When gynecologic cancer survivors are studied longitudinally, sexuality is the life area that undergoes major, or even permanent, change.95 There are several reliable and valid strategies to assess quality of life (e.g., SF-36, FACT)96 and sexuality,97 and research has documented the acceptability of such assessments in clinical trials.98 This discussion is divided into three areas: (1) review of sexual difficulties for gynecologic cancer survivors, (2) identification of medical and psychological variables related to morbidity risk, and (3) discussion of the merits of psychological interventions for cancer survivors.
In the literature on sexual functioning outcomes, reports often examined outcome as a function of treatment modality. Specifically, many studies provide data on surgery versus radiotherapy, usually in the treatment of cervical cancer. With one exception,99 randomized trials testing for sexual outcomes have not been conducted, even though it is likely that there are systematic differences between treatment modalities. Occasionally, ovary or endometrial cancer patients also may be included in the analysis. Although inclusion this adds to the variability in outcomes, the variations in the same modality seem to be less important in predicting sexual outcomes than the effects of different treatment modalities (e.g., surgery versus radiotherapy). For this reason, we review the retrospective data within treatment regimens, specifically surgery (e.g., radical hysterectomy), radiotherapy (external beam, with or without intracavitary treatment), and combination surgery and radiotherapy.95
Review of the Literature: Retrospective Studies
RADICAL HYSTERECTOMY AND RELATED SURGERIES.
Historically there have been conflicting reports on the sexually disruptive effects of hysterectomy per se. The vaginal shortening with radical hysterectomy may contribute to subjective feelings for the woman that the vagina is “too short” for intercourse. Nerve and vascular disruption to the pelvis (as may occur with lymphadenectomy) may result in loss of sensitivity and orgasmic disruption. The latter issues and other types of morbidity (e.g., bladder or bowel dysfunction) have led to the consideration of nerve-sparing techniques for radical hysterectomy.100
With few exceptions,101,102 the data on radical hysterectomy and related surgeries come from small sample reports, which are more subject to threats of internal validity, including subject selection (i.e., biased sample), history (i.e., the effects of events before and after cancer and cancer treatment), and maturation (i.e., the effects of normal developmental events, such as menopause). Although large sample sizes do not solve these research design flaws (i.e., the data have the same problems with validity), they may provide more reliable (stable) estimates of sexual outcomes. Review suggests that approximately 20% of women report being sexually inactive at follow-up, and of the women remaining active, approximately 30% reported significant declines in the frequency of intercourse. The rates of dysfunctions ranged from 25% to 40% for the sexually active women, with dyspareunia in particular a problem for 30% of women.
RADIATION THERAPY.
The effects of radiation on the vagina have been detailed.103 The rapid cell renewal system of the vagina’s epithelium provides for the natural exfoliation of the outermost layer of nondividing cells, which in turn makes the epithelium sensitive to the effects of radiation. The damage causes depletion of the cell supply, which is compensated by mucosal shrinkage. Significant vaginal shortening occurs.58 The slow occlusion of blood vessels and the gradual laying down of fibrosis in the submucosal tissues leads to tissue hypoxia and progressive narrowing of the vaginal canal with a significant decrease in elasticity and a decrease in sensation. The patients may have genital scarring and vaginal fibrosis. The thinning of the vaginal epithelium and lubrication loss increase susceptibility to trauma and infection. Pelvic stenosis and fibrosis cause decreased blood flow and vaginal secretions, which are coupled with decreases in vaginal length and diameter. It is estimated that radiation-induced tissue changes continue for 36 months after the completion of therapy.
Radiation to the pelvis can destroy ovarian functioning for the premenopausal woman, inducing menopausal symptoms and further adding to causing vaginal atrophy and lubrication difficulties. The exact dose required to cease ovulation permanently is unknown; however, the nearer to menopausal age of a woman, the lower the dose required. Because current standard doses for gynecologic tumors consists of 4000 to 6000 cGy to the whole pelvis,104 excluding the dosage received with intracavitary treatments [see Andersen et al53 for a discussion of the psychological aspects of this difficult treatment], cessation of ovulation is virtually certain for women of any age. Estrogen therapy after treatment can control menopausal symptoms such as hot flushes and aid in the healing of the vaginal epithelium,105 but dyspareunia still may occur. Estrogen replacement therapy has not been offered to many patients.
