals within those groups who are serologically negative for HBsAg and HBsAb. The efficacy of a serum-derived HBV vaccine was demonstrated initially on a large scale in a cohort of more than 1000 homosexual men in the United States; this trial showed an antibody (HBsAb) response in 96% of those vaccinated, with an overall protective efficacy of 88% against all HBsAg-positive events for vaccine compared with placebo.23 More recently, a recombinant vaccine consisting of purified HBsAg particles derived from yeast cells was licensed in the United States,24eliminating even the theoretical (but never proven) risk of transmitting other viral agents with a serum-based vaccine.25 Controlled trials in homosexual men showed an equivalent prevalence of acquired immune deficiency syndrome (AIDS) in groups receiving either the placebo or the serum-derived HBV vaccine.26
Estimates in the United States tabulate approximately 200,000 new primary cases of HBV infection per year, only 25% of which are associated with acute symptomatic infection.27 The dose of initial viral infection appears to correlate negatively with the risk of development of persistent disease. Survivors of fulminant hepatitis rarely have chronic infection, whereas experimental infections with low doses of virions result in longer incubation periods, milder clinical disease, and persistent antigenemia.28
Blood and blood products are the most thoroughly established sources of hepatitis B infection, although HBsAg has been demonstrated in a variety of body fluids. Of those, however, only serum, saliva, and semen have been associated consistently with transmission in experimental models.29,30,31 Despite the presence of HBsAg in feces, past attempts to produce infection using feces of experimentally infected subjects were unsuccessful, suggesting blood from the gastrointestinal tract to be the uncommon infectious vector present in feces of carrier individuals.32
Percutaneous transfer of the virus is the most obvious route of transmission in the medical setting, either through blood products or through needle-stick accidents. Contact of infectious material with broken skin or mucous membranes also can result in effective transmission. Recent surveys, however, show that approximately 50% of health care workers at risk for contracting HBV have not been vaccinated against the virus.33
Compared with other transmissible viruses, such as the human immunodeficiency virus (HIV), HBV is a fairly stable virus and remains infectious on household surfaces that may then contact mucous membranes, such as toothbrushes, baby bottles, razors, and eating utensils.34,35 Although transmission in households is more common through sexual contact than through fomite contact,36,37 nonsexual household transmission has been established as a route for HBV infection.38,39,40 In areas of the world with higher HBV carrier rates than the United States, nonparenteral transmission would be expected to constitute the major route of person-to-person HBV infection. Vertical transmission is a major source; investigators in Taiwan estimated that 40% to 50% of HBsAg carriers became infected in the perinatal period.21,41
Children born to carrier mothers who escape the neonatal period without evidence of infection are still at risk for childhood acquisition of HBV. One of the early vaccine trials conducted in Senegal showed that among children seronegative at the beginning of a randomized HBV vaccination trial, almost 10% acquired HBV infection in the absence of vaccination by the end of a 12-month follow-up period.42
Immunoprophylaxis regimens to prevent HBV transmission in the perinatal period, to be discussed later in the chapter, were a direct extension of the success of these therapies in high-risk adult populations. Postexposure immunization was first demonstrated through the use of immunoglobulin preparations with high titers of HBsAb, when administered within 4 hours of experimental infection with HBV.43 Before the development of an effective HBV-specific vaccine, transient preexposure prophylaxis was demonstrated using hyperimmune globulin (HBIG),44,45 although such use of HBIG is now of purely historical interest in terms of understanding the evolution of therapeutic standards. Currently, postexposure treatment consists of a single dose of HBIG administered as temporally as possible to the exposure. Immediate therapy is, of course, optimal for maximal protection, although 75% efficacy has been shown when HBIG is administered within 7 days of exposure.46 Although it does not increase the efficacy of HBIG therapy, a series of HBV vaccination also should be initiated if the exposure was within a setting of ongoing risk, such as a health care or institutional setting. This regimen consists of injections at 0, 1, and 6 months and results in high antibody titers in more than 90% of those younger than age 60 years.47,48,49 Administration of HBV vaccine simultaneously with HBIG does not diminish the immunologic response to the vaccine.50,51
