A generally accepted definition of microinvasive carcinoma is a lesion that is predominantly intraepithelial with a focus of invasion of microscopic dimensions confined to the superficial stroma. Gland involvement does not remove the lesion from the category of intraepithelial neoplasia.
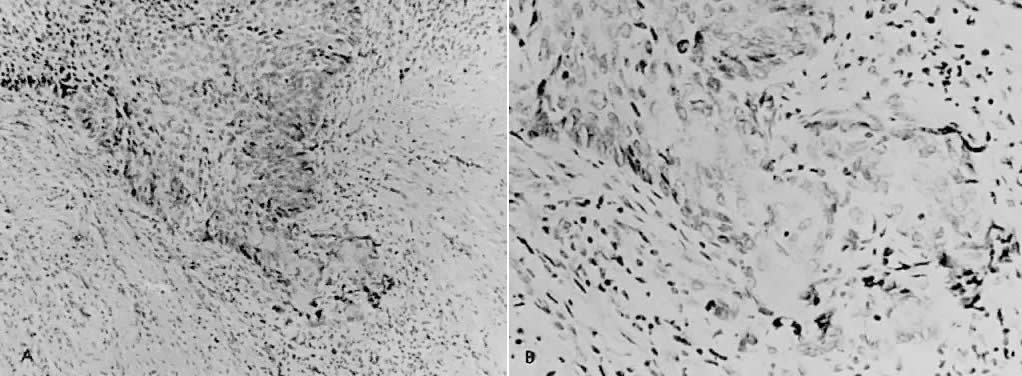
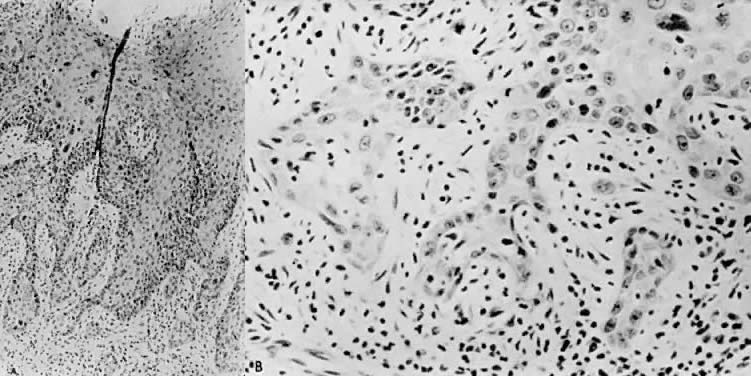
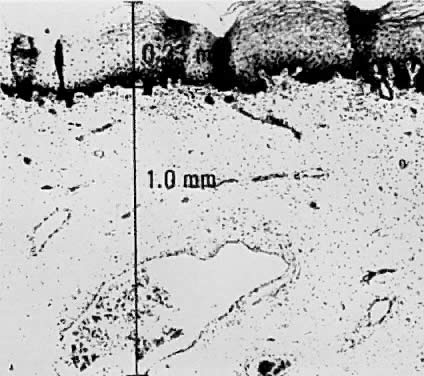
There is general agreement that disruption of the basement membrane is the criterion by which microinvasive carcinoma can usually be identified. This basement "membrane” has been demonstrated by Luiebel and colleagues52 using electron microscopy. He noted it to be complete in CIN III but straighter than in normal squamous epithelium. In invasive carcinoma, it was usually absent or present in an imperfect form. Where the membrane was absent, small cytoplasmic protrusions of carcinoma in the adjacent stroma could be seen, apparently representing invasion by malignant neoplastic cells. Ashworth and co-workers53 confirmed these electron microscopic findings and discussed histochemical procedures including the periodic acid-Schiff reaction alone or in combination with other techniques. He added that invasive cell groups are also found partially or completely invested by a basement membrane that appears to be newly formed by those cells. The typical histologic picture of early stromal invasion below CIN III may show some differentiation of the invasive peg. The cells are rich in cytoplasm and the nuclei larger and clearer than those of the intraepithelial portion of the lesion. There may be cells in various stages of degeneration with some leukocytic infiltration. In hematoxylin-eosin preparations, these pegs may appear eosinophilic and stand out well from the basophilic matrix (Fig. 1) with a dense round cell infiltration surrounding the invasive peg. Tapering or branching small tongues of epithelial cells, as well as the formation of narrow cell columns or the appearance of cell groups that seem to be invading the stroma may be considered criteria of invasion (Fig. 2). On the other hand, the formation of very large epithelial pegs can only be regarded as suspicious of early invasion.54
Disagreement occurs in the specific definition of microinvasive carcinoma regarding the exact depth of invasion, presence or absence of confluence, or vascular space involvement. There is fairly general agreement that the invasion should be 5 mm or less if the lesion is to be considered microinvasive. Averette and colleagues12 used penetration beneath the basement membrane of less than 1 mm as the criterion. Some authors consider confluence1,3,14,16,17 and vascular involvement55,56 as important factors that necessitate removing patients from the microinvasive category.