As with the surgery studies, several data are available. The percentages of women discontinuing sexual activity or reporting decreases in frequency are comparable to the surgery studies, with approximately 20% reporting no activity and 40% reporting significant decreases. The percentages of women reporting response cycle disruption are higher, however, in the range of 40% to 50%. Supporting descriptive data on pelvic examinations suggest the contribution of radiation effects on the vagina coupled with estrogen deficiency to the occurrence of sexual disruption.
COMBINED SURGERY AND RADIOTHERAPY.
There are two common scenarios in receiving combination therapy. Radiotherapy can follow surgery if the pelvic lymph nodes are positive for malignancy. When radiotherapy precedes surgery, the apex of the vagina, which is exposed to the largest dose of radiation when intracavitary treatment is included, may be removed during surgery. If this is done, the remaining portion of the vagina is less affected and, perhaps, less vulnerable to problems of dyspareunia. In the retrospective studies, researchers do not always mention the sequence of the combined treatment, making interpretation difficult and comparison of sequences impossible.
It is believed that these women would have greater risk for sexual morbidity because of the combination therapy. With the exception of higher rates of dyspareunia (in the range of 35%), however, the outcomes appear similar to those for the single-modality groups. Summary analyses suggest that approximately 15% reported no sexual activity, whereas 40% reported declines in frequency, and 25% to 50% of women reported difficulties with desire, excitement, or orgasm.
RADICAL SURGERIES.
Pelvic Exenteration.
This surgery is disfiguring and produces many functional problems with the obvious sexual sequelae.106 Clinical articles commonly have reported the cessation of sexual activity for most women (i.e., 80% to 90% of those surveyed). Available reports are from English-speaking countries only, including Canada,107 the United Kingdom,108 and the United States109,110,111,112,113; all reveal a negative scenario for sexual outcomes and significant psychological distress, including depression and anxiety-related problems. For most women and couples, the prospect of ending their sexual life (as most couples cease all sexual activity when intercourse becomes impossible) is distressing and may be a source of continuing marital discord and may lead to divorce.109
Vaginal reconstruction is possible for some and enables a woman to maintain sexual activity that includes intercourse; however, many sexual difficulties often remain, and some physicians have described the outcomes as disappointing.114 Some women have difficulties with the physical characteristics of the new vagina (e.g., the cavity is too large or too narrow); others have general problems with arousal or orgasm or specific impediments such as dyspareunia or bleeding with intercourse. Regardless of whether or not women with pelvic exenteration undergo vaginal reconstruction, these women face the greatest disruption to their body and functioning of any female cancer group. It is remarkable that little systematic descriptive or intervention work has been done with these women in view of the curative intent of this surgery.
Surgeries for Vulva Cancer.
Despite the sexual morbidity of vulva surgery, attention to the sexual or psychological outcomes for women is relatively recent, with the first substantive reports not appearing until 1983. As with pelvic exenteration, vulvar treatments can have a dramatic impact on sexuality and other life areas. After early observations on the benefits of conservative therapy115 and the advocacy of individualized treatment approaches,116 a paradigm shift in treatment occurred. In addition to less radical resection of the primary lesion, this shift has included selective omission of groin dissection and routine pelvic lymphadenectomy, the possibility of sentinel node biopsy rather than lymphadenectomy,117 preoperative radiation to obviate the need for exenteration in patients with advanced disease, and postoperative radiation to decrease the incidence of groin recurrence.118,119 The data for in situ and invasive disease are reviewed separately because of the considerable difference in the treatment regimens. Also, on average, in situ patients are younger than patients with invasive disease.
Andersen and colleagues120 provided extensive data on the sexual outcomes for women treated with wide local excision and related treatments for in situ disease. In situ patients are more likely to be sexually inactive at follow-up, whether or not they have available sexual partners, than age-matched healthy counterparts. If the woman has a sexual relationship, however, the rates of sexual dysfunction are only slightly higher than those for healthy women.
