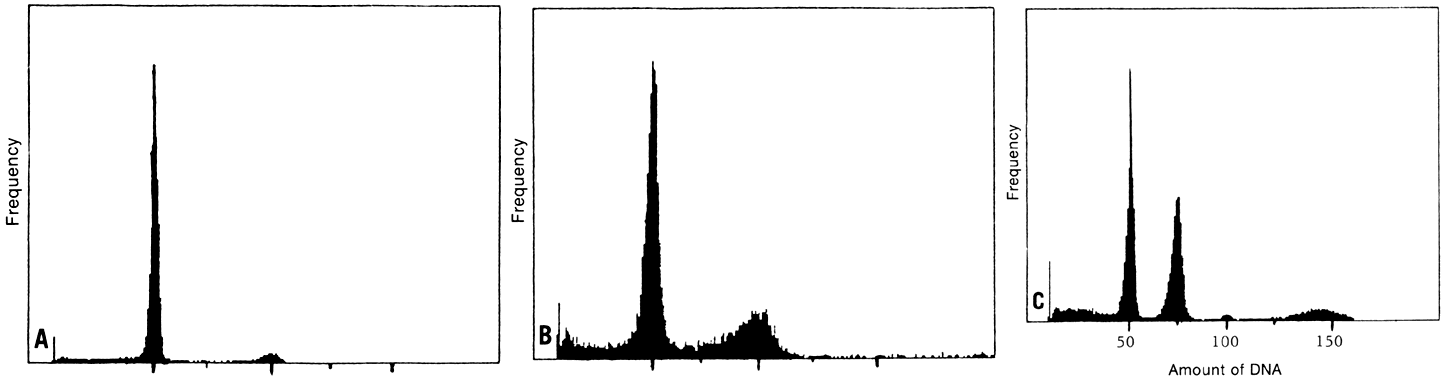
A clinically significant relationship between cytophotometrically determined
DNA-content abnormalities and aggressive biologic behavior has been
established in ovarian carcinoma.1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24 Kallioniemi and associates2 examined the DNA content of 157 ovarian malignancies (89 stages I and
II; 68 stages III and IV) and showed a significant negative association
of both abnormal DNA content and cell cycle distribution with survival (clinical
follow-up for censored observations 2 to 17 years). Tumors
were categorized as low-risk, intermediate-risk, or high-risk based
on a combination of both of these DNA histogram characteristics. Low-risk
tumors (20%) were nonaneuploid with a low S-phase fraction, whereas
high-risk tumors (32%) were characterized by either multiploidy or aneuploidy
combined with a hypertetraploid DNA index or a high S-phase
fraction. The remaining 48% of intermediate-risk tumors had DNA-content
histograms that did not meet either of these sets of criteria. The risk
of death for patients with high-risk and intermediate-risk DNA-histogram
type tumors was 9.6 and 5.6 times higher, respectively, than that
for patients with low-risk DNA-histogram type tumors. This association
between survival and DNA histogram type was retained even after the
effects of other significant covariants (e.g., stage, residual tumor, age, treatment, histopathologic type) were removed. In
addition, although the incidence of aneuploidy was higher in high-stage
tumors, the prognostic value of cytophotometric DNA analysis
was retained in both low-stage (I and II) and high-stage (III and IV) tumors. Khoo
and colleagues15 reported similar results for 125 ovarian adenocarcinomas in which multivariate
analysis identified only ploidy and postsurgical residual disease
as independent prognostic variables. The prognostic value of DNA-content analysis also has been confirmed when
only high-stage ovarian carcinomas are examined.4,5,6,7,8,9,10,15,16,17,18,21,22,23 Friedlander and co-workers10 examined the prognostic value of cytophotometric DNA-content analysis
in 91 stage III and IV ovarian epithelial malignancies with a median clinical
follow-up of 20 months (range, 9 to 36 months). Aneuploid cell
populations were resolved in 69% of tumors. These aneuploid tumors had
a median survival of approximately 55 weeks, whereas the 29 tumors without
resolvable aneuploid cell populations had not yet reached their
median survival at 100 weeks. Multivariate analysis of DNA content, stage, chemotherapy
group (sequential or combined chlorambucil and cis-platinum), histopathologic grade and type, amount of residual tumor, and
performance status showed that DNA content was the major determinant
of survival for these advanced-stage tumors, with stage and performance
status also remaining significant prognostic factors. Blumenfeld and associates4 reported similar results among 84 patients with stages II to IV ovarian
epithelial malignancies with an average clinical follow-up of 33 months (range, 16 to 88 months). They divided tumors into two risk groups
based on cytophotometric DNA analysis. High-risk tumors consisted of
those with nontetraploid aneuploid cell populations (52%). The remaining 40 diploid
and tetraploid tumors were categorized as low-risk. The
median survival for patients with high-risk and low-risk tumors was 19 and 48 months, respectively. Univariate analysis demonstrated that DNA
content, stage, age, histopathologic grade (grade 1 vs grades 2 and 3 combined), residual
disease after surgery, and single-agent versus triple-agent
chemotherapy were also significant prognostic factors. Multivariate
analysis of these factors, however, showed only DNA content, stage, and
age to be independent prognostic factors, DNA content being
the most important determinant of survival for stage III tumors. Kaern and associates21 also demonstrated DNA-content abnormality to be an independent prognostic
variable in 169 stage III and IV ovarian adenocarcinomas with a minimum
follow-up of 2 years. In a study of 184 stage III and IV ovarian
carcinomas, however, Pfisterer and colleagues22 reported that although aneuploidy was associated with decreased survival, they
did not find it to be an independent prognostic variable using
multivariate analysis. Although the incidence of aneuploidy in stage I and II ovarian carcinomas
appears to be significantly lower than the 50% to 80% reported in advanced-stage
disease,25 its prognostic value is retained.1,2,4,7,8,10,12,14,20,25 Kallioniemi and associates2 found that 42 of 89 (47%) stage I and II ovarian carcinomas were aneuploid
and that these aneuploid low-stage tumors had a significantly poorer
prognosis than the nonaneuploid tumors. This was most apparent for
multiploid tumors and for those aneuploid tumors with DNA indices greater
than 2. In addition, further prognostic information was obtained
when nonaneuploid tumors were divided on the basis of S-phase fraction. Similarly, Vergote
