Hemodynamic evaluation of the critically ill patient requires full understanding of cardiovascular structure and function. History taking and physical examination should be carried out efficiently, yet thoroughly. Assessment of maternal vital signs, mental status, urine output, and documentation of fetal well-being provides very important evidence of adequacy of circulation and represents the reference to which patient deterioration or effects of treatment will be compared. When rapid deterioration of cardiovascular status occurs, however, reliance on traditional clinical parameters may not allow for timely intervention. Reliable, continuous information may require the use of invasive monitoring, such as an arterial line (intravascular mean arterial pressure), and/or a pulmonary artery catheter (cardiac output and pulmonary arterial and wedge pressures).
Arterial Line
Blood pressure can be determined, in decreasing order of reliability, by intravascular catheters, digital oscillometers, and sphygmomanometers. Direct recording of intravascular pressure is recommended for all patients in the intensive care unit who require careful continuous monitoring of arterial pressure.18
Fluid-filled tubing transmits the pressure wave from the cannulated artery to the pressure transducer. Systolic, diastolic, and mean arterial pressures are measured. Mean arterial pressure (MAP) represents the driving pressure to the peripheral organs, and it can be either calculated (MAP = diastolic pressure + 1/3 pulse pressure) or measured by integrating the area under the arterial pressure waveform. The latter method is independent of heart rate and the assumption that diastole represents two thirds of the cardiac cycle.18
Some indications for arterial line placement may include: expectation of hemodynamic instability regardless of etiology; need for continuous blood pressure measurement when using vasoactive drugs (e.g., dopamine, nitroprusside); respiratory insufficiency; intubation for more than 4 to 6 hours; and the need for frequent arterial blood sampling. Some anesthesiologists recommend arterial line placement in situations in which the potential for rapid decrease in blood pressure exists. It has also has been recommended for morbidly obese patients for whom appropriate size cuffs are not available.
Cannulation of the radial artery must be preceded by evaluation of arterial supply to the hand by the Allen test.19 Palpate the radial and ulnar pulses. Ask the patient to make a tight fist with her hand in supine position. Occlude both radial and ulnar arteries with your thumbs. Ask the patient to open and close her hand multiple times (this will make the palm pale). Then release your thumb from one of the arteries. The palm should flush in no more than 5 seconds. Persisting pallor of the palm indicates insufficiency or occlusion of the released artery. Repeat the same procedure and test the other artery. Local anesthesia should be used, as it will both decrease the patient's pain and increase your success rate.
Thrombosis, infection, bleeding, vascular or nerve trauma, and accidental injection of intravenous drugs are potential complications.
Pulmonary Artery Catheter
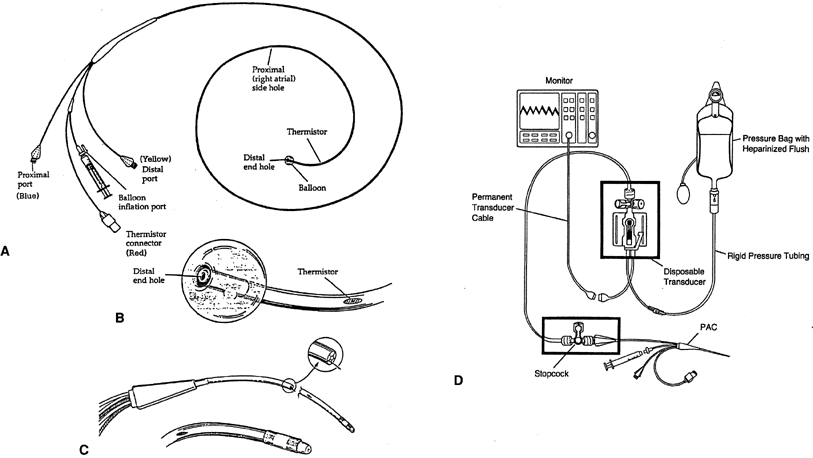
The pulmonary artery catheter (PAC), often called the Swan-Ganz catheter, is a triple lumen, flow-directed, balloon-tipped, thermodilution catheter (Fig. 1). The catheter's length ranges from 60 to 110 cm and is 2.3 mm (7 Fr) in outside diameter. The standard catheter has 3 ports (a proximal infusion port, a distal infusion port, and a balloon inflation port) and the thermistor connector in its proximal end. The catheter's shaft is mainly made of a polyvinyl chloride derivative and is radiopaque.
