Cell Type
In 1958 Wentz and Reagan12 divided cervical squamous carcinomas into three cell types: large cell keratinizing, large cell nonkeratinizing, and small cell. With the advent of electron microscopy and immunohistochemistry, it became apparent that what had been termed small cell carcinoma really represents a heterogeneous group of tumors. These include small cell squamous carcinoma, small cell anaplastic carcinoma, and small cell neuroendocrine carcinoma. Furthermore, a newly proposed classification has expanded the spectrum of neuroendocrine carcinoma to include those poorly differentiated large cell carcinomas demonstrating neuroendocrine differentiation by immunohisto-chem-istry.13
Large cell keratinizing squamous carcinoma is characterized by sheets and nests of cells with abundant cytoplasm, large pleomorphic nuclei and inconspicuous nucleoli. Keratin pearls and intercellular bridges are evident (Fig. 1). Mitotic figures are noted occasionally, and the growth pattern is largely infiltrative.14
Large cell nonkeratinizing squamous carcinoma has large cells of similar size and shape. The cytoplasm is moderate in amount, eosinophilic to amphophilic, some having individual cell keratinization with distinct cell borders (Fig. 2). By definition keratin pearl formation should be absent. Nucleoli are prominent and mitotic figures are common. The invasive edge is often smooth.14
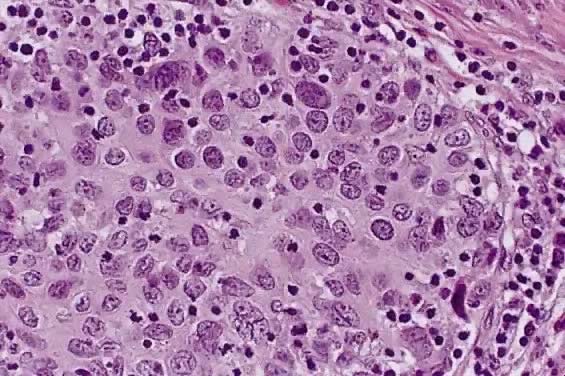
Small cell squamous carcinoma is characterized by loosely cohesive nests and sheets of small to medium sized cells with hyperchromatic nuclei, scant cytoplasm and small nucleoli. Keratinization is minimal or absent, and mitotic figures are abundant (Fig. 3). The nuclear chromatin is finely to coarsely granular, and small nucleoli are often evident (Fig. 3).15 Crush artifact and nuclear smudging are not prominent. The nuclear cytoplasmic ratio is lower than small cell anaplastic carcinoma. The cell borders are also more distinct. Rare cytoplasmic keratinization also belies the squamous nature of the lesion.15
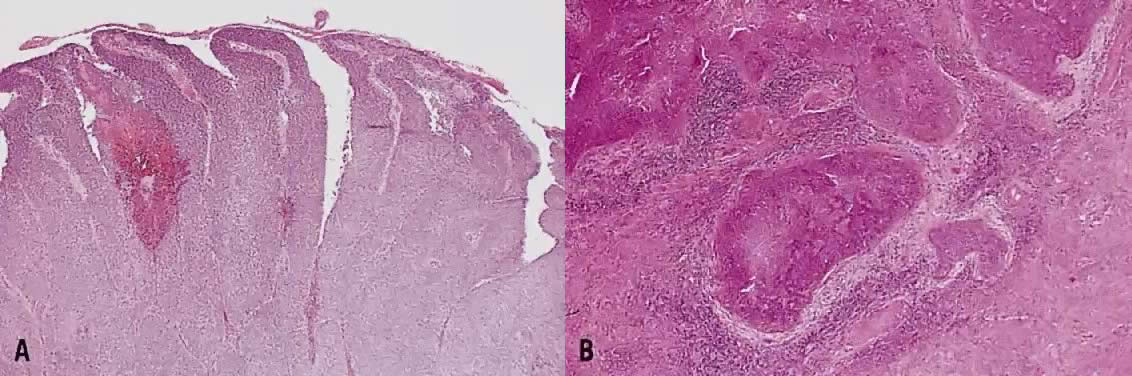
