Historical Background
The second-look laparotomy was introduced approximately 30 years ago by Wangensteen for periodic evaluation of patients who had undergone surgery for gastrointestinal carcinomas in which all gross tumor had been removed but the patients were at high risk for recurrence because metastatic disease had been found in regional lymph nodes.4 Asymptomatic patients without clinical evidence of carcinoma had a second-look operation performed in an attempt to find early recurrence when secondary resection still offered a chance of cure. About half of the patients were found to have recurrent carcinoma, and approximately 9% of these patients were apparently cured by the second procedure. This demonstrated that the prognosis of some patients with residual carcinoma could be altered favorably by a second-look procedure.
This second-look approach was later extended to the evaluation of ovarian cancer. Persistent or recurrent cancer was identified at operation in 11 of 14 patients. Two of these 11 were apparently cured following re-excision of tumor at the time of second-look. Of the three patients in whom no residual cancer was found at second-look operation, one subsequently developed recurrent disease. Therefore, 4 of 13 patients with ovarian carcinomas were apparently cured as determined by laparotomy and subsequent course.
An increasing number of complete clinical remissions resulted from the development of effective radiotherapy and chemotherapy for the treatment of metastatic ovarian cancer. A second-look laparotomy was often done before deciding to discontinue chemotherapy in patients with no residual ovarian cancer or to resect residual tumor masses and change therapy. In an early series reported by Rutledge and Burns from M.D. Anderson Hospital and Tumor Institute, 13 patients with advanced carcinoma of the ovary underwent re-exploration following treatment with melphalan chemotherapy.5 No cancer was found in 12 patients, treatment was discontinued, and recurrences subsequently developed in only two of these patients. This indicated that most patients who are found disease free at second-look operation can have their treatment discontinued with reasonable expectation for cure.
Tepper and associates6 reported a series of 17 patients with advanced ovarian carcinoma who had exploratory laparotomy with incomplete tumor removal, 3000 rads of abdominopelvic irradiation, and thiotepa chemotherapy, followed by second-look surgery. Irradiation and chemotherapy produced a significant reduction in tumor in all but three cases. Three patients showed no evidence of disease, and seven patients (41%) survived for more than 3 years. In nine patients, all gross tumor was removed at second-look; mean survival of these patients was 39 months, compared with 20 months for patients with gross residual disease. The second-look operation, therefore, served both diagnostic and therapeutic purposes in this series.
Wallach and associates7 employed second-look laparotomy in ten patients with advanced ovarian cancer who had responded favorably to alkylating-agent chemotherapy. Survival was improved by reoperation only in those patients who had had initial incomplete surgery and subsequent complete tumor disappearance with chemotherapy. The authors suggested that second-look surgery may be indicated to reduce residual tumor burden before a change in chemotherapy. They concluded that the development of newer chemotherapeutic agents may create an expanded role for second-look operation.
In 1976, Smith and associates8 reported on the results of systematically applying second-look laparotomy in the evaluation of 103 patients with advanced ovarian cancer. Analysis of their data revealed several important findings. First, the survival of all patients was directly related to the number of chemotherapy courses received. Patients receiving one to four courses of chemotherapy had a 5-year survival rate of 9%, compared to 32% for five to nine courses, and 80% for ten or more courses. All patients who had 12 or more courses of chemotherapy and were without evidence of disease at second-look laparotomy were alive at the time of the report. Second, survival could be affected by second-look surgery. Twenty-nine patients with cancer at second-look had complete tumor removal at that time. Of these, 14 (48%) were alive with no evidence of disease. Continuation of chemotherapy based on the finding of residual disease at second look resulted in increased survival, 30% surviving for 5 years. Third, patients with no evidence of disease at second look could have their chemotherapy safely discontinued. Of 23 patients with negative second-look laparotomies, 19 (82.6%) remained free of cancer. The four patients who developed recurrent disease following negative second-look laparotomy had received only four courses of chemotherapy before the operation.
