The International Federation of Gynecology and Obstetrics (FIGO) recognizes
two prognostic factors in its most recent staging (Table 1). For many years, grade and depth of invasion have been recognized as
important prognostic factors. In 1988, FIGO decided that endometrial cancer
should be surgically staged, thereby increasing our ability to determine
the true extent of disease.2 Before that time, clinical staging had been used, with replete data in
the literature detailing the large number of patients whose disease process
was considerably different than the clinical stage. More than 25% of
stage I patients had disease outside the uterus. In stage II, approximately
half had a different stage; in many instances, they actually
had only disease in the uterine fundus, which had tremendous treatment
implications. With the new staging, treatment can be better defined. Knowing
the depth of invasion and grade of tumor, even in stage I, allows
more precise determination of prognosis and treatment. Table 1. FIGO Staging, Endometrium
Stage | Findings |
IA G123 | Tumor limited to endometrium |
IB G123 | Invasion to less than half the myometrium |
IC G123 | Invasion to more than half the myometrium |
IIA G123 | Endocervical glandular involvement only |
IIB G123 | Cervical stromal invasion |
IIIA G123 | Tumor invades serosa and/or adnexae and/or |
| positive cytologic findings |
IIIB G123 | Vaginal metastases |
IIIC G123 | Metastases to pelvic and/or para-aortic lymph nodes |
IVA G123 | Tumor invasion of bladder and/or bowel mucosa |
IVB | Distant metastases, including intra-abdominal |
| and/or inguinal |
FIGO, International Federation of Gynecology and Obstetrics.
Phenotypic Status Classically, obesity, hypertension, and diabetes have described the typical
patient with endometrial cancer. Today, most studies suggest that
hypertension and diabetes, when corrected for weight and age, are not
necessarily risk factors. Obesity, nulliparity, and late menopause are
more suggestive of this type of patient. If these conditions are present
in excess, it is said that the risk of adenocarcinoma increases fivefold. This
patient usually has a well-differentiated lesion with superficial
invasive disease, an excellent prognosis, and treated with simple
hysterectomy and bilateral salpingo-oophorectomy. Some investigators
have designated this patient as type I. In contrast, the type II
patient usually is thin, parous, and black; has a poorly differentiated, deeply
invasive cancer with extrauterine disease, requiring more aggressive
treatment; and has a resultant poor prognosis. In the former
patient, unopposed estrogen may be the etiology, but this does not seem
to be a factor in the latter patient. Recently, the American Cancer Society (ACS) Task Force on Screening for
Endometrial Cancer evaluated risk factors to identify women who might
benefit from some type of screening. Multiple risk factors commonly said
to be associated with endometrial cancer were reviewed in depth. Women
with hereditary nonpolyposis colon cancer (HNPCC) account for only 2% to 10% of
all female colon cancers, yet approximately 5% of all endometrial
cancers occur in women with this risk factor. These women have 22% to 50% lifetime
risk of having endometrial cancer develop, and
the disease occurs at a younger age, approximately 15 years younger than
women without HNPCC. The greatest risk of HNPCC carriers having endometrial
cancer develop is between 40 and 60 years of age, with the absolute
risk greater than 1% per year. Only HNPCC was thought to be significantly
related to warrant consideration for screening. The ACS recommends
annual screening with endometrial biopsy should be offered to
this group of women beginning at age 35. Patients should be informed
about potential benefits, risks, and limitations of testing for early
endometrial cancer detection.6 Differentiation For more than a century, it has been known that tumor differentiation is
an important prognostic factor in endometrial cancer.3 This has been substantiated in many studies (Table 2). Patients with well-differentiated cancers have an excellent prognosis
with hysterectomy as their only treatment. Patients with poorly differentiated
tumors tend to have more deeply invasive cancers and a greater
tendency to have extrauterine disease, require more intense therapy, and
have a poor prognosis. In several large studies, it would appear
that approximately one third of all adenocarcinomas are well differentiated, with
approximately one quarter being poorly differentiated. Table 2. Five-Year Survival of Stage I Carcinoma of the Endometrium*
Grade | Surgical | Clinical |
G1 | 92% | 52% |
G2 | 87% | 60% |
G3 | 74% | 36% |
*Survival rates based on 3590 patients.
