Embryology Neural tube defects (NTDs) are a heterogeneous group of malformations resulting
from failure of neural tube closure between the third and fourth
week of embryologic development. Approximately 18 days after conception, the
neural plate folds inward to form a central neural groove and
bilateral neural folds. The neural folds then fuse in the midline to
begin formation of the neural tube. Fusion continues toward the cranial
and caudal ends simultaneously, with closure of the anterior neuropore
by day 24 of development and closure of the posterior neuropore by
day 26. The cranial end of the neural tube becomes the forebrain, midbrain, and
hindbrain, and a failure of closure results in anencephaly. The
caudal end of the neural tube becomes the spinal cord, and a failure
of posterior neuropore closure results in spina bifida. This classic
description of the mechanism of spinal cord closure has been challenged
by Van Allen and colleagues.9 Using reviews of previous published clinical reports, this group has shown
evidence of simultaneous multisite neural tube closure based on five
common sites for NTD lesions. Anencephaly, encephalocele, and spina bifida are the three most common
forms of NTDs. Anencephaly is the most severe of these lesions. With failure
of brain development, the cranium does not form (called acrania), and
the remaining neural elements are covered by a thin membrane. Encephaloceles (Fig. 3) are much less common than anencephaly or spina bifida. They are cystic
extensions of the brain through an overlying scalp and skull defect, somewhat
analogous to a spina bifida. 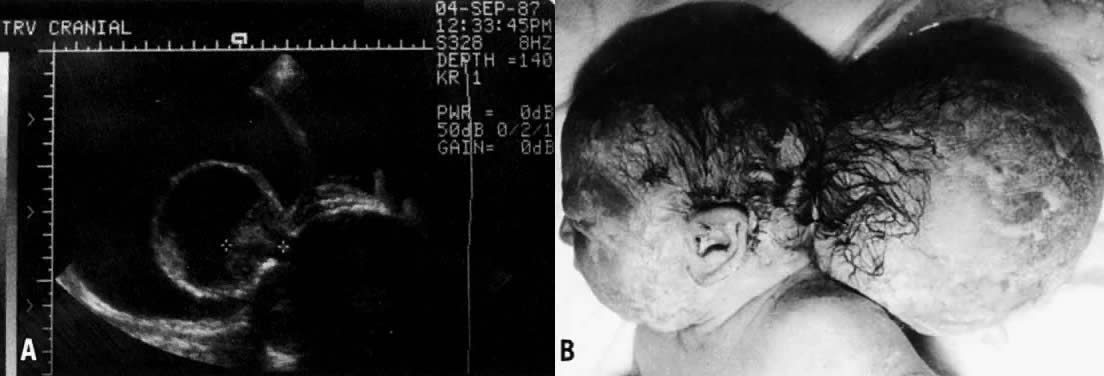 Fig. 3. A. An encephalocele diagnosed by ultrasound. Notice the cursors identifying
the small amount of extruded brain tissue through the cranial defect. B. The infant after delivery. Fig. 3. A. An encephalocele diagnosed by ultrasound. Notice the cursors identifying
the small amount of extruded brain tissue through the cranial defect. B. The infant after delivery.
|
A disruption of the vertebral arches often accompanied by underlying spinal
cord defects is collectively called spinal dysraphism or spina bifida (Fig. 4). They are classified as spina bifida occulta if the disruption involves
only bony structures and spina bifida cystica if there is a saccular
defect involving neural elements. Meningomyeloceles constitute 90% of
spina bifida and are composed of neural tissue covered by meninges that
extrude through the vertebral column. Alternatively, a fluid filled
sac (not containing neural elements) covered by meninges that protrudes
through the bony defect is called a meningocele. Although spinal dysraphism
can occur at any region of the vertebral column, the most common
site for these defects is the lumbosacral area.  Fig. 4. Spina bifida by ultrasound (A) and after delivery (B). Fig. 4. Spina bifida by ultrasound (A) and after delivery (B).