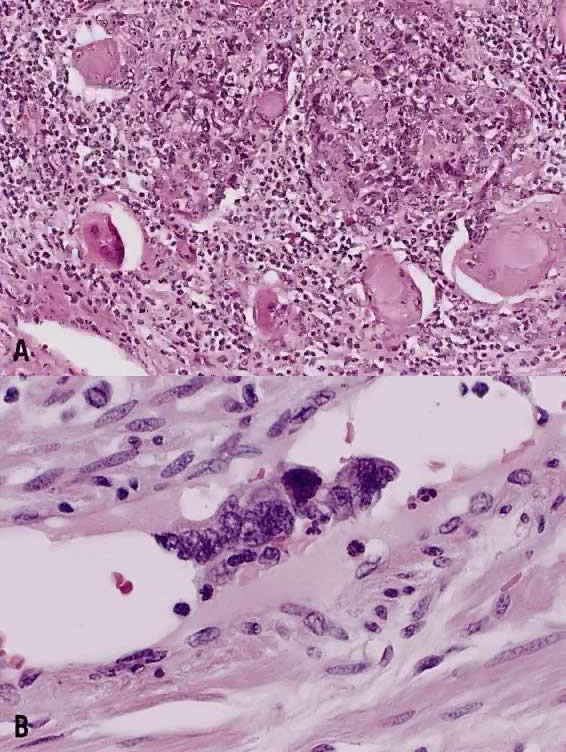
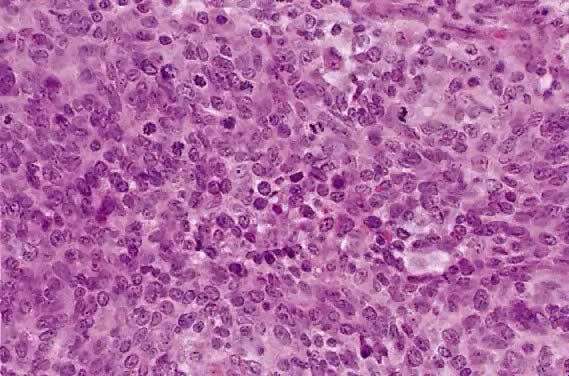
The category of small cell anaplastic carcinoma should be reserved for those cervical tumors that resemble small cell or intermediate cell anaplastic carcinomas of the lung.16–20 Small cell anaplastic carcinoma is made up of sheets and cords of cells diffusely infiltrating a delicate fibrovascular stroma, simulating lymphoma (Fig. 4A). The tumor cells have round, oval to elongated, small to intermediate sized nuclei. The nuclear chromatin ranges from coarsely granular to dark and sometimes smudged (Fig. 4B). Nucleoli are inconspicuous and mitotic figures are frequent. The cytoplasm is scant resulting in nuclear molding and high nuclear cytoplasmic ratios. Necrosis and crush artifact are common. Desmoplastic reaction of the stroma is minimal or absent.
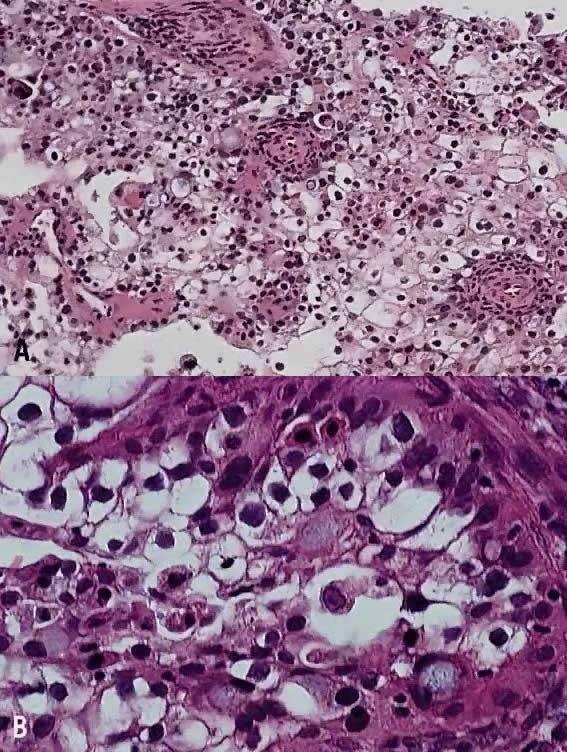
In recent years, it has become clear that neuroendocrine carcinomas are not limited to the small sized tumor cells. Poorly differentiated large cell carcinomas may express neuroendocrine differentiation by immunohistochemistry. These large tumor cells have the nuclear characteristics common to neuroendocrine carcinoma, namely coarsely granular chromatin (Fig. 5A). However, the nucleoli vary from indistinct to medium size. In addition to diffuse infiltrative pattern, tumor cells are also arranged in trabeculae, ribbons and rosettes (Fig. 5B), and the cytoplasm varies from scant to moderate in amount. In a recently proposed classification, neuroendocrine carcinomas are divided into small and large cell types.13
All poorly differentiated small cell and large cell carcinomas with the above morphologic features should be studied by immunohistochemistry for neuroendocrine markers (neuron-specific enolase, chromogranin, synaptophysin; Fig. 5C). By immunohistochemical stains, 20% to 100% of small cell anaplastic carcinomas were reported to express neuroendocrine differentiation.13,18 This subset is classified as small cell neuroendocrine carcinoma. Neuroendocrine carcinomas sometimes coexist with adenocarcinoma or squamous carcinoma (Fig. 5D).