(Pecorelli S [ed]: Annual Report on the Results of Treatment
in Gynecologic Cancer, p 65, Vol 24. Milan, 2000.)Depth of Myometrial Invasion As depth of invasion increases, prognosis worsens (Table 3). This factor may be even more predictive of prognosis than differentiation
of tumor. Depth of invasion appears to be an indicator of tumor
volume, which is a most important prognostic factor in any cancer. In
a large study carried out by the Gynecologic Oncology Group (GOG), approximately 15% of
patients with clinical stage I cancer had disease limited
to the endometrium and 40% had significant (middle or deep third) myometrial
invasion.3 There appears to be a correlation between grade and depth of invasion. In
general, as depth of invasion increases, there is a greater likelihood
that the disease process is poorly differentiated. More than one
third of grade 3 lesions tends to be deeply invasive; only 7% are limited
to the endometrium. In contrast, only 10% of grade 1 lesions are deeply
invasive. It appears that patients with grade 3 disease limited
to the endometrium have a better prognosis than do patients with grade 1, deeply
invasive disease. Table 3. Relationship Between Depth of Myometrial Invasion and 5-Year Survival
Rate (Stage I)
Stage | Surgical Stage |
IA | 89% |
IB | 90% |
IC | 81% | (Pecorelli S [ed]: Annual Report on the Results of Treatment
in Gynecologic Cancer, p 65, Vol 24. Milan, 2000.)Extent of Disease Proper evaluation, both surgical and pathologic, must be performed to evaluate
the patient's cancer fully. Without adequate evaluation at
the time of surgery, surgical staging is no better than clinical staging, with
its inherent problems already discussed. Proper treatment can
be applied only when the extent of disease is known. Spread of disease
from the corpus to the endocervix not only changes the stage but can
also affect survival (Table 4). If the disease extends into the endocervical canal, the incidence of
pelvic lymph node metastases increases considerably. In fact, there are
considerably more patients with metastases to the pelvic nodes in a
corpus et colli than there are patients with primary cervical cancer
with disease limited to the cervix. Approximately 10% of stage I endometrial
cancers metastasize to the adnexa. Occasionally, this is appreciated
because of the enlarged adnexa noted on pelvic examination, but
in many instances, discovery is at the time of surgery. Histologic confirmation
is required for a patient to be placed into stage III disease
based on adnexal metastasis. Obviously, treatment must be adequate for
this extrauterine disease. Table 4. Five-Year Survival in Endometrial Cancer*
Stage | Surgical | Clinical |
I | 87.4% | 53.8% |
II | 76.3% | 41.4% |
III | 56.6% | 23.1% |
IV | 17.8% | 12.0% |
*Survival rates based on 6085 patients.
(Pecorelli S [ed]: Annual Report on the Results of Treatment
in Gynecologic Cancer, p 65, Vol 24. Milan, 2000.)Intraperitoneal disease, although unusual, can occur when disease clinically
is thought to be limited to the uterus. Complete evaluation of the
intraperitoneal space is, therefore, imperative. In the large GOG study, 6% of
patients with clinical stage I disease were found to have
intraperitoneal disease. Lymph Node Involvement For many years, it was thought that endometrial cancer did not go to the
lymph nodes, particularly the pelvic nodes. During the 1960s and even
before, there were data suggesting that pelvic lymph nodes could be
involved with metastases, even with early-stage disease. These data were
based on patients who had been primarily treated with radical hysterectomy
and pelvic lymphadenectomy. In the early 1970s, an increased amount
of information became available to substantiate the fact that patients
with clinical stage I cancers had a significant chance of having