The proximal infusion port exits 30 cm from the tip of catheter and after correct insertion should be located in the patient's right atria. This port is used to infuse fluids or medications, and when connected to a transducer with a heparinized line, it is used to continuously monitor the right atrial pressure (RAP), which is equivalent to the central venous pressure (CVP).
The distal infusion port exits at the tip of the catheter, and after correct insertion, it should rest in a branch of the patient's pulmonary artery. This port is connected to a transducer with a heparinized pressure line (continuous flush of 3 mL per hour of heparinized solution in a bag at a pressure of 300 mmHg) to maintain the lumen patent and continuously monitor the pulmonary artery pressure (PAP) when the balloon is deflated, and intermittently the pulmonary capillary wedge pressure (PCWP) when the balloon is inflated. Both the proximal and the distal lumina of the catheter may be used to withdraw samples of venous blood for laboratory studies.
The lumen of the balloon's inflation port ends in small latex balloon, located 0.5 cm from the catheter's tip. The balloon is inflated at the time of catheter advancement and when PCWP is needed. The fully inflated balloon (1.5 mL) creates a recess for the tip, preventing the tip from perforating vessels, valves, or chambers as the catheter is advanced.18 While floating the catheter, there is no limit to the duration of inflation; however, once wedged, it should generally not remain inflated for more than 1 minute.
The thermistor is a temperature sensor and transducer, located in the outer surface at 4 cm from the catheter's tip that can be attached to a thermodilution cardiac output computer. When cardiac output measurement is desired, 10 mL of injectate (usually normal saline at room temperature or a known cold temperature) is infused into the proximal port. The thermistor will determine the blood's temperature at the tip of the catheter before and after the injectate infusion, and the computer will calculate the cardiac output.
Conventional PAC measure pressure and flow is appropriate for assessment of left ventricular function, but right ventricular function, which is more dependent on volume than pressure, is not assessed.20 The standard PAC has been modified to accomplish other functions. The oxymetric PAC, in conjunction with a bedside microprocessor, provides continuous determination of mixed venous blood's hemoglobin oxygen saturation (SvO2), which allows continuous calculation of oxygen consumption (VO2). Another enhanced PAC can measure right ventricular end-diastolic volume and ejection fraction through a combination of electrode sensing and thermodilution.21 Right ventricular ejection fraction catheters allow recognition of right and left ventricle interdependence.22 New thermodilution catheters (with a thermal filament) can measure cardiac output continuously.23 These modified catheters are 7.5 Fr and require an 8.0-Fr introducer. There are other catheters with additional ports that exit 14 cm from the tip through which medication can be given or for passing temporary pacemaker leads into the right ventricle. However, the small size of these ports renders them ineffective for resuscitation with infusion of large volumes of fluid or blood.
INDICATIONS FOR PAC PLACEMENT IN OBSTETRIC PATIENTS
Currently, the PAC is used in obstetrics in the management of critically ill patients or those at risk for sudden decompensation and in clinical investigations that attempt to define the pathophysiology of various disease processes or evaluate therapy of a specific condition.
A list of obstetric conditions for which invasive hemodynamic monitoring may assist in their management was published by the American College of Obstetrics and Gynecology (ACOG) in 1992.24 Invasive monitoring is not needed in every patient with one of these conditions, nor is this an all-inclusive list. This list can be divided into:
- Obstetric patients with acute critical condition or whose condition is
subject to rapid deterioration:
- Sepsis with refractory hypotension or oliguria (septic shock);
- Unexplained or refractory pulmonary edema, heart failure, or oliguria;
- Severe preeclampsia with pulmonary edema or persistent oliguria;
- Intrapartum or intraoperative sudden cardiovascular decompensation (i.e., amniotic
fluid embolism);
- Severe hypovolemic shock (massive hemorrhage);
- Adult respiratory distress syndrome;
- Shock of undefined etiology.
- Sepsis with refractory hypotension or oliguria (septic shock);
- Obstetric patients with chronic cardiovascular or metabolic diseases:
- New York Heart Association class III and IV cardiac disease*;
- Cardiomyopathy irrespective of etiology;
- Uncontrolled hyperthyroidism or pheochromocytoma;
- Unstable coronary artery disease.
- New York Heart Association class III and IV cardiac disease*;
*Particularly dangerous structural or physiologic disturbances include primary pulmonary hypertension, Eisenmenger's physiology, aortic coarctation, and mitral stenosis. More precise assessment of their base line condition, both early in pregnancy to assess the risk of continuing the pregnancy and during labor and delivery, as well as prompt evaluation of complications and subsequent therapeutic decisions, should favorably affect both management and outcome.24
CONTRAINDICATIONS FOR PAC PLACEMENT
In general, contraindications are25:
- Right heart valvular disease: Tricuspid or pulmonary valvular stenosis
or prosthetic valves.