Small cell anaplastic carcinomas are believed to derive from multipotential cell or argyrophilic cells in the basal cell layer of the endocervical mucosa.21 This tumor has propensity to occur in young women (mean age, 36 years vs 50 years in patients with small cell squamous carcinoma),22 strong association with HPV type 18,23 and aggressive behavior.22,24 When treated with radical hysterectomy and pelvic nodal dissection, 4 of 6 (67%) patients with small cell anaplastic carcinoma had pelvic nodal metastasis and 5 of 8 (63%) patients developed tumor recurrence, compared with 18% nodal metastasis and 9% tumor recurrence in women with small cell squamous carcinoma.22 Spread beyond the uterus and pelvis is common even for stage I tumor. It is recognized as one of the most aggressive types of cervical cancer with a 5-year survival rate of 14% in one study.24
Sevin and colleagues25 reported 12 women with small cell anaplastic carcinoma, including one FIGO stage IA, 10 stage IB, and one stage II. When compared with stage IB and II cervical squamous carcinoma and adenocarcinoma combined, small cell anaplastic carcinoma had a higher frequency of vascular lymphatic space invasion (82% vs 62%), more frequent lymph node metastasis (45.5% vs 18.9%), and lower 5-year survival rate (36.4% vs 71.6%). Only 42% (5 of 12) of patients were disease free at the time of report. In view of these findings, a combined therapy of surgery, radiotherapy, and cytotoxic chemotherapy is recommended.25
The value of separating squamous carcinoma by cell type was evaluated using the data of the Gynecologic Oncology Group (GOG). Among women with stage I squamous carcinoma treated surgically, the cell type was not predictive of pelvic nodal metastasis and outcome.26,27 The percentages of patients progression-free at 5 years were 84% for large cell keratinizing and 74% for large cell nonkeratinizing (p = not significant), and 75% for grade 1, 82% for grade 2, and 78% for grade 3 (p = not significant).26
The consistency and reproducibility among pathologists in separating large cell keratinizing and nonkeratinizing tumors based on cervical biopsy specimens cause problems in interpretation. In the GOG study of women with IIB to IVA squamous carcinoma treated by radiation therapy, when the histologic criteria were modified to include all tumors with individual cell keratinization in the large cell keratinizing category, this group had a significantly higher recurrence/death rate than the large cell nonkeratinizing group (65.8% vs 53.5%, p = .0074).28
Histologic Grade
The histologic grade reflects the degree of differentiation of the tumor cells. The most commonly used grading system for squamous carcinoma is a modification of the original Broders' system consisting of three grades based on the amount of keratin, the degree of nuclear atypia, and the mitotic activity. Grade 1, well-differentiated lesions exhibit abundant intercellular bridging, cytoplasmic keratinization, and keratin pearls. The cells are relatively uniform with minimal nuclear pleomorphism. The mitotic rate should be less than 2 per high-power field.15 Grade 2, moderately differentiated lesions show primarily individual cell keratinization, moderate nuclear pleomorphism, and up to four mitotic figures per high-power field.15 Grade 3, poorly differentiated lesions show little evidence of squamous differentiation. The tumor cells are immature, with marked nuclear pleomorphism, scant cytoplasm, and more than four mitotic figures per high-power field.15
In surgically treated patients with stage I and II squamous carcinomas, some studies have found histologic grade to influence prognosis and pelvic nodal metastasis.27 In the study of stage I squamous carcinoma from GOG, the histologic grade was correlated with the frequency of pelvic nodal metastasis. Pelvic nodal metastasis occurred in 9.7%, 13.9%, and 21.8% of grade 1, grade 2, and grade 3 tumors, respective.27 A comparable results were reported by Fuller and colleagues29 (Table 2). Some investigators of stage IB and IIA carcinomas have found that grade 3 tumors were larger and had a higher incidence of lymph node metastasis than lower-grade lesions.29 Fuller and colleagues29 found that 25% of grade 3 tumors had pelvic lymph node metastases, compared with 9% and 16% in grades 1 and 2 tumors, respectively.