Clinical Disease and Pregnancy
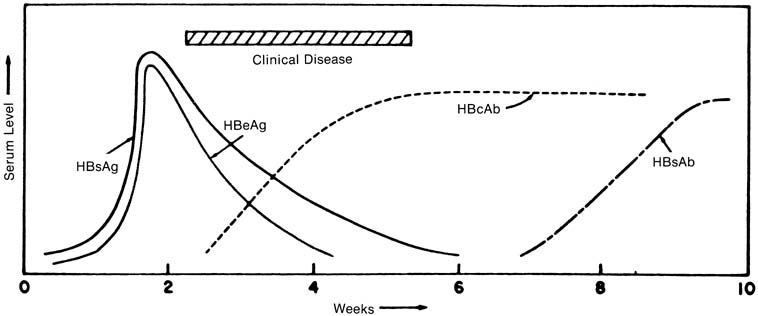
Hepatitis B is distinguished from the other viral hepatitides by its long incubation period (1–6 months), by the presence of extrahepatic symptoms in up to 20% of patients (arthralgia, rash, and myalgia thought to be a result of antigen–antibody complex deposition),52,53 and, eventually, by the detection of HBV-specific serum markers (Fig. 2). The appearance of HBsAg usually predates any clinical symptoms by 4 weeks, on average, and remains detectable for 1 to 6 weeks in most patients.54 In the 90% to 95% of patients in whom chronic infection does not develop, HBsAg titers decrease as symptoms diminish. The appearance of HBsAb defines the absence of the carrier state; titers increase slowly during the clinical recovery period and may continue to increase up to 10 to 12 months after HBsAg is no longer detectable. In most patients with self-limited, acute hepatitis B, HBsAb is detectable only after HBsAg titers in serum disappear.55,56 A “window” of time has been described in which a patient still with clinical hepatitis is negative for both HBsAg and HBsAb. During this time, HBV infection still can be diagnosed by the detection of HBcAb, which begins to appear 3 to 5 weeks after HBsAg does. HBcAb titers may decrease in the first 1 to 2 years after infection, although the antibody is still detectable years after acute disease in most patients.55 The appearance of HBeAg parallels that of HBsAg; in self-limited infections, HBeAb is detectable soon after the time that HBeAg disappears.
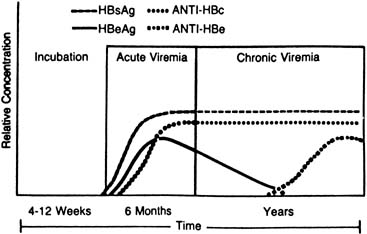
The chronic HBV carrier state usually can be predicted by HBsAg seropositivity for 20 weeks or more (Fig. 3). A test for HBV–DNA polymerase activity is positive in 50% of persistently HBsAg-positive patients, indicating ongoing viral replication57; Dane particles also can be identified in serum from these patients through electron microscopy.58 HBcAb is detectable in the serum of carriers at levels higher than those seen in either acute or recovering self-limited infections, and e-antigen markers are variable. HBeAb develops, with the disappearance of HBeAg, in one half to three quarters of carriers,59,60 and its presence is inversely related to the relative infectivity index of a patient's serum.61
|
Acute HBV infection during pregnancy is treated mainly by supportive measures, as in the nonpregnant state. An increase in fulminance and mortality rates with acute HBV infection during pregnancy has been demonstrated in some HBV-endemic areas,62,63 although other investigators in Western countries have suggested that these adverse outcomes were related more to health care conditions and maternal malnutrition.64,65 No teratogenic association has been established for maternal HBV infections,64,66 even though evidence of HBV infection at birth in children of HBV-carrier women have suggested the possibility of transplacental leakage of HBV-infected blood from mother to fetus in utero.67,68,69 Encouragement is necessary to maintain adequate nutrition during the early symptomatic phase, and liver-metabolized drugs, if not avoidable, need to be monitored carefully through blood levels. Phenothiazine may be used, if needed, to control nausea and vomiting. In addition, household and sexual contacts of patients should be offered passive immunization with HBIG after their HBsAg seronegativity is established.
Universal screening protocols for prenatal patients have been advocated by a number of groups and are discussed more fully in the next section. Routine screening with HBsAg testing detects both chronic carriers and asymptomatic, acutely infected patients. A positive HBsAg result in early pregnancy should be followed-up by tests for liver function, as well as HBeAg and HBeAb; HBcAb is not helpful in distinguishing acute from chronic disease, and HBsAb rarely is present if HBsAg is still circulating. Repeating the tests for HBsAg and liver function later in pregnancy, however, does help to make the diagnosis and guide the need for perinatal prophylaxis of the neonate. Although a recent multicenter study indicated that treatment of chronic HBV carriers with alfa-interferon was effective in achieving remission, both biochemically and histologically, in one third of patients,70 such therapy cannot be recommended in pregnant HBV carriers. No information currently exists to guide the use of genetic prenatal diagnostic procedures in HBsAg-positive women and the potential risk of producing in utero infection, although studies have demonstrated that such infection is possible in the face of preterm labor or placental abruption.67,68,69 HBIG, administered periprocedurally to HBsAg-positive women, may protect the fetus from infection in a manner comparable with the use of Rh-immune globulin in Rh-negative women, but again, such statements are purely speculative and await further investigation before they take the shape of recommendations.