- Right-to-left shunt: By definition, pulmonary blood flow is reduced, and
the balloon-tipped catheter is more likely “to be floated” into
the systemic side of the shunt. Even if placed in the pulmonary
artery, the inflated balloon can significantly increase the shunt.
- Previous pneumonectomy: Balloon inflation may precipitate an unacceptable
rise in pulmonary vascular resistance, and although a rare complication, pulmonary
artery rupture in such a patient would almost certainly
be fatal.
- Latex allergy: Unless latex-free catheter is available.
- Anticoagulation or severe coagulopathy: This is a relative contraindication
that can be bypassed by avoiding percutaneous venepuncture and cannulation. In
these cases, venous cut down of the left basilic vein is
recommended.
- Patient at risk of severe arrhythmias: Although not common, complete AV
block can occur in patients with preexisting left bundle branch block. In
these cases, the appropriate transthoracic pacing equipment must
be available.
Central Venous Access
The two most common vascular approaches are, in order of preference:
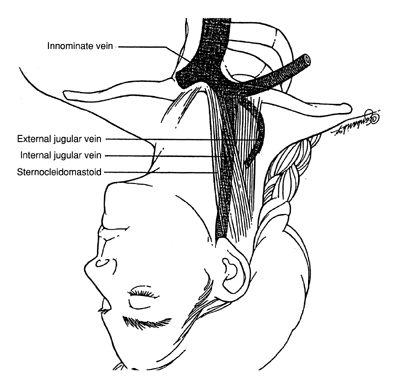
- Right internal jugular vein: Provides a nearly straight access to right
atria and ventricle, and offers a low risk of creating a pneumothorax.
- Left subclavian vein: Provides a more direct access to the vena cava and
offers less risk of thoracic duct damage, as compared with the left
internal jugular vein.
- The femoral and antecubital veins are used less frequently because of greater
difficulty in positioning the catheter. Additionally, use of the
inguinal area in obstetrics may limit access to and manipulation of
the catheter at critical times such as during delivery.26
INSERTION TECHNIQUE
- Set up. At the time of publication, a video is available at this address:http://www.manbit.com/PAC/videos/video1.wmv
- Check patient's allergies record (especially iodine and latex).
- Patient must be monitored with continuous EKG (V2 is recommended) and EFM.
- The catheter's proximal and distal lumina should be flushed and purged
of air and the balloon checked for symmetrical inflation, absence
of an air leak, and ease of spontaneous deflation.
- Check all tubing, stopcocks, and transducer connections for a tight fit. Supervise
establishment of zero reference point, flushing of pressure
tubing, and calibration of pressure transducer.
- If the internal jugular approach is selected, the patient is positioned
in slight Trendelenburg position (head tilted below the horizontal) with
a wedge under the ipsilateral hip and asked to turn her head to the
side opposite the vessel selected for cannulation.
- The selected insertion site is then prepared and draped in a sterile fashion. If
a povidone-iodine preparation (Betadine) is used, it should
be left in contact with the skin at least 2 minutes.
- The operator should be gowned and gloved.
- A resuscitation cart should be available at the bedside.
- Check patient's allergies record (especially iodine and latex).
- Central vein percutaneous cannulation: Catheter-over-guidewire modified
technique. At the time of publication, a video is available at this address:http://www.manbit.com/PAC/videos/video2.wmv
- Inject the skin and make a wheal made over the carotid artery (Fig. 2) with 1% Xylocaine and a 21- or 22-gauge 5-cm needle.
- The needle (“finder needle”) is then directed into the patient's
neck and toward the ipsilateral nipple at a 45° angle, aspirating
for blood as a tract toward the vessel is anesthetized. Often
the vein walls will be collapsed, one against the other, by the pressure
of the needle, and the vessel is completely traversed without blood
return. Accordingly, if a tract of the entire length of the needle
has been anesthetized and no blood has returned, the operator should
slowly withdraw the needle, maintaining a small amount of negative pressure
on the syringe. Failure to achieve blood return merits repositioning
the patient and further attempts, each time redirecting the angle
by approximately 5° medial or lateral to the prior attempt.