TABLE 2. Frequency of Lymph Node Metastasis in Surgically Treated Cases
| Delgado et al27 (745 stage I squamous carcinoma >3 mm in depth) | Fuller et al29 (431 IB and IIA squamous carcinoma, adenocarcinoma, and others) | ||
FIGO stage | I | 15.5% | IB | 15% |
|
|
| IIA | 22% |
Stromal invasion | 3–5 mm | 3.4% | <1/3 | 0% |
| 6–10 mm | 15.1% | 2/3 | 12% |
| 11–15 mm | 22.2% | >2/3 | 24% |
| 16–20 mm | 38.8% |
|
|
|
| 22.6% |
|
|
LVSI | Absent | 8.2% | Absent | 14% |
| Present | 25.4% | Lymphatic | 36% |
|
|
|
|
|
Parametrial disease | Absent | 13.5% | Not evaluated |
|
| Present | 25% |
|
|
Tumor size | Occult | 8.9% |
|
|
| Gross | 20.9% |
|
|
Histologic grade | Grade 1 | 9.7% | Grade 1 | 9% |
| Grade 2 | 13.9% | Grade 2 | 16% |
| Grade 3 | 21.8% | Grade 3 | 25% |
Not significant |
|
|
|
|
Cell type | LCK | 17.2% | Squamous carcinoma | 18% |
| LCNK | 17.2% | Adenocarcinoma | 18% |
| Small cell and others | 17.6% | Adenosquamous carcinoma | 17% |
LCK, large cell keratinizing; LCNK, large cell non-keratinizing.
Among 445 patients with stage IIB through IVA squamous carcinoma treated by radiation therapy following GOG protocols, the histologic grade had no impact on prognosis.28 Similar findings were reported by others.30,31
In the early 1980s, a malignancy grading system (MGS) was proposed as an alternative grading system.32 In the MGS system eight morphologic parameters of a tumor, consisting of four characteristics of the tumor cell population (structure [i.e. papillary vs solid], degree of cell differentiation, nuclear pleomorphism, and mitotic activity] and four characteristics of the tumor-host relation (mode of invasion, stage of invasion, extent of vascular invasion, degree of host inflammatory response [lymphoplasmacytic]) are evaluated and scored on a one- to three-point scale with minimum score of 8 points and a maximum score of 24 points.32 When this grading system was applied to 445 IIB to IVA squamous carcinoma patients treated by radiation therapy, the recurrence/death rates were 32.9% for women whose tumors scored up to 12 points, 57.3% for tumors scoring 14 to 16 points, and 64.8% for neoplasms with scores of more than 18 points (p = .004).28
Variants of Squamous Carcinoma
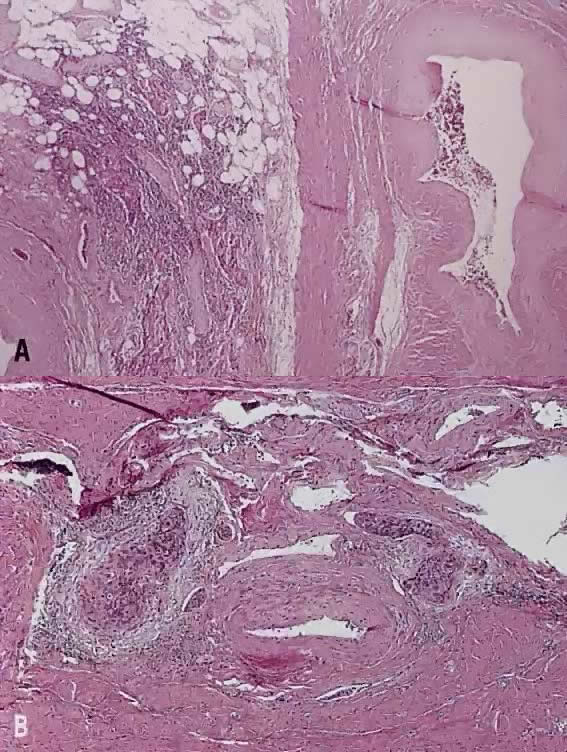
Verrucous squamous carcinoma of the cervix, like that of other sites, represents a special variant of well differentiated squamous carcinoma. Grossly, these tumors appear exophytic and warty, and may simulate a condyloma acuminatum. Histologically, the cells in this variant show orderly maturation and lack cytologic atypia. The tumor grows by expansion with smooth pushing margins, as opposed to the infiltrating pattern of conventional squamous carcinoma.
