Over the past five decades, a variety of techniques have been developed to treat CIN each based on the concept of ablation of or excision of lesions. Ablative techniques include cryosurgery and carbon dioxide (CO2) laser vaporization. Cryosurgery, as the name implies, uses a freezing technique to destroy the abnormal epithelium. It is an older technique that remains popular because it is easy to perform, requires minimal equipment or maintenance, and is associated with minimal patient discomfort in an outpatient/office setting. Either carbon dioxide gas or nitrous oxide is used as the refrigerant for the cryosurgery unit. A thin layer of water-soluble lubricant on the cryotherapy probe allows for a more uniform contact between the probe and the cervix and facilitates a complete freeze. The probe must cover the entire lesion and should create a 4- to 5-mm ice ball beyond the edge of the probe. The use of a flat cryoprobe without a nippled tip that extends into the cervical canal, as seen in Figure 1, will reduce the possibility of stenosis and is less likely to cause the squamo-columnar junction to recede into the endocervical canal, making follow-up evaluations difficult. The use of a double-freeze technique with 3-minute intervals each of freeze–thaw–freeze appears to improve the efficacy. Local lidocaine anesthesia reduces patient discomfort at the time of the procedure.18 Because the desiccated tissue at the transformation zone is not removed with the procedure, it will slough as a clear watery discharge for 10 to 14 days after treatment; however, this may persist for as long as 4 to 6 weeks and women should be prepared for this via pretreatment education.
CO2 laser vaporization of the cervix gained popularity in the mid 1980s. It is also a procedure that is easy to perform in the outpatient clinic. Generally, a CO2 laser-mounted on a colposcopy unit is used and the outline of the lesion demarcated. The energy of the laser is absorbed by the water in the tissue and destruction occurs mainly by vaporization. Most instruments have varying power settings and the rate of tissue destruction is dependent on the power setting, the laser spot size, and the duration of tissue exposure. Unlike cryotherapy, the laser allows for very precise tissue destruction and can spare unnecessary destruction of normal tissue by conforming the treatment to the shape of the lesion. Vaporization to a depth of 6 to 7 mm is required to remove the entire transformation zone and its associated endocervical glands. The treatment causes only minor discomfort in the form of uterine cramps and can be generally performed with local infiltrative anesthesia or a paracervical block. Advantages of the procedure include the precision of the instrument to treat the entire lesion to the appropriate depth and the ability to treat coexisting preinvasive changes of the vagina and vulva, as seen in Figure 2. The disadvantages of the CO2 laser over other techniques include increased procedure time, the expense of the laser unit (and its maintenance), and increased discomfort and bleeding complications.
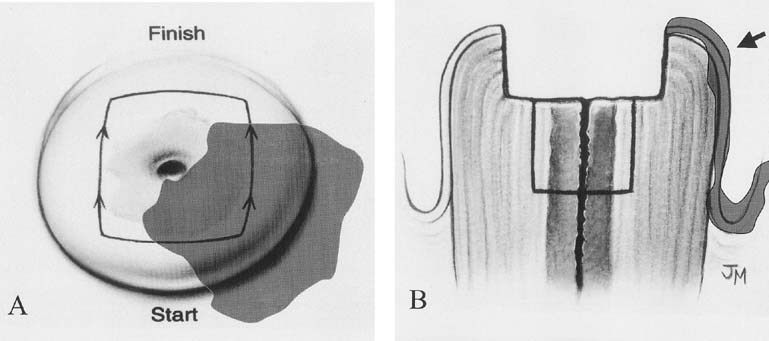
Ablative or destructive techniques for treating the cervical transformation zone are appropriate only when the extent of the dysplasia is known, colposcopy with directed biopsy is consistent with preinvasive cervical disease, and invasive carcinoma is not suspected. If these criteria are not met, an excisional (cervical conization) procedure must be performed. Although an excisional technique can be selected as therapy for most CIN cases cervical conization is first and foremost considered a diagnostic procedure. The indications for cervical conization are outlined in Table 1. Excisional techniques include cold knife conization, the loop electrosurgical excision procedure (LEEP) as a cone, and CO2 laser conization. The use of a scalpel to excise a cone-shape piece of tissue or cold-knife conization is the classic technique and has the advantage of allowing cone biopsies of various shapes and sizes to fit the clinical scenario (Fig. 3). A disadvantage of this technique is the need for regional or general anesthesia in an outpatient surgical suite.