- Once the vessel is located (free-flowing blood returns), the finder needle
is left free in the patient's neck to serve as a guide for recannulation
of the vessel with an 18-gauge needle (“insertion needle”), following
which a vascular J-tipped guidewire is passed
into the vessel. The wire should pass freely and without resistance and
should never be withdrawn through the cutting edge of the needle for
fear of severing the wire.
- After the wire is passed into the vessel, the needle is withdrawn over
the wire.
- A scalpel is used to make a 3- to 4-mm cut about the wire, following which
the larger 7.5- or 8-Fr vascular access catheter is passed, with the
assistance of a vein dilator, over the wire.
- The wire and dilator are then withdrawn and ready withdrawal of blood through
the catheter introducer should be achieved. If there is any doubt
as to whether the blood is arterial or venous, blood gas analysis can
be performed.
- Inject the skin and make a wheal made over the carotid artery (Fig. 2) with 1% Xylocaine and a 21- or 22-gauge 5-cm needle.
- Intracardiac passage and intrapulmonary positioning. At the time of publication, a
video is available at this address:http://www.manbit.com/PAC/videos/video4.wmv
- Connect the distal port of the catheter to the pressure transducer and
oscilloscope monitor.
- The catheter is next advanced through an introducer at the venous access
site.
- Fully inflate the balloon (1.5 mL of air) after the tip enters the vessel. This
will be marked by the appearance of oscillations in the monitor
reflecting the pressure in the vein (normal jugular, subclavian, and
superior vena cava pressure is 1 to 6 mmHg). In an average size individual, this
is obtained after insertion of approximately 15 cm of the
catheter. If resistance to balloon inflation is encountered, the catheter
should be advanced further into the vessel and a repeat attempt made
at inflation. This will usually be successful, as the caliber of the
vessels increases as the catheter is advanced centrally.
- Monitor continuously the pressure tracings to determine tip location (Fig. 3). By slowly advancing the balloon-tipped catheter, it will be carried
along and directed into position by venous blood flow.18,25,26
- The pressure tracing in the superior vena cava, its tributaries, and the
right atria is the same. It is a low-amplitude pressure tracing that
varies with the respiratory cycle (i.e., pressure falls with inspiration
and rises with expiration).
- Continued advancement of the catheter tip across the tricuspid valve into
the right ventricle results in a spiking waveform, representative of
the pulsatile systolic pressure (normal right ventricular systolic pressure
is 15 to 30 mmHg). The pressure in the right ventricle during
diastole should be the same as the pressure in the right atrium.
- Subsequently, as the pulmonary artery is entered, another spiking waveform
of lower amplitude is identifiable, measuring now diastolic pressures
above 0 mmHg (normal pulmonary artery diastolic pressure is 6 to 12 mmHg), whereas
the systolic pressure, in normal conditions, should be
equal to the right ventricular systolic pressure.
- The next waveform obtained, a damped tracing with respiratory variation, is
the pulmonary capillary wedge pressure. If the balloon is deflated, a
pulmonary artery tracing reappears. The wedge pressure is the same
as the diastolic pressure in the pulmonary artery, unless there is pulmonary
hypertension. A true pulmonary capillary wedge pressure is verified
by: conversion of a pulmonary artery pressure tracing to a pulmonary
capillary wedge pressure tracing when the balloon is inflated; the
presence of respiratory variation in the pressure tracing; and a calculated
mean pulmonary artery pressure higher than the pulmonary capillary
wedge pressure.
- The pressure tracing in the superior vena cava, its tributaries, and the
right atria is the same. It is a low-amplitude pressure tracing that
varies with the respiratory cycle (i.e., pressure falls with inspiration
and rises with expiration).
- Routine chest radiography to verify catheter positioning is not necessary
if the insertion was uncomplicated and the surgeon is certain the chest
cavity was not entered. Verification of proper positioning of the
pulmonary artery catheter is dependent on obtaining a high quality and
appropriate pressure tracing, not by chest radiograph.
- Connect the distal port of the catheter to the pressure transducer and
oscilloscope monitor.
The ability to obtain accurate tracings over prolonged periods can be enhanced by the use of two devices, which are particularly useful in situations that mandate catheter repositioning. A catheter introducer system containing a one-way valve allows a malfunctioning catheter to be advanced, withdrawn, or changed without loss of the venous access. Additionally, sheaths are available that fit over the catheter itself and attach to the introducer port. The sheaths maintain sterility of a segment of the pulmonary artery catheter, allowing its advancement or withdrawal as necessary to obtain accurate pressure tracings.