To differentiate verrucous squamous carcinoma from either condyloma, pseudoepitheliomatous hyperplasia or typical squamous carcinoma, full-thickness biopsy specimens are necessary. Some squamous carcinomas have a verrucous appearance, but show severe nuclear atypia and foci of invasion by nests or single cells. These tumors behave like conventional squamous carcinoma and should be identified as such. Condyloma acuminatum has prominent koilocytosis and delicate fibrovascular cores, as opposed to the compressed cores and confluent epithelial growth pattern seen in verrucous squamous carcinoma. Condylomas also lack the expansile, endophytic extension into the stroma seen in verrucous squamous carcinomas.
None of at least 18 cases of verrucous carcinoma reported in the literature had lymph node metastasis. Direct extension into the vagina occurred in 6 women and the endometrium in 3 cases. About 40% to 50% had local recurrence, due to either incomplete excision or resistance to radiation therapy.33
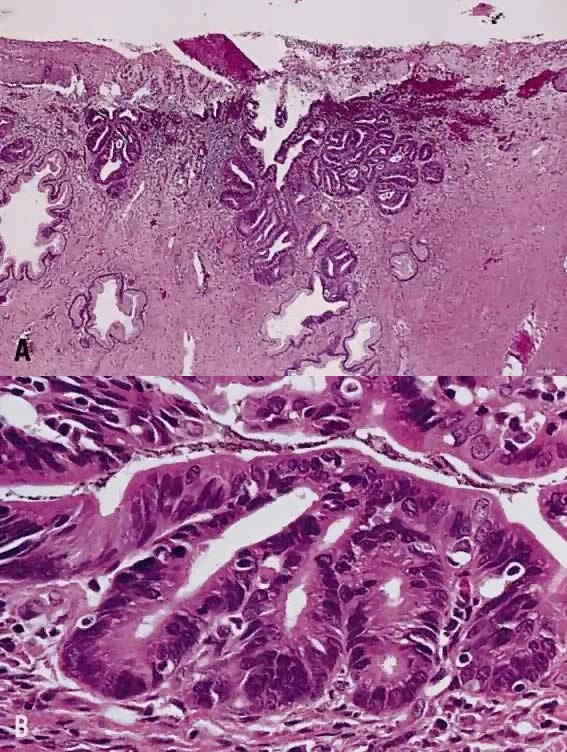
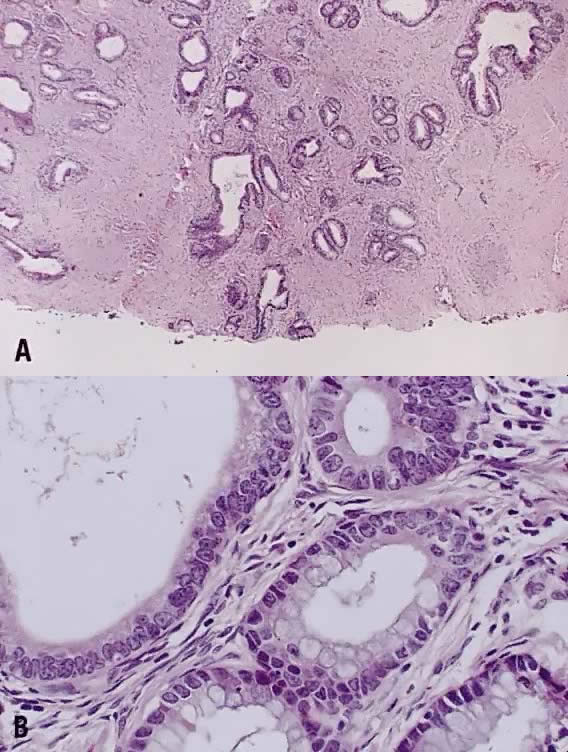
Papillary squamous carcinoma of cervix is characterized by highly dysplastic squamous cells forming papillary fronds with thin fibrovascular cores (Fig. 6A). Not surprisingly, the gross appearance of this lesion may be warty or fungating as in verrucous squamous carcinoma. In recent years, terms such as papillary squamotransitional cell carcinoma and transitional cell carcinoma were used.34,35 Although there is some morphologic resemblance to the poorly differentiated transition cell carcinoma of the urinary bladder, by immunostains, only 2 of 21 (9.5%) tumors expressed a distinct marker of transitional cells, cytokeratin 20. Thus these tumors are squamous cell type.34
In a series of 32 women, their age varied from 22 to 93 years (mean, 50 years).34 They presented with abnormal bleeding or abnormal cervical smears. The tumor size ranged from 0.7 cm to 6 cm, mean 3.0 cm. The diagnostic problems are indicated by the fact that only 20 of 32 (63%) specimens were considered to be adequate. The remaining 37% were too superficial to determine in situ or invasive carcinoma. Of those with suitable specimens, 90% (18 of 20) had stromal invasion.34 Full-thickness biopsy specimens are necessary to distinguish in situ from invasive lesions. This is because invasion is evident only in the stroma beneath the papillary surface components (Fig. 6B).
