The exenteration consists of three important and distinct components: determination of resectability, resection, and reconstruction. Although thorough preoperative evaluation will limit the number of patients considered for exenteration, resectability ultimately can be determined only by surgical evaluation. This criterion previously necessitated an initial exploratory laparotomy; however, operative laparoscopy, as reported by Plante and colleagues,32,33 has recently been used successfully to assess patients with recurrent cervical cancer. As more gynecologic oncologists gain experience with laparoscopic retroperitoneal surgery, this technique may decrease the number of unnecessary laparotomies. If laparoscopy is performed and no contraindications to exenteration are noted, one then proceeds to laparotomy. After the abdomen is opened, determination of resectability includes inspection and palpation for intraperitoneal disease, evaluation of nodal status, and determination of the presence or absence of sidewall fixation. Evidence of disease on any peritoneal surfaces or metastatic disease to the periaortic nodes is an absolute contraindication to surgery.
The appropriateness of performing an exenteration with positive pelvic lymph nodes is debatable. Stanhope and Symmonds34 reported a 5-year survival rate of 23% in patients with positive nodes who underwent this procedure, thus arguing in favor of proceeding with surgery. Unfortunately, however, no other investigators have even approached this survival rate. Rutledge and coworkers35 reported a survival rate of only 7% in patients with positive pelvic nodes; Shingleton and associates12 also reported a poor survival. Morley and coworkers22 reported a 0% 5-year survival rate in patients with positive regional lymph nodes, citing this finding as a contraindication to exenteration in such patients. In another series, the 5-year survival rate was 9.2% for those patients with positive lymph nodes.36 Interestingly, Shingleton and associates12 were unable to demonstrate a difference in survival between those patients who underwent pelvic node dissection and those who did not. This finding suggests that lymph node dissection is not a mandatory component of the operation when no clinically suspicious nodes are encountered. From the available data, it would seem that positive pelvic nodes are a contraindication to pelvic exenteration.
Sidewall fixation should be evaluated and, if found, is a contraindication to surgery. This can be determined by opening the paravesical and pararectal spaces and performing a biopsy, if necessary, to confirm the presence of disease. Shingleton and associates12 reported a 0% 2-year survival in patients treated with exenteration in the presence of sidewall fixation. This poor survival rate was confirmed by Creasman and Rutledge.37
Once resectability has been determined, a decision regarding the feasibility of anterior exenteration versus total pelvic exenteration should be considered. Reports by Orr and coworkers31 and Symmonds and associates18 have shown that anterior exenteration can be performed in carefully selected patients with excellent survival and low morbidity. Hatch and colleagues38 reported a 5-year survival of 53% and an operative mortality of only 1.6% in 69 patients who underwent anterior exenterations. They were also able to develop criteria for identifying appropriate candidates for anterior exenteration. Women with cancer recurrence more than 1 year after radiotherapy, a lesion less than 3 cm in diameter, and no evidence of bladder invasion were believed to be ideal candidates for anterior exenteration. These criteria would imply that patients who are being considered for anterior exenteration should undergo a cystoscopy to rule out bladder mucosal involvement.
The major potential intraoperative complication with this procedure is hemorrhage. Blood loss generally varies from 2000 to 4000 mL but can be much greater. Ketcham and coworkers16 reported increased mortality in patients with intraoperative blood loss greater than 3000 mL. Intraoperative monitoring with a pulmonary catheter should be considered in all patients undergoing pelvic exenteration. This has been shown to aid considerably in the management of intraoperative fluid replacement, which can exceed 1500 mL/hr.39
After resection of the tumor, attention must be given to reconstruction. This includes construction of a urinary conduit, covering of the denuded pelvic floor, reconstruction of the vagina, and consideration of reanastomosis of the colon and rectum, where appropriate.
Construction of a Urinary Conduit
The first attempts at urinary diversion consisted of wet colostomies and rectal bladders. These were soon abandoned because of serious metabolic side effects and infectious complications owing to fecal contamination of the urinary tract.