Perinatal Hepatitis B Virus Transmission
Discussion of perinatal HBV infection focuses on the following three major areas: (1) the transmissibility of the virus from mother to fetus; (2) the sequelae of neonatal infection; and (3) the effectiveness of currently available modalities for prophylaxis.
Finally, the extensive variance in prevalence rates worldwide requires that the feasibility of prenatal screening programs to identify carrier mothers be addressed.
The potential for vertical transmission of HBV at birth is significant. Most infants born to carrier mothers are HBsAg-negative at birth experience seroconversion in the first 3 months after delivery, suggesting acquisition of the virus at birth.71,72,73,74 Mothers positive for both HBsAg and HBeAg are at highest risk for transmitting the virus; 85% to 100% of their offspring become infected, with 70% to 90% becoming chronic carriers. Mothers who are HBsAg-positive but HBeAg-negative, presumably indicating lower levels of replicating virus, do have a lower risk of transmitting the virus, but up to 35% of their children still will become carriers in the absence of neonatal therapy.75,76,77,78 In addition to the long-term risks of HBV-related sequelae in chronic carriers, such as cirrhosis and hepatocellular carcinoma, both fulminant fetal neonatal hepatitis79,80,81 and childhood-onset hepatic carcinoma,82 have been described in children born to HBsAg-positive mothers.
Early attempts at interrupting the perinatal transmission cycle used HBIG alone, administered in the neonatal period. Globulin alone had a protective efficacy against the carrier state of 70% to 75%, although the protection was not permanent, and many children eventually became infected after the passively acquired antibody was cleared, undoubtedly through household contact.83,84,85 With the advent of the hepatitis B vaccine, trials were established to test its efficacy when administered in the newborn period, both alone and in conjunction with HBIG. A combination of HBIG and vaccine in the newborn period conferred significantly greater protection against perinatally transmitted HBV than even the vaccine alone, increasing efficacy from a range of 75% to 85% up to 90% to 95%.42,77,83–92 The small but identifiable percentage of babies who become infected, despite even combined HBV therapy at birth, is believed to represent in utero infection.93,94 HBV DNA has been identified in abortus tissue extracted from an HBsAg-positive mother,95 and other reports show evidence of intrauterine infection in clinical situations, increasing risks for transplacental leakage, such as preterm labor associated with placental abruption.68,69 Still, combination HBV-specific immunotherapy provides the best opportunity to prevent the chronic carrier state in the offspring of HBsAg-positive mothers. In the United States alone, approximately 16,500 births occur in HBsAg-positive women each year, approximately 4300 of whom are also HBeAg-positive.96 Infants born to these women should receive HBIG (0.5 mL) intramuscularly (IM), ideally within 12 hours of birth. HBV vaccine should be administered concurrently at a different site (0.5 mL IM) or can be administered up to 7 days after birth if it is not immediately available.96 The timing of HBIG appears to be more critical than that of vaccine in achieving maximal effectiveness of passive–active therapy. Subsequent vaccination is performed, also 0.5 mL IM, at ages 1 month and 6 months. Follow-up for these infants is crucial, because one recent study confirms the concern that in the United States, groups at highest risk for HBV infection are also least likely to be compliant with follow-up care.97
Hepatitis B Screening in Pregnancy
The unique opportunity to provide almost complete protection against perinatally acquired HBV infection makes antenatal identification of HBV carriers critical so that combined neonatal prophylaxis can be administered in a timely fashion. In nonendemic areas such as the United States, screening protocols were organized initially to test pregnant women who are in the HBV risk groups, as defined by the United States Public Health Service (Table 2).98 Such recommendations, however, were not without problems. Reports from a number of groups working in geographically diverse areas around the country found that using risk groups alone for prenatal HBV screening would miss 40% to 60% of all HBsAg-positive parturients.99,100,101,102,103,104,105 Overall, in these studies, the HBsAg-positive rate ranged from 0.3% to 1.5% (Table 3). Even if risk factors were to be used to identify these women, however, evidence from one survey shows that only 60% of obstetricians could name more than two HBV risk groups, and less than 30% knew the recommended treatment for infants born to carrier mothers.106
| Asian, Pacific-Island, or Alaskan Eskimo descent, whether immigrant or born in the United States |
| Born in Haiti or sub-Saharan Africa |
| History of acute or chronic liver disease |
| Rejection as a blood donor |
| Staff or patient in a hemodialysis unit |
| Staff or patient in an institution for the mentally retarded |
| Occupational exposure to blood in medical/dental settings |
| Repeated blood transfusions |
| Household contact with HBV carrier or hemodialysis patient |