Smith and associates,8 Wallach and colleagues,7 and Tepper and co-workers6 showed that about 60% to 75% of patients in apparent clinical remission following therapy for advanced ovarian cancer will be found to have residual disease at the time of a second-look surgical procedure. Thus, second-look operations were valuable for deciding whether to stop or continue chemotherapy. These early reports on second looks also suggested that resecting residual disease and continuing chemotherapy improved survival. The timing of the second-look procedure seemed to be important; only patients who had completed at least ten courses of chemotherapy and had had an apparent complete clinical response benefited from the operation. Second-look laparotomy, therefore, is employed in patients who are clinically free of disease and have completed a prescribed program of chemotherapy, its purpose being to determine the presence or extent of persistent disease and allow resection of disease if present. Patients who are found free of disease may have their chemotherapy discontinued with reasonable expectation of cure, whereas patients with residual disease should continue on some type of therapy.
Technique
A second-look operation should be a carefully planned procedure. Preoperative evaluation, including physical examination, chest radiography, pap smear, serum chemistries, CA 125 testing, and computed tomographic scanning should be negative for evidence of persistent or recurrent disease. Patients should be placed on a preoperative bowel preparation, since bowel injury may occur or intestinal resection may be required to remove persistent cancer.
Laparoscopy can be a useful adjunct at the time of second look, sparing a significant number of patients a more extensive surgical procedure. Much of the peritoneal cavity can be viewed, and selected biopsies and washings from the pelvis and each paracolic gutter can be obtained for frozen-section determination. These patients have usually had previous extensive abdominal operations, often involving gastrointestinal surgery, widespread intra-abdominal carcinomatosis, and occasionally abdominopelvic radiotherapy, all of which leave omental and bowel adhesions to the anterior abdominal wall. Such conditions increase the risk of intestinal injury when the laparoscopic trocar is inserted and may sometimes interfere with visualization. Despite these problems, few major complications from laparoscopy have been reported.
Rosenoff and co-workers9 described only 2 of 235 laparoscopies for malignant disease as unsuccessful because of extensive adhesions; there were no bowel perforations, and none of the five “significant” complications resulted in death or long-term morbidity. Mangioni and co-workers10 reported problems with adequate visualization in 19 (20.6%) of 92 patients undergoing laparoscopy for evaluation of ovarian cancer, the pelvis being the most frequently obstructed site. The morbidity in this series was unremarkable (4%), and only one serious complication requiring laparotomy was encountered. No complications were observed in 95 laparoscopies performed on patients with ovarian cancer in the report from Spinelli and associates.11 Ozols and co-workers12 also found laparoscopy to be a safe and feasible procedure in patients with ovarian cancer who had prior laparotomies. The procedure could not be performed for technical reasons in only 6% of the 99 patients; there were few serious complications, and in only 2.5% of cases was medical therapy required to remedy a complication. On the other hand, Berek and associates13 reported that 27% of procedures were unsuccessful and that major complications requiring laparotomy, most commonly bowel perforation, occurred in 14% of patients.
If biopsy-proven residual disease that is not considered resectable is found or if cytology is positive at laparoscopy, laparotomy may be deferred. However, if visualization is inadequate, no disease or resectable disease is seen, or histologic and cytologic specimens are negative for tumor, it is appropriate to proceed with laparotomy. A vertical midline incision that extends above the umbilicus should be employed. Upon entry into the peritoneal cavity, any fluid present should be aspirated and sent for cytologic examination. If no free fluid is present, the pelvis, right and left paracolic spaces, and right subdiaphragmatic area are individually irrigated with 50 ml to 100 ml of saline and the aspirates sent for cytologic evaluation. A careful inspection of all peritoneal surfaces should be performed. Each diaphragm should be carefully palpated and inspected; a laparoscope inserted through the incision may be a useful adjunct. The abdominal and pelvic retroperitoneum should be palpated for enlarged lymph nodes. The entire bowel and its mesentery, as well as adhesions, should be inspected carefully. The pelvis must be well visualized.
An attempt should be made to resect all gross tumor found at second-look laparotomy. When discrete, bulky tumor masses are found, resection may be more readily accomplished than when diffuse seeding is present. The smaller the maximum diameter of the residual tumor left after second-look surgery, the better the prognosis and the longer the survival.