|
Spina bifida is frequently accompanied by the Arnold-Chiari malformation. This
anomaly results from a downward displacement of the medulla, fourth
ventricle, and cerebellum through the foramen magna into the region
of the cervical spine. This downward displacement of the hindbrain
can hinder the egress of cerebrospinal fluid from the brain, causing
an enlargement of the ventricles. This accounts for the 70% to 90% incidence
of hydrocephalus associated with spina bifida.10 Other, less common defects include exencephaly (i.e. exteriorization of an abnormally formed brain) and iniencephaly (i.e. defect of the skull base, cervical spine, and underlying neural tissue). Myeloschisis, usually
seen as an early fetal defect, describes an open
flat neural plate that may be extensive.11 Myelodysplasia, or occult spinal dysraphism, describes less obvious malformations
of the cord resulting from maldevelopment of the caudal region
of the neural tube. These defects are often associated with lipomas
or cutaneous changes overlying the region such as dimpling, sinus tracts, or
hairy patches. These probably result from an embryologic mechanism
similar to NTDs and may be associated with neurologic or orthopedic
disabilities. Schut and coworkers12 have extensively reviewed the clinical aspects and variations of these
defects. Etiology, Incidence, and Recurrence Risks Eighty-five percent of NTDs occur by multifactorial inheritance, a genetic
predisposition from an interplay between various genes and environmental
factors. The etiologic heterogeneity of NTDs was best illustrated
by Holmes and coworkers, who reported that, of 106 liveborn or stillborn
infants with an NTD, about 12% had identifiable causes.13 A small proportion of NTDs occur because of single-gene disorders, chromosomal
aneuploidy, and teratogen exposure. Meckel syndrome is the most
common of the single-gene disorders associated with a NTD. This autosomal
recessive syndrome includes posterior encephalocele, polydactyly, cleft
palate, and cystic dysplasia of the kidneys. Because the recurrence
risk for such a defect is 25%, it underscores the need for careful
evaluation of all infant with NTDs, because recurrence risks depend
on the cause of the malformation. Chromosomal aneuploidy also accounts for a small percentage of NTDs. Of
the various types of NTDs, encephaloceles and spina bifida are more likely
to be associated with triploidy; with trisomies 13, 18, and 21; and
with various unbalanced translocations. The recurrence risk for these
disorders varies with the mechanism responsible for the aneuploidy. For
example, the recurrence risk for a trisomy is approximately 1%,, and
triploidy is thought to be a sporadic event with a negligible recurrence
risk. Recurrence estimates for translocations depend on the specific
nature of the translocation and whether they are maternally or
paternally transmitted. Several teratogens have been implicated in the cause of NTDs. Two anticonvulsant
medications in current use, carbamazepine and valproic acid, have
been demonstrated to cause these defects. Robert and Guibaud14 originally reported an association between valproic acid and NTDs, noting
a 1% risk for NTDs in patients taking this medication. This observation
has been substantiated in several animal models.15–17 Carbamazepine also is associated with a 1% risk of spina bifida.18 Children of mothers with insulin-dependent diabetes mellitus have a 1% to 2% risk
of NTD and a twofold to threefold (4% to 9%) increased incidence
of congenital malformations compared with the general population.19 Although glycemic control may not be the sole etiologic factor in malformations
in infants of diabetic women, careful preconceptional control
is believed to decrease the prevalence of NTDs and other anomalies in
these patients. The causes of NTDs are listed in Table 1. TABLE 1. Causes of Neural Tube Defects
Multifactorial inheritance
Single-gene (autosomal recessive) disorders Meckel syndrome (most common)
Robert syndrome
Jarcho-Levin syndrome
Median facial cleft syndrome
HARDE (Walker-Warburg) syndrome
Oculo-auriculo-vertebral (Goldenhar) syndrome
Chromosomal aneuploidy Trisomy 18
Trisomy 13
Trisomy 21
Triploidy
Unbalanced translocations, markers, ring chromosomes
Teratogens Valproic acid
Carbamazepine
Aminopterin
Thalidomide
Amniotic band sequence
Cloacal extrophy
Sacrococcygeal teratoma
Maternal insulin-dependent diabetes mellitus
NTDs are the second most common fetal malformation in the United States, surpassed
only by congenital heart defects. The incidence of NTDs varies
with race, geographic location, and various other predisposing factors. In
the United States, the incidence is approximately 1 to 2 cases
per 1000 live births, whereas the incidence in Britain is about four
times greater. Families who have had a child with an NTD have a 10-fold
increase in their recurrence risk. In the United States, a family
with an affected child has a 2% recurrence risk of another child with
an NTD. If the defect in the first affected pregnancy was anencephaly, the
family has a higher risk for recurrence of anencephaly than for recurrence
of spina bifida. The risks for other affected U.S. populations
are listed in Table 2. Between 90% and 95% of NTDs occur in families without a prior family
history of an NTD. TABLE 2. Estimated Incidence of Neural Tube Defects Based on Specific Risk
Factors in the United States
Population | Incidence/1000 Live Births |
Mother as reference | |
General incidence | 1.4–1.6 |
Women undergoing amniocentesis for advanced maternal age | 1.5–3.0 |
Women with diabetes mellitus | 20 |
Women on valproic acid in first trimester | 10–20 |
Fetus as reference | |
One sibling with NTD | 15–30 |
Two siblings with NTD* | 57 |
Parent with NTD | 11 |
Half sibling with NTD | 8 |
First cousin (mother's sister's child) | 10 |
Other first cousins | 3 |
Sibling with severe scoliosis secondary to multiple vertebral defects | 15–30 |
Sibling with occult spinal dysraphism | 15–30 |
Sibling with sacrococcygeal teratoma or hamartoma |  15–30 15–30
|
NTD, neural tube defect.