| Multiple episodes of veneral disease |
| Percutaneous use of illicit drugs |
(Immunization Practices Advisory Committee: Postexposure prophylaxis of hepatitis B. MMWR Morb Mortal Wkly Rep 33:285, 1984)
| HBsAg-Positive (%) | Detected by Risk Group (%) | |
| Palm Beach (FL), 198699 | 1.1 | 38 |
| Miami, 1987100 | 1.2 | 53 |
| Cleveland, 1987101 | 0.5 | 45 |
| New Orleans, 1987102 | 0.9 | 50 |
| Chicago, 1987103 | 1.4 | 38 |
| El Paso, 1990104 | 0.8 | 50 |
| San Antonio, 1990105 | 0.3 | 42 |
Such findings have led to recommendations by the Public Health Service96 and, most recently, by the American College of Obstetricians and Gynecologists107 that HBsAg screening be performed as part of routine prenatal testing in all pregnant women. An elegant cost-analysis study by Arevalo and Washington108 shows that such a program, taking into account both acute and long-term costs of neonatally acquired HBV disease, is cost-effective at a prenatal population prevalence for HBsAg of only 0.06%. In countries where HBsAg carriage is endemic, funding for medical screening programs tends to be limited. In these settings, especially as the cost for HBV vaccine begins to decrease, workers have advocated consideration of empiric vaccination for all newborns.109,110 Such a policy also has been recommended for children of Southeast Asian immigrants to the United States to prevent both perinatal and household acquisition of HBV.111
However, protocols establishing prenatal HBsAg screening policies do not address the problematic issue of deliveries in inner-city populations among women with minimal to no prenatal care. Maternal HBsAg status, in the absence of prenatal testing, then can only be known in the 1 to 2 days after delivery, and the newborn may miss out on maximally effective HBV prophylaxis. Investigators have recognized this problem because most hospital laboratories perform HBsAg testing at best on a daily basis.112 This fact is particularly important because evidence suggests that HBIG administered as perinatal prophylaxis may have limited efficacy if it is not administered as soon as possible after birth.113 A recent study has shown the rate of HBsAg carriage to be significantly higher among such unregistered women than in a comparison group enrolled in an inner-city clinic (7.8% vs 0.8%) and that the increase was specifically related to substance abuse. Among unregistered women with positive urine drug screens, moreover, the HBsAg-positive rate was 15%, and a maternal urine drug screen was suggested as a rapid screening test to target neonates at highest risk for HBV infection for prophylaxis, before the 24 to 48 hours required awaiting maternal HBsAg status.114
Impact of Perinatal Hepatitis B Virus Vaccination Programs
The efficacy of perinatal HBV vaccination programs in preventing infection in children born to carrier mothers has led to the inclusion of the HBV vaccine series in the American Academy of Pediatrics' current recommendations for childhood vaccines for the general population.115 Still, investigators have demonstrated that even in high-risk groups for perinatal HBV transmission, appropriate neonatal surveillance is critical. Among 426 children born to HBsAG-positive mothers in one longitudinal series, only 68% were completely vaccinated with the full three-dose sequence. Among the children followed-up, it was shown that the third vaccine dose was least likely to be received (64%). Serologic evaluation of the children in this well-conceived surveillance program showed 4% to have acquired chronic carrier status, with an additional 10% having evidence of resolved natural infection, as demonstrated by positive tests for antiHBc. Not surprisingly, incompletely vaccinated children were more likely to be HBsAg-positive than those completing the series (12% vs 1%; relative risk 7.9 [confidence interval = 1.5–41.2]).116 These findings underscore the need for reinforcement of complete vaccination for all children enrolled in the HBV vaccine series, particularly those born to carrier or other high-risk mothers.
The beneficial impact of adequate childhood vaccination for HBV recently has been demonstrated dramatically in reports from Taiwan, where large-scale mass vaccination programs were begun in 1984. Researchers there have conclusively proven a link between HBV and hepatocellular carcinoma (HCC) by showing a significant decrease in the average annual incidence of childhood HCC since the institution of the program.117,118 The decrease in the rate of childhood HCC was also paralleled by a decrease in the rate of HBsAg carriage among children born before the vaccination program was started, suggesting a herd immunity effect from the mass inoculation of children in the much more infectious younger birth cohorts, and resulting in a lower rate of horizontal HBV infection among the older unvaccinated children.117 These researchers had demonstrated previously that 5 years into the institution of the vaccination program in Taipei, the HBsAg carrier rate in children younger than age 5 years had decreased from 9.3% in 1984 to 2% in 1989.119 These results even further bolster the need to identify HBsAg carrier mothers and provide timely and complete HBV vaccination to their children.