and co-workers14 demonstrated that nondiploidy could be resolved with flow cytometry in
archival tumor tissue in 49% of 279 stage I ovarian carcinomas and that
it was a powerful independent prognostic factor of disease-free survival
in these patients. In addition to the demonstrated association between aneuploidy and prognosis
in ovarian adenocarcinoma detailed above, an association between
aneuploidy and high histopathologic grade has been reported by most,1,11,14,15,21,22,25,26,27,28 but not all,4,29 investigators, including those who based the histopathologic assessment
on quantitative morphology.5,6,7,24 A significant association of cytophotometric DNA content and cell cycle
abnormalities with histopathologic grade was reported in ovarian carcinoma
by Kallioniemi and associates.25 This association was stronger when DNA-histogram type was compared with
histopathologic grading based on nuclear characteristics (e.g., nuclear-to-cytoplasmic ratio, nuclear chromatin pattern, and nucleolar
abnormalities) than when histopathologic grading was based on architectural
features (e.g., percentage of microscopically solid tumor). This relationship between
nuclear grade and aneuploidy was retained for both low-stage and high-stage
tumors. In addition, a significant relationship between tumor histopathologic
type (World Health Organization histopathologic types: serous, mucinous, clear
cell, endometrioid, and undifferentiated) and the
incidence of aneuploidy was reported by these investigators. This was
due primarily to a lower than average incidence of aneuploidy among
mucinous tumors (39%) and a higher than average incidence of aneuploidy
in undifferentiated tumors (75%) as compared with serous, clear cell, and
endometrioid tumors (61%, 54%, and 61%, respectively). This association
between histopathologic type and DNA-histogram abnormalities
was not, however, independent of nuclear grade and was eliminated when
groups were adjusted for histopathologic nuclear grade. This relationship
between histopathologic type and DNA-histogram abnormalities has
been confirmed by most4,10,14,22,23,26 but not all15 investigators. Although the prognostic values of histopathologic and cytophotometric DNA
analysis are interrelated in ovarian carcinoma, cytophotometric DNA
analysis is frequently the stronger prognostic variable.1,2,4,10,15,21 Although no significant relationship between cytophotometric DNA analysis
and histopathologic grading was found for the 84 advanced-stage ovarian
carcinomas reported by Blumenfeld and associates,4 univariate analysis showed a significant prognostic value for both of
these factors. However, only DNA analysis retained its prognostic value
when analyzed simultaneously with stage, age, residual disease after
surgery, chemotherapy, tumor type, tumor size, and surgical treatment. Similarly, Kallioniemi
and associates2 reported that although DNA analysis, architectural histopathologic grading, nuclear
histopathologic grading, and mitotic index were all significant
prognostic variables in ovarian carcinoma, only cytophotometric
DNA analysis, nuclear histopathologic grading, and mitotic index retained
prognostic value after they adjusted for covariance due to stage, residual
tumor, histopathologic type, age, and chemotherapeutic treatment. In
addition, when they analyzed all factors simultaneously, only
cytophotometric DNA analysis and stage remained independent prognostic
variables. Finally, the incidence and prognostic value of cytophotometric ploidy determination
has been reported to be associated with residual disease.15,17,18,22 Multivariate analysis of 125 ovarian carcinomas reported by Khoo and associates15 showed not only that the amount of residual disease and the ploidy status
were independent prognostic variables, but that the prognostic value
of ploidy analysis was particularly significant in patients with minimal
residual disease. Although the prognostic value of cytophotometric DNA-content analysis is
well established in ovarian carcinomas, its clinical value in epithelial
tumors of low malignant potential (LMP) remains undemonstrated.30,31,32,33,34,35,36,37 Conclusions from limited reports include the following: - The incidence of aneuploidy appears to be lower among LMP tumors compared
with fully malignant ovarian carcinomas.1,11,30,31,32,34,37
- Mucinous LMP tumors may have a higher incidence of aneuploidy than serous
LMP tumors.37
- The incidence of aneuploidy may be higher in advanced-stage disease.1,11,30,31
In addition to the lower incidence of aneuploidy in ovarian LMP tumors, DNA
indices in these tumors have been reported to be generally lower
than those in ovarian carcinomas, clustering in the near-diploid region.1,34 Finally, relatively little is known about the possible clinical utility
of cytophotometric DNA-content analysis in nonepithelial ovarian neoplasms. Although
investigations of its prognostic value in granulosa cell
tumors have been reported,38,39,40,41 overall numbers are small and conclusions contradictory. |