In a series of 9 cases, 1 tumor was an in situ lesion, whereas the remaining 8 cases ranged from stage I to stage IV invasive carcinoma.36 In another study, the FIGO stage was known in 11 women, including 2 stage 0, 3 stage IA, 2 stage IB, 1 stage IIA, 1 stage IIB, and 2 stage IIIB.34 In this series, only 12 women had follow-up information, 3 patients died all stage IIB or higher, mean survival 13 months after diagnosis.34 One patient developed ovarian metastasis, 1 patient had vaginal recurrence 12 years later, and in 1 patient, adenosquamous carcinoma of the endometrium occurred. Five women were alive and well, and 3 died of other causes. Late recurrence and metastasis were known to occur.36
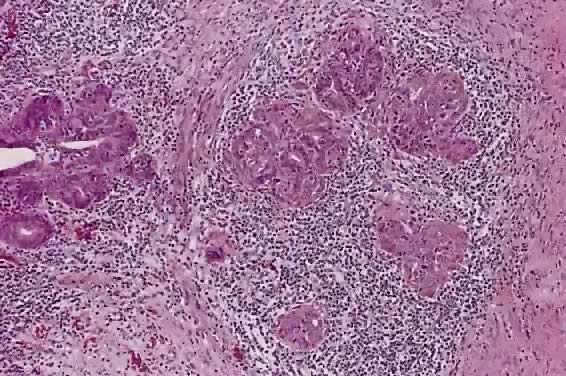
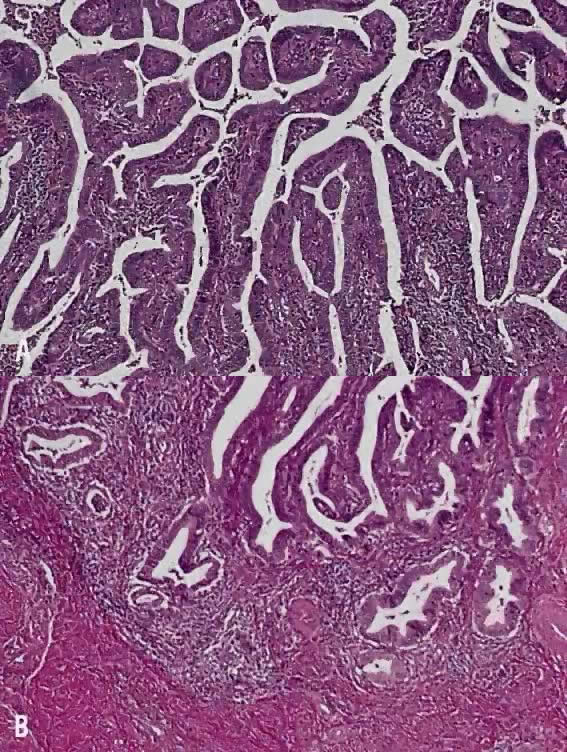
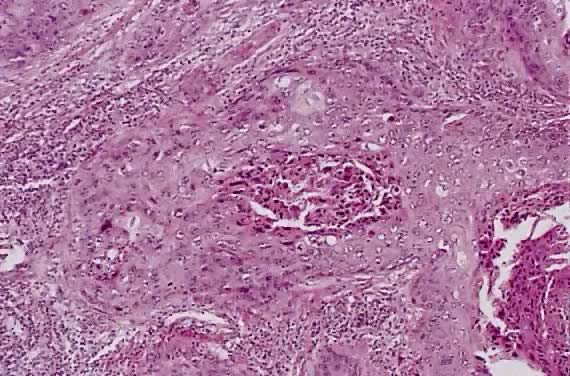
Circumscribed carcinoma of the uterine cervix was described in 1977 by Hasumi and associates37 In their study, the tumors were characterized by solid cords of cells with neither squamous nor glandular differentiation, surrounded by a dense lymphocytic infiltrate, which occasionally contained a considerable number of eosinophils and plasma cells. The tumor cells were fairly monomorphic, with large nuclei, one or more nucleoli, and clear to eosinophilic, granular cytoplasm. Many mitotic figures were seen. All 39 cases reported measured larger than 5 mm in depth, but only 2 (5%) had lymph node metastases at the time of surgery, compared with 18% of ordinary squamous carcinomas of comparable stage. Improved 5 year survival was also seen in these cases (97% vs 79%, p < .05).37
Studies of similar, if not identical, tumors use the term lymphoepithelioma to indicate the histologic likeness to the lymphoepithelioma of the nasopharynx and the malignant lymphoepithelial lesions of the salivary glands.38,39 In a study by Tseng and associates,40 15 such tumors were compared with the conventional squamous carcinomas by polymerase