The finding of no gross tumor at second-look laparotomy does not mean that the patient is free of cancer. It is the surgeon's responsibility to adequately sample the abdominal and pelvic peritoneum, the contents of the peritoneal cavity, and the retroperitoneum, and to remove any remaining reproductive organs and omental tissue in order to afford the best opportunity of determining the status of the patient's cancer. Peritoneal surfaces must be sampled from the diaphragm to the cul-de-sac. Any palpable or visualized irregularity is subjected to biopsy. Biopsy of the diaphragm may be readily accomplished with a Kevorkian biopsy instrument or a laparoscopic biopsy forceps. A diaphragmatic pap smear obtained with a sterile tongue blade may also be helpful. Strips of peritoneum should be obtained from the paracolic spaces, lateral pelvic walls, anterior bladder peritoneum, and cul-de-sac. Bowel serosa and mesentery are sampled at sites of adhesions. Any residual omental tissue should be removed. Hysterectomy or oophorectomy should be performed if the uterus or ovaries were not removed at the initial operation. Biopsies should be obtained from the stumps of the infundibulopelvic ligaments and round ligaments. Pelvic and para-aortic lymph node sampling should be performed, since these may be the only sites of persistent disease in some patients.14 Particular attention should be directed to and biopsies obtained from sites where residual tumor remained after the initial operation. These areas, as well as the omentum, residual ovaries, and pelvic peritoneum, are the most common sites of persistent epithelial ovarian cancer at second-look laparotomy. A minimum of 20 separate sites should be subjected to biopsy to ensure adequate sampling in patients with no gross evidence of disease.
Second-Look Laparoscopy
Laparoscopy has been proposed as an initial procedure at the time of second look to obviate laparotomy in patients found to have diffuse intraperitoneal cancer or positive peritoneal cytology. Several series have documented its usefulness and limitations (Table 1). Rosenoff and associates9 performed second-look laparoscopy in patients in clinical remission after at least 1 year of chemotherapy. Five of 16 patients (31.2%) were found to have residual, unsuspected tumor at laparoscopy and were continued on chemotherapy. Four of the 11 patients who had negative second-look laparoscopies had laparotomies, and two were found to have small foci of residual tumor among pelvic adhesions. Three of the remaining seven patients who had no evidence of disease at second-look laparoscopy relapsed 12, 15, and 19 months later. The remaining four patients were in remission 10 to 24 months later. In the Spinelli series, two of seven patients in clinical remission were found to have residual neoplastic disease at laparoscopy; one patient with a negative second-look laparoscopy relapsed 7 months after chemotherapy was stopped.11 Mangioni and co-workers10 reported that 6 of 18 patients with adequate negative second-look laparoscopies showed residual disease at major surgery.
TABLE 1. Second-Look Laparoscopy in Ovarian Cancer
| Negative | |||
Positive | Laparoscopy/Positive | |||
Laparoscopy | Laparotomy | |||
Series | No./Total | % | No./Total | % |
Rosenoff9 | 5/16 | 31.2 | 2/4 | 50 |
Spinelli11 | 2/7 | 28.6 |
|
|
Mangioni10 | 5/34 | 14.7 | 6/18 | 33.3 |
Piver15 | 8/22 | 36.3 | 2/10 | 20 |
Smith12 | 8/24 | 33.3 | 5/11 | 45.5 |
Ozols12 | 33/66 | 50 | 10/18 | 55.6 |
Total | 61/169 | 36.1 | 25/61 | 41 |
Piver and co-workers15 performed second-look laparoscopy in 22 patients with stage IIB, III, and IV ovarian adenocarcinoma after a median of 23 months of chemotherapy. Eight patients (36.3%) had documented evidence of persistent ovarian cancer and were thus spared a laparotomy. Ten of the remaining 14 women underwent a second-look laparotomy and eight (80%) had no evidence of persistent malignancy.
Second-look laparoscopy was performed by Smith and associates in 24 patients with no clinical or radiographic evidence of disease at the completion of a chemotherapy program for metastatic ovarian cancer.16 Laparoscopic-detectable cancer was present in eight patients (33.3%). Visualization was inadequate in five patients, and one of these five subsequently had persistent ovarian cancer at laparotomy. Of the 11 patients with adequate visualization and no evidence of disease at laparoscopy, five had biopsy-proven residual ovarian cancer at laparotomy, a false-negative rate by laparoscopy of 45%. Overall, 14 (58.3%) of the 24 patients who were clinically free of disease immediately prior to second-look operation were found to have residual tumor and required continued chemotherapy.