The first urinary conduit was described by Butcher and associates.40 The ileal conduit and sigmoid conduit have been used extensively since this first report. These intrapelvic bowel segments have often received significant amounts of radiation before the procedure, resulting in a high rate of intestinal and conduit complications.41 Therefore, some surgeons use a segment of nonirradiated transverse colon. Orr and colleagues41 showed a significant reduction in urinary leaks and gastrointestinal complications with transverse colon conduits; this is now the bowel segment used most often at many institutions. Morley and coworkers22 prefer the sigmoid conduit because it avoids an anastomosis. Several authors have recommended the routine use of ureteral stents to decrease technical error during conduit construction and to protect against urinary strictures.41,42
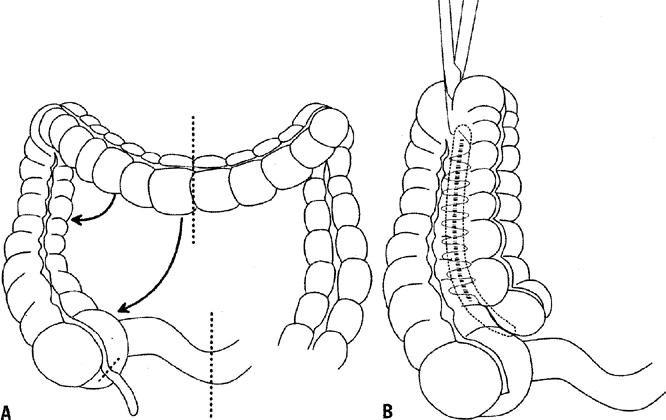
At several institutions, ileal and colonic conduits have been replaced with continent urinary diversions. These continent conduits more closely simulate the normal lower urinary tract system with a low-pressure system and a urine capacity approximating that of a normal bladder. They do not require an external collection device and, ideally, are not subject to reflux. Such techniques are the results of refinements of the pioneering works of Gilchrist and Merricks43 and Kock and associates.44 Expanding on these works, Rowland and colleagues45 demonstrated that the ileocecal valve could be used for recurrent, intermittent catheterization. Numerous variations of continent urinary diversions have subsequently been developed, including the Kock, Mainz, Mainz II, Indiana, Miami, Penn and supracecal colonic continent urostomy (SCCCU) pouches.44,46,47,48,49,50,51 One of these techniques, the Miami pouch, is illustrated in Figure 1. None of these procedures appears to be superior to the others,52 and experience with these technically challenging operations may be the best guide for choosing a particular approach.
Early and late complications of continent urinary reservoirs appear to be similar for all types described. Common complications include ureteral stricture/obstruction (early and late), anastomotic leak (early), sepsis (early), and urinary stone formation (late). Operative mortality rates from early complications have been reported to be as high as 9%.53 Importantly, these operations require judicious patient selection, despite the suggestion that they can be performed in half of patients undergoing exenteration.47 Appropriate candidates should be highly motivated and possess the manual dexterity to care for the continence mechanisms. In addition, continent diversions are inappropriate for patients with compromised renal function.52 Whichever continence mechanism is employed, the benefits derived from these procedures must be weighed against the disadvantages of more extensive surgery, a longer length of operation, and a greater rate of surgical and postoperative complications.
Covering the Denuded Pelvic Floor
After the exenterative procedure, the denuded raw pelvic floor must be covered to avoid infectious and gastrointestinal complications. Although grafts constructed of foreign material have been used for covering the denuded pelvic floor, optimal closure of the pelvis is probably accomplished with a technique that brings in a new blood supply. Transposition of an omental pedicle, as demonstrated by Powers and colleagues,54 meets this criterion and has been associated with a decreased rate of major small bowel complications. Other methods of closing the pelvis include reanastomosis of the colon and rectum, as reported by Berek and associates,55 and use of a myocutaneous flap as part of vaginal reconstruction, as reported by Hatch.56 Others have used a peritoneal patch or graft with good results.22
Vaginal Reconstruction
Consideration of vaginal reconstruction should be made before the operation. This must be discussed with the patient to determine the desirability of maintaining sexual function. Lack of sexual desire, advanced age, or lack of a sexual partner may lead the patient to decide not to undergo vaginal reconstruction. The following are among the numerous methods of postexenteration vaginal reconstruction: split-thickness skin grafts, myocutaneous flaps, sigmoid vaginostomy, vulvovaginoplasty, ileal vagina, and amnion grafts. Recently, the inferior gluteal flap has been described with excellent results and no late complications.57 In addition, the rectus abdominis myocutaneous flap has been modified, with an improvement in restoring sexual function in as many as 84% of patients in the study by Goldberg and coworkers58 Potential complications related to vaginal reconstruction include infection, hemorrhage, graft necrosis, and prolonged hospitalization, all of which have not significantly changed in recent years.59
Reanastomosis of the Colon and Rectum
In patients who have undergone total pelvic exenteration, consideration should be given to reanastomosis of the colon and rectum. The development of the end-to-end circular stapling device has allowed the anastomosis of the colon to as little as 3 to 4 cm of rectal stump. Berek and associates55 reported good results with this technique, but recommended a diverting colostomy in previously irradiated patients. Barakat and coworkers60 noted a greater than 50% rate of anastomotic leaks in patients undergoing concomitant low rectal anastomosis.60 Hatch and associates61 reported success with this technique, but recommended bowel rest with 14 days of total parenteral nutrition instead of a diverting colostomy. An omental wrap of the anastomosis site was also recommended. They noted that most leaks were posterior and believed that it was important to cover this area with the omentum. The reservoir capacity of the rectum is reduced, and frequent defecation can be a problem after surgery. Hatch and associates61 recommended diphenoxylate and atropine sulfate (Lomotil) or loperamide (Imodium) every 2 hours until stool frequency is satisfactory. This problem usually diminishes with time, making consideration of reanastomosis reasonable. Frequent defecation as well as rectal tenesmus, which is another side effect of a low end-to-end anastomosis of the colon to rectum, can be reduced through the construction of a rectal J-pouch low-pressure reservoir, possibly obviating the need for antidiarrheal medication.62