*Risk is higher in British studies. Risk increases further for three or
more siblings or combinations of other close relatives.
Main DM, Mennuti MT: Neural tube defects: Issues in prenatal diagnosis
and counseling. Obstet Gynecology 67:1–15, 1986.
Screening and Diagnostic Tests ALPHA-FETOPROTEIN LEVELS. In 1972, Brock and Sutcliffe measured AFP in the amniotic fluid of 31 pregnancies
with anencephaly and 6 pregnancies with spina bifida, hydrocephaly, or
both conditions.21 All of the cases of anencephaly and most of the spina bifida cases before 30 weeks' gestation
demonstrated amniotic fluid AFP levels that were
markedly elevated during pregnancy. When the fetus has an open (not
skin covered) NTD, AFP leaks from the fetal circulation into the amniotic
fluid. In 1974, Wald and coworkers performed a case-controlled study
comparing maternal serum AFP levels in seven pregnancies with open
NTDs with 14 control pregnancies matched for maternal age, parity, and
gestational age.21 Maternal serum AFP levels in the affected pregnancies were significantly
higher than those of the control population. This led to the hypothesis
that there would be a role for measuring MSAFP in screening for NTDs. The
U.K. Collaborative studydemonstrated the utility of this test
for prospective open NTD screening in 1977.7 In anencephaly, the malformed skull is not completely covered by overlying
skin, and it is therefore the lesion most accurately detected with
MSAFP screening. More than 90% of anencephaly cases can be detected by
MSAFP screening, and 99% can be detected by ultrasound examination. Approximately 99% of
anencephaly cases can also be detected by amniotic
fluid AFP and acetylcholinesterase (AChE) testing. In contrast, most
encephaloceles are skin covered and therefore are less likely to be identified
by MSAFP screening or amniocentesis and are most often detected
by ultrasound. Spina bifida and anencephaly occur with equal frequency. Approximately 80% of
spinal cord defects are open—the tissue overlying the defect
is not skin covered. The remainder of spinal cord defects are covered
by skin or by a thick membrane and are not detectable by screening. In
general, MSAFP screening programs detect approximately 85% of open
fetal NTDs: 80% of open spina bifida and 90% of anencephaly. Almost
all of these open lesions can then be diagnosed by amniotic fluid testing. The
object of any screening program is to maximize detection at an
acceptable false-positive rate. A screening test cutoff point is a balance
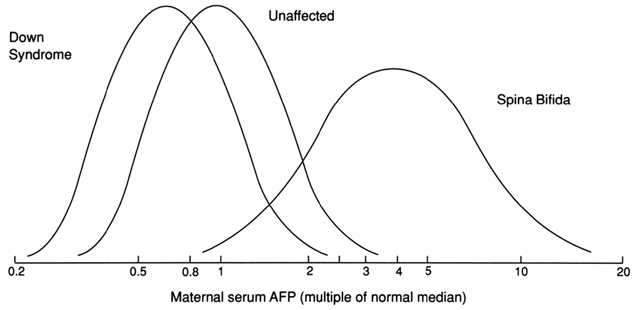
between these two factors. The correct MoM value for MSAFP can
only be calculated after all the appropriate information regarding the
patient is taken into account. This includes weight (at the time the