In 1981, Ozols and colleagues12 updated the experience at the National Cancer Institute with laparoscopy for postchemotherapy evaluation in ovarian cancer. Laparoscopy was performed in 66 patients who were without evidence of disease after receiving 6 to 12 months of chemotherapy for advanced ovarian cancer. Residual disease was detected at laparoscopy in 33 patients (50%); 7 patients (11%) were noted to have progressive disease requiring a change in therapy, whereas 24 patients (36%) had small residual disease indicating a need to continue presently effective chemotherapy. In 28 patients (42%) there was no evidence of residual disease at laparoscopy, and 18 of these patients underwent second-look laparotomy. In ten patients (56%), disease was detected at laparotomy that had not been observed at laparoscopy. The pelvis was the most common site of disease that was missed by laparoscopy. Laparoscopic examination was unsuccessful in only five patients (7.6%). Residual cancer was detected in 83.6% of patients who had second-look laparoscopy/laparotomy.
These six series involved a total of 169 women who underwent second-look laparoscopy when they were in complete remission after extensive chemotherapy for advanced ovarian cancer. Thirty-six percent were found to have persistent malignancy at laparoscopy and were thus spared laparotomy at that time. Laparoscopy usually afforded good visualization of the diaphragms, liver surface, abdominal wall peritoneum, omentum, and bowel surfaces. The pelvis was the most difficult area to assess adequately. Biopsies from suspicious areas and peritoneal cytologic washings added significantly to the accuracy of the laparoscopic evaluation. In fact, malignant peritoneal cytology provided the only evidence of persistent tumor at laparoscopy in 6% to 18% of patients.
The presence of visible tumor, positive biopsy results, or cytologically positive peritoneal washings at laparoscopy obviates a more extensive operative procedure. Because of the significant relapse rate following negative second-look laparoscopy as noted by Rosenoff and associates and the high false-negative rates in the Smith, Mangioni, and Ozols reports, however, all patients having negative or inadequate laparoscopic examinations should have immediate laparotomy with multiple peritoneal and nodal biopsies and cytologic washings so that residual tumor will not be overlooked and chemotherapy prematurely stopped.9,10,12,16
Second-Look Laparotomy
In the last decade, second-look laparotomy has become an accepted, integral part of the treatment program for patients with advanced ovarian cancer. Yet the place of the procedure in the evaluation of early ovarian cancer, the timing of the operation, the effect of histology and tumor grade, and the relationship between the initial surgical procedure and the findings at second look remain controversial. Most importantly, the effect of second-look procedures on survival is difficult to assess from available data.
In 1980, Schwartz and Smith17 presented data on a series of second-look laparotomies performed at the M.D. Anderson Hospital between 1960 and 1974. One hundred eighty-six patients with epithelial ovarian cancers underwent exploratory operation to assess the status of the cancer after a course of treatment. There was no evidence of disease in 58 of the 186 patients (31.2%). This included 18 (66.7%) of 27 stage I patients, 14 (45.2%) of 31 stage II patients, and 26 (20.3%) of 128 patients with stage III and stage IV disease. The only evidence of disease in 13.4% of patients was microscopic foci of tumor in random biopsies. Disease regression with macroscopic tumor was present in 41.5% of patients, whereas progressive or stationary disease was found in 23%. The most common sites of persistent disease were the pelvic peritoneum, the ovaries, and the omentum.
The amount of chemotherapy that patients received before second-look surgery had a significant effect on findings. Only 14.6% of patients who completed two to nine courses of melphalan were found free of disease at second look, whereas 56.4% who received ten or more courses of chemotherapy were without evidence of cancer. The volume of residual tumor left at the initial operation correlated directly with the incidence of negative second-look operations. Negative second-look operations occurred in 30.8% of patients who had all of their tumor removed at the time of the initial operation, in 31.5% of patients with residual tumors smaller than 2 cm, and in 16.7% of patients with residual tumors larger than 2 cm. The percentage of negative second-look operations was higher for mucinous and endometrioid than for serous adenocarcinomas. The histologic grade of tumor did not seem important.