metastases to the pelvic lymph nodes. In the large GOG study, 9% of
patients with clinical stage I had metastases to the pelvic lymph nodes. Almost
without exception, the metastases were microscopic and could
not be determined on gross evaluation. Palpation of the lymph nodes in
both pelvic and para-aortic areas is extremely unreliable in endometrial
cancer. The incidence of lymph node metastases is directly related
to grade and depth of invasion (Tables 5 and 6). Only 3% of patients with well-differentiated cancers have pelvic node
involvement; 18% of patients with poorly differentiated tumors have
lymph node metastases. Only 1% of patients with disease limited to the
endometrium disease have pelvic node metastases, whereas 25% of patients
with deep invasion have pelvic nodes involved. It appears that depth
of invasion may be more predictive of pelvic lymph node metastases
than grade.3 Table 5. Grade Versus Positive Pelvic and Aortic Nodes
Grade | Pelvic | Aortic |
(n) | No. (%) | No. (%) |
G1 (180) | 5 (3) | 3 (2) |
G2 (288) | 25 (9) | 14 (5) |
G3 (153) | 28 (18) | 17 (11) | (Modified from Creasman WT, Morrow CP, Bundy L et al: Surgical pathological
spread patterns of endometrial cancer. Cancer 60:2035, 1987.)Table 6. Maximal Invasion and Node Metastasis
Maximal Invasion | Pelvic | Aortic |
(n) | No. (%) | No. (%) |
Endometrium only (87) | 1 (1) | 1 (1) |
Superficial muscle (279) | 15 (5) | 8 (3) |
Intermediate muscle (116) | 7 (6) | 1 (1) |
Deep muscle (139) | 35 (25) | 24 (17) | (Modified from Creasman WT, Morrow CP, Bundy L et al: Surgical pathological
spread patterns of endometrial cancer. Cancer 60:2035, 1987.)For many years, it was stated that if lymph node metastasis occurred in
endometrial cancer, it was to the para-aortic nodes. There were few data
to substantiate that impression until large cooperative group studies
indicated that approximately 5% of patients with clinical stage I
cancer had metastases to the aortic nodes. Although lymph node metastases
can be present without pelvic nodes involved, two thirds of patients
with metastases to the para-aortic nodes also have pelvic nodes involved. Nodal
involvement is directly related to differentiation of the
tumor and depth of invasion. In the GOG study, 11% of patients with clinical
stage I cancer had metastases to the pelvic, para-aortic, or both
lymph node groups. Peritoneal Cytology Malignant effusions are a poor prognostic sign. Even when ascitic fluid
is not present in the peritoneal cavity, cytologic evaluation can be
performed. It appears that the presence of malignant cells in the peritoneal
cytology is extremely important in evaluating the extent of disease
and therefore prognosis. If no ascitic fluid is present when the
peritoneal cavity is entered, 100 mL of saline can be injected into the
pelvic cavity. The saline is allowed to admix in the cul-de-sac and
then is withdrawn and sent to the cytologic laboratory. The possible prognostic role of malignant peritoneal cytology in endometrial
cancer has come under considerable discussion during the past several
years. Most of these studies suggest that in 10% to 20% of cases
thought to be clinical stage I (pre-1988 FIGO stage), malignant cells
were present in the peritoneal cytology. On multivariate analysis, this
finding has been shown to be highly predictive of other poor prognostic
factors, such as intraperitoneal disease and metastases to the lymph
nodes.3 Most studies in the literature suggest that the finding of malignant peritoneal
cytology is itself a poor prognostic factor. Is malignant peritoneal
cytology a poor prognostic factor if there is no other evidence
of disease outside the uterus? Several studies suggest it is important; others
have not found this relation. | 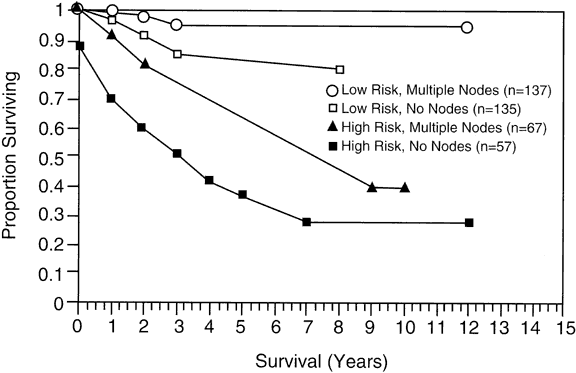
 .026; high-risk group, p = .0006.
.026; high-risk group, p = .0006.