blood sample was obtained), gestational age, and race and considers whether
the patient has insulin-dependent diabetes mellitus. An MSAFP level
is considered elevated if the value is greater than 2.0 or 2.5 times
the median value (2.0 or 2.5 MoMs) for normal controls at the same
week of gestation. MSAFP screening is most accurate when performed between 16 and 18 weeks' gestation, but
testing can be performed between 15 and 22 weeks. Screening
earlier or later than the optimal gestational age decreases the
sensitivity of the test. Screening should be voluntary and should be
performed after the patient has been fully informed regarding the benefits
and limitations of the test. The patient should understand that a
normal MSAFP result does not ensure a child without an abnormality (including
an NTD), and that an elevated MSAFP level does not specifically
diagnose an abnormality. Instead, an elevated value places the patient
in a high-risk group that necessitates further evaluation. The most
common causes of false-positive and false-negative MSAFP results are
listed in Table 3. TABLE 3. Common Causes of False-Positive and False-Negative Maternal Serum
Alpha-Fetoprotein Levels
False-Positive Levels
Inaccurate gestational dating (patient has a more advanced gestation than
estimated)
Multiple gestation
Race (black patients have higher levels than white patients)
Underweight patients (less than 90 pounds)
Spontaneous fetal to maternal bleeding
False-Negative Levels
Inaccurate gestational dating (patient has less advanced gestation than
estimated)
Maternal insulin-dependent diabetes mellitus
Obesity
Consider the hypothetical example given with the protocol in Figure 5. A cohort of 10,000 consecutive women present for MSAFP screening with
a level of risk comparable to the U.S. population. About 10 to 15 of
these pregnancies would be affected with an NTD. MSAFP screening would
detect 8 to 12 of these defects. At a cutoff point of 2.0 MoMs, the false-positive
rate for this test is 4%, but a screen positive test result
would only imply a 3% risk of having a fetus affected with an open
NTD. A positive screening test result increases these patients' risks
from 1.5 per 1000 to 3 per 100. Conversely, 97% of pregnancies with a
positive screening test result are unaffected. If a screening cutoff
point of 2.5 MoM is used, the false-positive rate is approximately 2%. 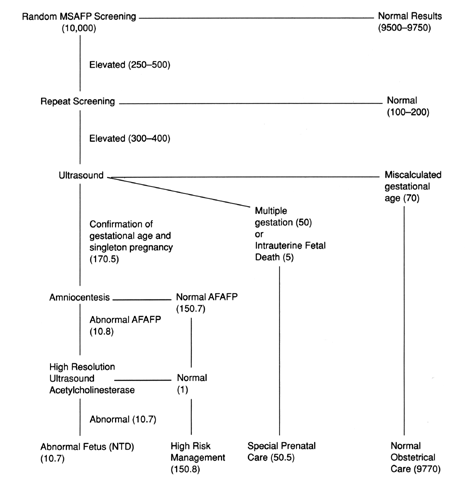 Fig. 5. Anticipated results for MSAFP screening of 10,000 prenatal patients.(Adapted from Haddow JE: Screening for spinal defects. Hosp Pract 17:128–138, 1982.) Fig. 5. Anticipated results for MSAFP screening of 10,000 prenatal patients.(Adapted from Haddow JE: Screening for spinal defects. Hosp Pract 17:128–138, 1982.)