Rates of survival after second-look laparotomy varied directly with the volume of tumor found at operation and the amount of tumor left behind. The 5-year survival rate after second-look operation was 72% for patients with no evidence of disease, 38% for patients with microscopic disease only, and about 16% for all patients with macroscopic tumor. Resection of residual tumor at the second-look operation influenced subsequent survival. The 2-year survival rate for patients who underwent total removal of residual tumor was 47.5%, compared with 29.5% for patients who had partial removal leaving residual tumors smaller than 2 cm and 9% for patients in whom residual tumors exceeded 2 cm in diameter. Seven patients (5.5%) who had cancer at the time of second-look operation survived 5 or more years free of disease. Patients who received chemotherapy after positive second-look procedures had 2-year and 5-year survival rates of 34.3% and 26.7%, respectively, compared with survival rates of 24.4% and 16.1% in patients who received radiation therapy.
Since this early study, many series of patients undergoing second-look laparotomy have been reported. In general, more than half of these patients with no clinical evidence of disease will have demonstrable cancer at the time of second look. Sites particularly likely to harbor residual carcinoma at second-look surgery are areas where tumors were known to have been left behind at the completion of the initial laparotomy, such as the pelvic peritoneum and omentum, and residual reproductive organs. In a majority of these series, the most important factors correlating with negative second-look operations are the stage of the disease, the histologic grade of the cancer, and the amount of residual disease left at the initial operations.
The chances of finding residual ovarian cancer at second-look laparotomy increase with advancing stage of disease. Although approximately 80% of patients with stage I disease have negative second-look operations, only about 30% of patients with initial advanced disease (stages III and IV) who are clinically free of cancer at the time of reoperation will have no pathologic evidence of disease (Table 2).17,18,19,20,21,22,23,24,25,26,27,28,29
TABLE 2. Second-Look Laparotomy Findings Related to Stage of Ovarian Cancer
| Negative Second | ||
| Total | Look |
|
Stage | Patients | No. | % |
I | 209 | 170 | 81 |
II | 146 | 99 | 68 |
III and IV | 825 | 268 | 32 |
Compiled from multiple sources
Histologic grade has been considered to be an important prognostic factor in ovarian cancer. A number of reports have examined the relationship between histologic tumor grade and findings at second-look laparotomy. Patients with grade 1 or grade 2 tumors have twice as high a proportion of negative findings (57%) as patients with grade 3 tumors (29%) (Table 3).17,19,21,24,28,30,31
TABLE 3. Second-Look Laparotomy Findings Related to Histologic Grade of
Ovarian Cancer
| Total | Negative Second Look | |
Grade | Patients | No. | % |
Borderline | 27 | 17 | 63 |
1 | 107 | 61 | 57 |
2 | 132 | 75 | 57 |
3 | 247 | 72 | 29 |
Complied From multiple sources
The volume of residual tumor left at the completion of the initial operation for ovarian cancer correlates with the incidence of negative second-look procedures (Table 4).17,18,19,20,21,22,24,25,26,27,28,31,32,33,34 Patients with small amounts of residual tumor following initial surgery have a higher likelihood of having a negative second-look operation. Whereas about 71% of patients with no remaining disease after the original operation have negative second-look operations, approximately 47% of patients with residual tumors smaller than 2 cm and 19% of patients with residual tumors larger than 2 cm have negative second-look laparotomies. This relationship emphasizes the importance of aggressive primary cytoreductive surgery in patients with ovarian cancer.
TABLE 4. Second-Look Laparotomy Findings Related to Residual Ovarian Cancer
After Initial Surgery
|
| Negative Second | |
Residual | Total | Look | |
Cancer | Patients | No. | % |
None | 321 | 227 | 71 |
<2 cm | 374 | 176 | 47 |
| 442 | 83 | 19 |
Compiled from multiple sources
Schwartz and Smith17 and Webb and colleagues21 noted a positive correlation between the amount of chemotherapy administered and negative second-look results, as well as survival. Most authors have been unable to demonstrate any effect of chemotherapy type or duration on outcome at second-look laparotomy.