chain reaction (PCR). Epstein-Barr viral gene sequences were found in 11 of 15 tumors (73%), compared with 4 of 15 (27%) of the usual squamous carcinomas (p = .001). Interestingly, HPV 16 and 18 types were detected in 20% (3 of 15) of these tumors, compared with 80% (12 of 15) of the usual squamous carcinomas (p = .001).40
Although the total number of cases in the literature is too small for complete understanding of these neoplasms, they appear to have a better prognosis than the conventional squamous carcinoma. After radical hysterectomy all 15 patients were alive and well.40
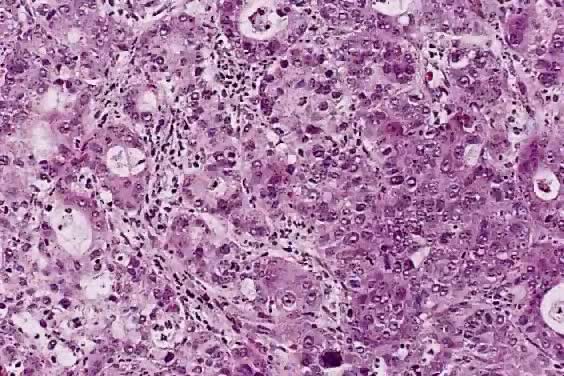
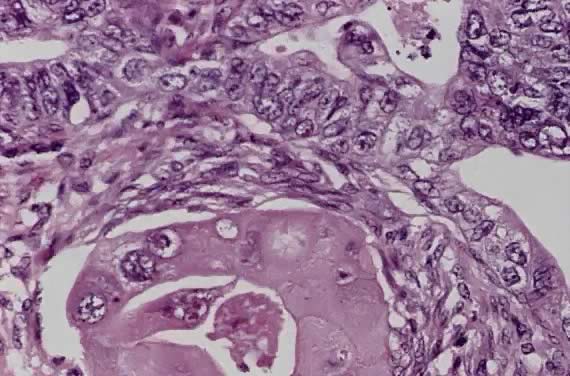
Spindle cell squamous carcinoma is a rare variant of poorly differentiated carcinoma that may be confused with either melanoma or sarcoma.5,41 This tumor is composed of cells with large, spindle shaped, or oblong nuclei arranged in fascicles (Fig. 7). Keratin formation and the nesting pattern typical of epithelial tumors may be absent. Stromal changes such as heavy collagen deposition may give the appearance of a fibrosarcoma or osteosarcoma. When confronted with such a lesion electron microscopic examination or immunohistochemistry is often required to identify the epithelial nature. A positive immunohistochemical stain for cytokeratin and the demonstration of desmosomes and tonofilaments by electron microscopy distinguish these lesions from mesenchymal tumors.
One may also rarely see abnormal spindle cells in the cervical stroma adjacent to typical squamous carcinoma. This so called pseudosarcoma was reported by Watty and colleagues42 who described individual atypical stromal cells with elongated, pleomorphic nuclei and frequent multinucleation. Rare abnormal mitotic figures were seen. The authors felt that this change represented a response to the nearby tumor and noted that similar lesions have been reported in squamous carcinomas of the head and neck region.