|
AMNIOTIC FLUID ALPHA-FETOPROTEIN AND ACETYLCHOLINESTERASE. Amniocentesis is often used to differentiate the disorders responsible
for a maternal serum AFP elevation. If there is an amniotic fluid AFP
elevation, a secondary test for the presence or absence of the AChE enzyme
by gel electrophoresis is performed on the fluid. AChE is not normally
identified in amniotic fluid. Tissues containing AChE are red blood
cells, muscle, and neural tissue. Concentrations of AChE are much
higher in fetal cerebrospinal fluid than in fetal serum. If the fetus
has an open NTD, amniotic fluid AFP and AChE are usually both elevated
and the high concentration of AChE in cerebrospinal fluid transudates
across the defect into the amniotic fluid. AChE is a sensitive test
for confirming an open NTD. Fetal blood contamination is the most common source of falsely elevated
AFP levels in amniotic fluid, and the amniocentesis performed to obtain
the sample is the most common cause of fetal blood in the fluid. In
such cases, amniotic fluid AFP is usually in the 3 to 5 standard deviation
range. AChE is not detected in 90% of cases because of the relatively
low AChE concentrations in the fetal blood. In congenital (Finnish) nephrosis, a
rare autosomal recessive disorder, amniotic fluid AFP
levels may be very high, and AChE is not identified. At the time of amniocentesis for elevated MSAFP, karyotype analysis should
also be performed regardless of the amniotic fluid AFP result. Omphaloceles
and NTDs are both associated with chromosomal aneuploidy. Even
when the amniotic fluid AFP level is normal, the addition of chromosome
analysis allows more informative counseling regarding perinatal outcome. ULTRASONOGRAPHIC DETECTION. Ultrasound is an integral part of the management of patients with an elevated
MSAFP level. It should be used as part of the initial fetal evaluation
to exclude improper gestational dating, multiple gestation, and
fetal demise. It can also identify other reasons for an elevated MSAFP
value (Table 4). Although amniocentesis is usually performed to explain elevated MSAFP
levels, a number of investigators have suggested that high-resolution
ultrasound alone is an acceptable alternative for diagnostic evaluation
of elevated MSAFP levels, particularly those in the range of 2.0 to 3.0 MoM.24,25 However, the accuracy of NTD diagnosis may be limited by the location
or extent of the lesion, fetal position, quality of the images, and experience
of the sonologist. TABLE 4. Other Abnormalities Identified by the Alpha-Fetoprotein Screening
Process
Ventral wall defects Omphalocele
Gastroschisis
Triploidy
Trisomies: 18, 13, 21
Unbalanced translocations
Amniotic band sequence
Pentalogy of Cantrell: omphalocele, lower sternal defect, deficiency of
diaphragmatic pericardium, intracardiac abnormality, anterior diaphragm
defect
Renal agenesis
Fetal demise
Multiple gestation
Congenital nephrosis (Finnish type)
Sacrococcygeal teratoma
Dermatologic disorders
Epidermolysis bullosa
Congenital icthyosiform erythroderma
Chorioangioma
Maternal hepatoma
Maternal ovarian teratoma
The use of ultrasound to detect spina bifida was addressed by Platt and
colleagues through the California Maternal Serum Alpha-Fetoprotein Screening
Program.26 Through this program, all patients with an elevated MSAFP level were offered
an ultrasound evaluation with an experienced sonographer at one
of 20 specialized prenatal diagnosis centers in the state of California. Of
the 161 cases of spina bifida identified, 148 (91.9%) were detected
by ultrasound. Because this detection rate at experienced prenatal
diagnosis centers is well below 100%, in the absence of an ultrasonographic
finding, amniocentesis for measurement of amniotic fluid AFP is
recommended. Three routine transverse views of the cranium are recommended by Nyberg.27 The biparietal diameter involves obtaining a transverse view of the fetal
skull at the level of the thalami; this view includes the cavum septum
pellucidi and the frontal horns of the lateral ventricles. The transventricular
view, above the plane of the biparietal diameters (BPD), contains
the lateral ventricles and choroid plexus. Both views are essential
in imaging the ventriculomegaly that is found in 80% of NTD cases. The
transcerebellar view, obtained by angling through the posterior
fossa, demonstrates the cisterna magna and the cerebellum. The fetal
spine should be imaged in sagittal, transverse, and coronal axes to
detect the presence of an NTD and any disruption in the overlying soft
tissues. Several cranial ultrasound markers are useful in identifying NTDs. Nicolaides
and coworkers28 first described the “banana” and “lemon” signs
in a retrospective study of 70 patients with open spina bifida between 16 and 24 weeks' gestation. In 100% of cases, the lemon sign was present, whereas
in cases for which the posterior fossa could be evaluated, 95% had
an absent or banana-shaped cerebellum. The lemon sign (Fig. 6) is caused by a scalloping of the frontal bones secondary to decreased
intracranial pressure from extrusion of the NTD. The cerebellar or banana
sign is a consequence of the Arnold-Chiari malformation. It is attributed
to a downward displacement of the cerebellum that is caused by
protrusion of the neural contents through the foramen magnum, which
results from the NTD. With advancing gestation there can be a further
herniation of the brain through the cisterna magna with an inability to
image the cerebellum. 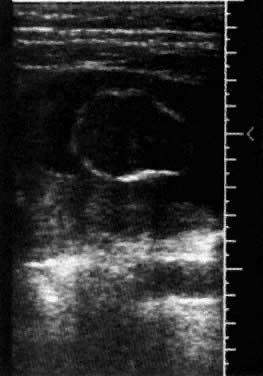 Fig. 6. Biparietal diameter with the lemon sign at 15 weeks' gestation. Fig. 6. Biparietal diameter with the lemon sign at 15 weeks' gestation.