Table 1. Indications for Cervical Conization
| An intraepithelial lesion or microinvasive carcinoma is present in the endocervical curettings |
| Cytologic assessment indicates an abnormality that is not consistent with the tissue diagnosis |
| The entire transformation zone is not visible |
| Microinvasive carcinoma is diagnosed by direct biopsy |
| Cytologic or biopsy evidence of premalignant or malignant glandular epithelium is detected |
(From American College of Obstetricians and Gynecologists: Technical Bulletin, No. 183, 1993)
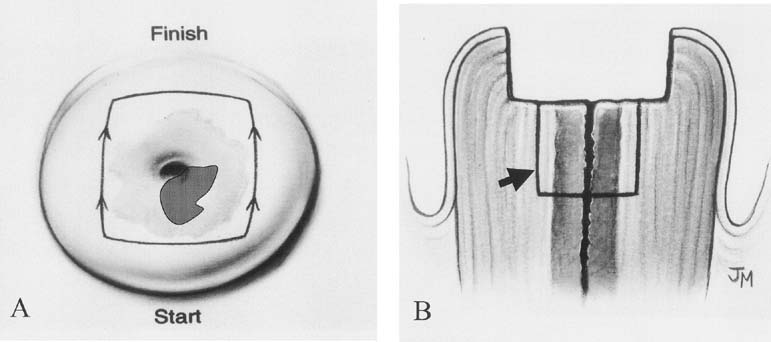
Electrocautery was used extensively in other countries as a destructive technique; the development of thin wire loops that excise the tissue and provide a histopathologic specimen is a more recent development (Fig. 4). The terms large loop excision of the transformation zone (LLETZ) and LEEP have both been used to describe a technique to excise the transformation zone with electrocautery current. This technique is alternatively called diathermy loop excision. It was initially described in Europe and has been extensively used in Great Britain.19 It has become the therapy of choice for many clinicians because it treats the transformation zone similar to ablative techniques and can be used in those clinical situations; however, it also provides a tissue specimen for histopathologic evaluation similar to surgical conization.
|
A cervical LEEP procedure can generally be performed in an outpatient office or clinic setting. Local infiltration of the cervix or paracervical block with lidocaine is recommended. When infiltrating the cervix itself, the use of lidocaine with 1:100,000 epinephrine causes local vasoconstriction that facilitates coagulation of small vessels in the cone bed, thus reducing bleeding from the procedure. Performing the procedure under colposcopic guidance facilitates visualization of the area to be removed. An appropriate loop size is selected to excise the transformation zone to a depth of 6 to 7 mm, extending 4 to 5 mm beyond the affected area. A 60- to 80-watt setting in the cut mode on the electrocautery unit will allow smooth excision and decrease tissue resistance (Fig. 5). In some cases, to avoid using excessively large loops, excision of the anterior and then the posterior portion of the transformation zone can be performed in two separate passes, with a third smaller loop to excise a specific endocervical specimen. Loops less than 20 mm in width and 15 mm in depth are helpful to avoid the excision of a large amount of normal cervical tissue. After removal of the specimen, bleeding areas are cauterized with the ball electrode using coagulation current, and then Monsel's solution (ferrous subsulfate) is applied to the cervix.
While LEEP is most often accomplished in the outpatient office setting, certain circumstances warrant the use of general anesthesia. Indications include cases in which good visualization and access to the cervix are difficult or impossible secondary to patient anatomy or discomfort, an atrophic or stenotic cervix flush with the vaginal wall, and large lesions that extend wide on the cervix or onto the vaginal epithelium.
As the CO2 laser gained popularity in the 1980s, techniques to perform laser conization were also developed. This technique is burdened by the need for regional or general anesthesia in an ambulatory surgery suite, although in some cases it can be performed in the outpatient setting. In addition, the laser cone surgical specimens may have significant cautery artifact which make histopathological evaluation cumbersome. Because the technique of LEEP is more time-efficient, has widespread applicability, and is more easily mastered, it has replaced laser cone in most centers. With appropriate power settings and technique LEEP excision produces minimal cautery artifact.20