The findings at second-look operations have important prognostic and therapeutic
implications. Although patients with a negative second-look
laparotomy have prolonged survival compared with patients in whom residual
cancer is discovered, anywhere from 15% to almost 60% of patients
who have a negative second look will develop recurrent disease (Table 5).24,25,26,27,28,29,31,32,33,35,36,37,38,39,40 The rate of recurrence is related to the initial stage of the patients' cancer, the
tumor grade, and the type of chemotherapy. Rubin and co-workers41 found that only 3.7% of patients with stage I disease had a recurrence
compared with 35.7% of patients with stage II, III and IV disease. They
also determined that the rate of recurrence increased with increasing
tumor grade, from 14.3% in grade 1, to 35.5% in grade 2, to 64.7% in
grade 3 tumors. In addition to these factors, patients with a negative
second-look laparotomy after cisplatin-based chemotherapy had a much
higher recurrence rate (50%) and a shorter median interval to recurrence (14 months) compared
with a recurrence rate of 25% and a median interval
to recurrence of 23 months in patients not treated with cisplatin. This
higher recurrence rate after cisplatin-based chemotherapy probably
relates to the higher response rates and shorter duration of administration
of this type of chemotherapy compared with single alkylating
agent or noncisplatin combination chemotherapy. Miller and colleagues33 determined that factors associated with prolonged disease-free status
at second-look laparotomy were stage (I and II = 48% vs III and IV = 18%), nonserous
histology (45% vs 11%), and size of the largest residual
tumor mass after primary operation (none = 52% vs <2 cm = 15% vs >2 cm = 14%). The
most important factor associated with disease recurrence
after a negative second look was grade (19% for grades 0, 1, and 2 vs 59% for
grade 3). Podratz and colleagues26 identified advanced patient age (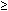 67 years) as well as undifferentiated tumor histology and
67 years) as well as undifferentiated tumor histology and 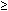 2 cm residual tumor burdens at primary surgery as factors contributing
to recurrence of tumor after a negative second look.
2 cm residual tumor burdens at primary surgery as factors contributing
to recurrence of tumor after a negative second look.
TABLE 5. Recurrences Following a Negative Second- Look Laparotomy for Ovarian
Cancer
|
| Negative |
| ||
|
| Second Looks | Recurrences | ||
Series | Patients | No. | % | No. | % |
Barnhill et al24 | 96 | 48 | 50 | 6 | 13 |
Berek et al32 | 56 | 18 | 37 | 4 | 22 |
Gershenson et al35 | 246 | 85 | 35 | 20 | 24 |
Cain et al27 | 177 | 104 | 59 | 17 | 16 |
Smirz et al25 | 88 | 35 | 40 | 8 | 23 |
Podratz et al26 | 135 | 63 | 47 | 12 | 19 |
Miller et al33 | 88 | 38 | 43 | 16 | 42 |
Dauplat et al36 | 51 | 24 | 47 | 4 | 17 |
Gallup et al28 | 65 | 34 | 52 | 8 | 24 |
Carmichael et al37 | 173 | 53 | 31 | 30 | 57 |
Chambers et al29 | 67 | 38 | 57 | 7 | 18 |
Heintz et al31 | 51 | 16 | 31 | 5 | 31 |
Creasman et al38 | 84 | 41 | 49 | 11 | 27 |
Podczaski et al39 | 102 | 49 | 48 | 15 | 31 |
Ghatage et al40 | 155 | 77 | 50 | 15 | 19 |
The apparent high false-negative rate for second-look laparotomy has led several investigators to propose additional consolidation therapy in an attempt to prevent recurrences in patients with surgically confirmed complete remissions. Whole abdominal irradiation, intraperitoneal radioactive chromic phosphate (32P), intraperitoneal chemotherapy, and additional intravenous chemotherapy have all been proposed as possible treatment modalities. While some claims of efficacy have been made, there are no prospective randomized trials demonstrating any of these modes of therapy superior to no treatment in preventing recurrences after a negative second look. In a small trial reported by Mangioni and colleagues, the best survival for patients with a negative second look was achieved by continuation of intravenous chemotherapy and the worse survival resulted from treatment with intraperitoneal 32P. There were no significant differences in survival between patients who received consolidation therapy and those who did not.42