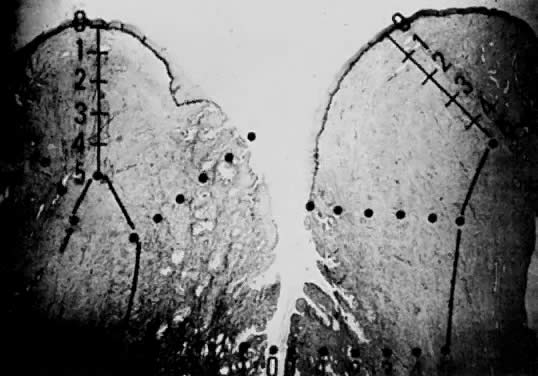
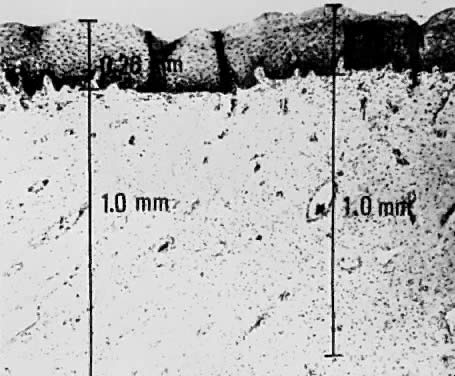
Using a hysterectomy specimen and a micrometer, Averette12 demonstrated the variable distances into the stroma from the basement membrane of ectocervix, squamocolumnar junction, and distant endocervix (Fig. 3). He showed the variable thickness of normal epithelium, which may cause variations in the relative stromal depth when a 1-mm point is measured from the basement membrane (Fig. 4). In addition, vascular channels are shown less than 1 mm beneath the epithelium (Fig. 5). These figures suggest the following:
The depth into the stroma may vary depending on the point of measurement.
Five-millimeter depth is significant in terms of total cervical thickness.
There may be a relative difference in stromal depth when 1 mm is measured
from two separate points.
Vascular channels are present within 1 mm of the stroma and are potentially
a conduit for spread even in very early invasive lesions.
Duncan and Walker57 found vascular involvement in only 2 of 76 patients with invasion of less than 3 mm, whereas vascular involvement was apparent in 8 of 15 patients who had stromal invasion in the 3 mm to 5 mm depth range. Only 2 of 91 patients had metastatic disease, both with vascular involvement. Vascular involvement was present in only 6 of 135 patients reported by Hasumi and colleagues58: there was no vascular involvement in patients with invasion up to 1 mm but 3 of 45 (6.7%) with invasion of 1.1 mm to 3 mm and 3 of 29 (11.1%) with invasion of 3.1 mm to 5 mm had vascular involvement. None of the six cases with vascular invasion had nodal metastasis. Sedlis and colleagues21 reported vascular space invasion by tumor in 31 of 132 patients (23%); this increased with depth of penetration. There was a strong correlation between both residual tumor in the hysterectomy specimen and vascular space involvement with deep stromal penetration and extensive lateral spread. No positive lymph nodes were observed in the 74 patients treated by radical hysterectomy, and recurrence was noted in two patients with extensive vascular involvement.
Simon20 and Creasman59 reviewed the recent literature in regard to lymph vascular space and lymph node involvement. In the study by Simon,20 54 (14.3%) of 378 women subjected to radical surgery and pelvic lymphadenectomy had lymph vascular space involvement and only 1 of the 54 (1.9%) had a node metastasis. None of the 213 women with long-term follow-up had either local recurrence or died from tumor. In Creasman's59 analysis of 267 women treated in a similar surgical fashion and with microinvasive tumors invading below the basement membrane to 3 mm or less, 39 had lymph vascular space involvement and 1 (without lymph vascular involvement) had lymph node metastasis. Of 105 women with tumors invading between 3.1 and 5 mm, 49 (47%) had lymph vascular permeation, 1 of which had lymph node metastasis. Of the 56 patients without lymph vascular involvement, 6 (11%) had positive nodes. Thus, it appears that in relationship to microinvasive carcinoma of the cervix, the presence of tumor cells in lymph vascular spaces does not support the theory that lymph node metastases are imminent. Additionally, there is disagreement as to what constitutes a lymph vascular space.
Confluence appears to increase with increasing depth of penetration.21,58,60 However, several authors believe this factor to be nonprognostic.21,60
Burghardt19 calculated tumor volume by measuring the depth, width, and length of the tumor. There were no lymph node metastases in 12 patients with an area of up to 99 mm2, which corresponded to 1000 mm3. In larger tumors, the frequency of metastasis increased in relation to tumor size. The smallest tumor showing metastasis had an area of 120 mm2.