|
Other investigators have suggested that the presence of the lemon sign
is related to gestational age.29 Among 50 cases with open spina bifida, the lemon sign was found in 24 (89%) of 27 fetuses
examined before 24 weeks, in 8 (50%) of 16 fetuses
between 24 and 34 weeks, and in none of the fetuses at 35 weeks' gestation
or more. Van den Hof and colleagues30 prospectively evaluated 130 fetuses with open NTDs. The lemon sign was
present in 98% of fetuses at less than 24 weeks' gestation but found
only in 13% of fetuses at more than 24 weeks' gestation. Cerebellar abnormalities
were present in 95% of fetuses irrespective of gestational
age. Growth retardation and ventriculomegaly significantly worsened with
gestation, but the head circumference remained disproportionately
small throughout pregnancy. Loss of the lemon sign may occur because of
compensatory bony changes as gestation increases or because of cerebral
ventriculomegaly that may compensate for the loss of brain through
the cisterna magna. Fetuses with open spina bifida have smaller BPDs by approximately 2 weeks' gestation
than expected for their gestational age. This finding increases
the detection rate for NTDs. If the gestational age is based on
BPD alone (not a composite of BPD and femur length), the MSAFP MoM is
increased when interpreted 2 weeks earlier than the correct gestational
age.31,32 Between 16 and 18 weeks, the detection of an open NTD increases to 90% at
a screening cutoff of 2.5 MoMs when gestational age as determined
by BPD measurement is used to interpret the result. | 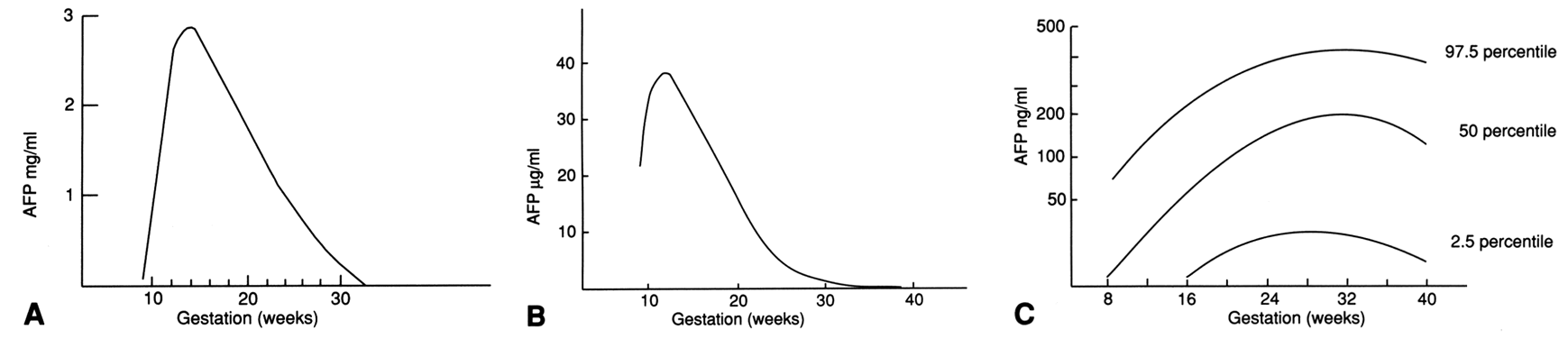


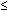 15–30
15–30