Patients in whom only microscopic cancer is found at the time of the second-look procedure have a survival that does not differ substantially from patients with a negative second look. Copeland and Gershenson43 reported follow-up for at least 4.5 years after second-look laparotomy in 50 patients with microscopic tumor and 85 patients with no evidence of tumor. Recurrences developed in 43% of evaluable patients with microscopic disease compared to 38% of patients who had had a negative second look. None of the well-differentiated tumors recurred in either group. At Memorial Sloan-Kettering Cancer Center, estimated actuarial 5-year survival for patients found to have microscopic disease at the time of second look was 62%.44 Other reports have documented disease-free survivals between 50% and 100% in this group of patients at 2 years after second-look laparotomy.25,34,38,39,45,46,47
On the other hand, patients with gross residual disease at second look have a poor prognosis. Approximately 80% of these patients will be dead within 2 years, with a median survival of about 14 months.17,25,26,34,39 Some authors have found that survival after a second-look operation is directly related to the amount of tumor left behind, indicating that survival could be altered by tumor removal at second-look. Schwartz and Smith reported a 5-year survival of 29% following total removal or reduction of tumor to less than 2 cm versus 9% if residual tumor was greater than 2 cm.17 Greco and associates18 also reported that five (56%) of nine patients who had all known residual cancer resected at second-look laparotomy remained free of detectable tumor at the time of their report. Berek and co-workers48 were able to perform significant cytoreductive surgery in only 38% of patients with gross residual disease at second-look surgery. Patients who benefited from secondary cytoreduction were those with small tumor burdens left behind at the original procedure and small volume residual disease which could be almost completely resected. Lippman and associates34 also noted that resection of residual tumor masses to less than 2 cm resulted in significantly greater survival. Dauplat and colleagues36 reported an improved survival in patients who had all macroscopic residual disease removed at second-look operation compared with patients in whom only partial removal could be accomplished. Hoskins and co-workers44 reported that 16 (37%) of 43 patients with macroscopic cancer at second look were cytoreduced to microscopic residual disease. Five-year survival was 51% in this group compared to 10% in patients with gross residual disease at the end of second-look laparotomy.
Most studies have not demonstrated a significant benefit to debulking procedures performed at the time of second-look operation. Raju and co-workers,49 Mead and co-workers,50 Luesley and co-workers,51 and Miller and co-workers52 found that removal of all macroscopic residual disease at second-look operation did not improve survival expectancy compared to patients in whom residual disease was still evident at completion of the procedure. Podratz and colleagues26 suggested minimal value in further aggressive tumor reduction unless complete removal of all macroscopic disease could be attained. Podczaski and associates39 emphasized that although significant secondary debulking of tumor may be theoretically beneficial, it is technically feasible in only a very few patients.
Second-look laparotomy is useful in evaluating response to different chemotherapy protocols, in determining eligibility for investigational treatment modalities, and in predicting prognosis, but it has not been proven to have any effect on overall survival. In 1983, Cohen and colleagues noted comparable survival between 73 patients having second-look operations and 43 eligible patients not having second-look operations, indicating that a second-look procedure may not have any effect on survival.22 Since then several other investigators have reported a similar lack of survival advantage for patients having second-look laparotomy over patients who did not.29,38,53,54,55,56 The ultimate impact of second-look procedures on survival will become apparent only over an extended period of time, as new secondary treatment modalities are explored that are likely to have maximum benefit in patients who have small residual tumor discovered at second look. The effects of further tumor resection at second-look laparotomy and new therapeutic interventions, such as intraperitoneal therapy or high-dose chemotherapy with autologous bone marrow transplantation, need to be assessed in a systematic fashion based on the findings at second look. Until further information is forthcoming, a second-look laparoscopy/laparotomy should be undertaken only as a part of a carefully planned re-evaluation in a patient with advanced epithelial ovarian cancer who has completed a prescribed program of chemotherapy, who is clinically free of disease, and who is eligible for an investigational treatment based on the results of the procedure.