 200.)
200.)
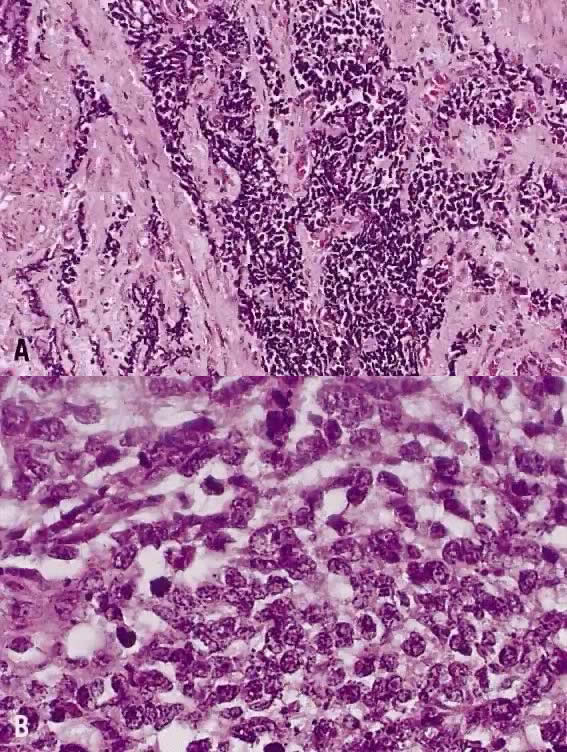
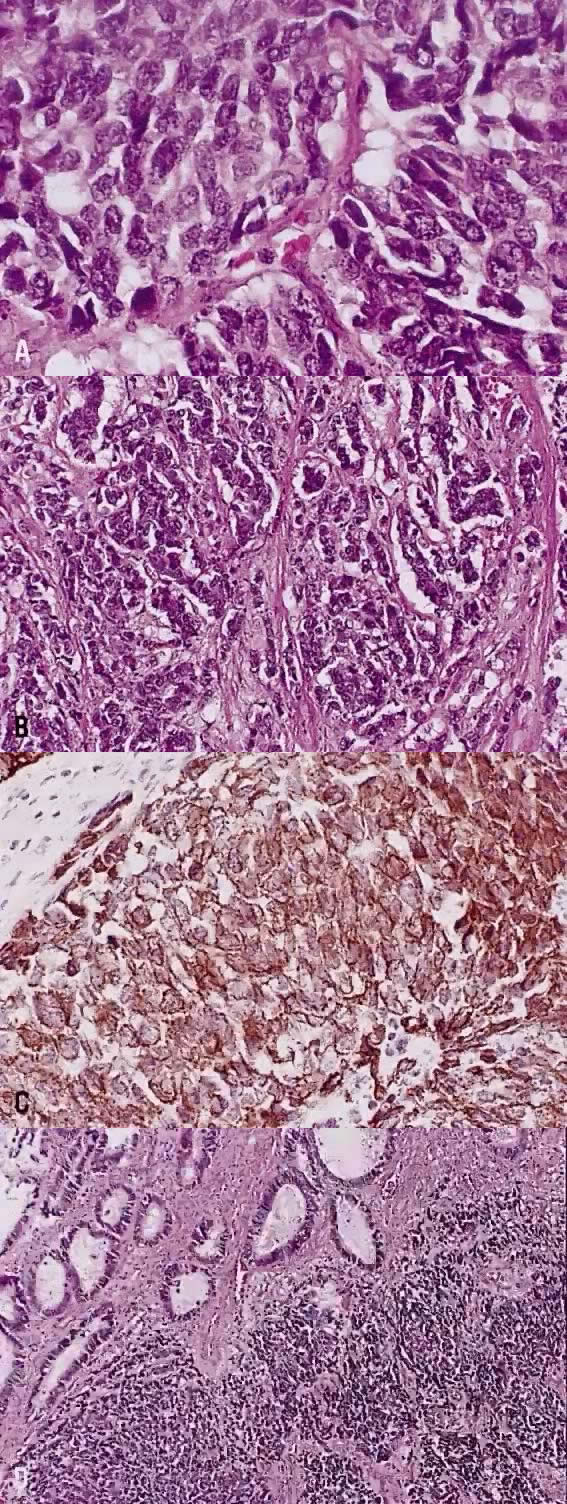
 21 mm
21 mm