The outcomes for women with in situ disease contrast markedly with the outcomes for women with invasive disease, many of whom are treated with radical vulvectomy, with or without groin dissection. The early retrospective studies, conducted in the 1980s, were limited by their small sample sizes (range, 9 to 52) and retrospective evaluations, but the trends were consistent.109,121,122,123 At least 30% to 50% of patients became sexually inactive, and of the women remaining active, 60% to 70% had multiple sexual dysfunctions. Research efforts with larger samples confirmed these findings and documented the high frequency of sexual dysfunctions, including ones that are difficult to treat (e.g., sexual aversion and hypoactive sexual disorder). In the Green report,124 advanced age, depression, and lower performance status were correlates of risk for sexual dysfunction. Reasons women and their partners end intercourse include the physical changes to the body and severe dyspareunia, such as may occur with a narrowed introitus. Rather than being resigned to this circumstance, most women would have preferred to remain sexually active.109
Review of the Literature: Prospective Studies
In the prospective designs, women have been recruited for study participation shortly after diagnosis and assessed longitudinally. These studies usually have detailed assessments of sexual functioning. Some studies also have included women treated for benign disease to estimate the magnitude of sexual problems with gynecologic disease and treatment and healthy women to estimate the base rate of sexual problems. The latter designs are most powerful when longitudinal assessment is included for all groups.
SEXUAL DISRUPTION WITH THE INITIAL APPEARANCE OF SYMPTOMS AND SIGNS OF CANCER.
The sexually disruptive effects of early signs and symptoms were described by Andersen and coworkers.125 In the United States, 41 women with early-stage cervical or endometrial cancer were studied before treatment, and their responses were compared with a matched group (i.e., age, menopausal status, and sexually active status) of healthy women in no gynecologic distress. Because 75% of the women with cancer experienced a substantial change in sexual functioning, it is likely that such obvious and disruptive sexual problems influenced the women to interpret their gynecologic disease signs and symptoms negatively and to seek medical consultation. These controlled data replicate an extension of the findings of Harris and colleagues,126 also in the United States, who interviewed 63 recently diagnosed women (stage of disease not specified). Although 85% of the women were satisfied with their sexual life before the appearance of symptoms, only 48% felt similarly afterward. Of the women, 50% stopped having intercourse, and another 30% reduced the frequency. Of women remaining sexually active, the percentage of women able to experience orgasm dropped from 58% to 15%. Finally, women reported that they initiated the changes in intercourse frequency because of bleeding, pain, and anxiety.
SINGLE-GROUP LONGITUDINAL DESIGNS.
The only experiment ever to have been conducted to test for differential sexual outcomes was reported by Vincent and associates99 in 1975 in the United States. A total of 50 women with early-stage cervical disease were randomly assigned to receive either radical hysterectomy or radiotherapy. The groups were matched for age, education, socioeconomic status, marital status, parity, race, and disease stage. In contrast to the results from the retrospective studies, the changes in sexual desire and activity from pretreatment to 6 months post-treatment were comparable: Estimates of diminished desire were obtained from 24% of the radiotherapy patients and 20% of the surgical patients. Decreased frequency of intercourse was reported by 29% of the radiotherapy patients and 33% of the surgical patients. This experiment provides convincing evidence that, in general, the rates of sexual behavior disruption and dysfunction were comparable for the particular treatment options offered in 1975.
Schover and coworkers127 reported sexual outcomes for 61 women treated for stage Ia (8%), Ib (85%), or IIa (7%) cervical cancer. Of women, 26 received radical hysterectomy and 35 received radiotherapy; however, 19 of the latter also received surgery. There was a 20% refusal rate for study participation. Although data were gathered at 6 and 12 months after therapy, data were available only for 48% and 42% of subjects. The report does not indicate significant sexual disruption. Rates of loss of sexual desire are reported as 24% for the initial assessment and 25% at 6 months and 25% at 12 months. Similarly the rate of excitement problems at 12 months was 20% and 27% for orgasmic dysfunction. Despite these findings, the rates of sexual complaints were high. At 12 months, 21% noted that vaginal penetration was painful, 45% indicated deep thrusting was painful, and 24% reported postcoital vaginal pain. Also, 29% reported continued vaginal bleeding at 12 months. Sexual problems of this type have been reported to be significantly distressing and meriting diagnoses of sexual dysfunction in other studies.
Flay and Matthews128 studied 16 New Zealand women treated with surgery or radiotherapy for stages I through III cervical cancer. By 14 weeks after the end of radiotherapy, approximately two thirds of the women were moderately to extremely dissatisfied with the low frequency of sexual activity and their low levels of desire, arousal, and satisfaction. Women indicated the following problems as contributing to their difficulties: vaginal shortening (64%), dryness (43%), narrowing (43%), and bleeding (36%). Dyspareunia was reported by 43% of the sample.
Hansen129 in Denmark provided sexual outcome data for women treated with surgery, radiotherapy, or combined treatment for stages Ib or IIa cervical cancer. The outcome data are combined, and results are reported only for 78 women who had no sexual difficulties before therapy. Of this subset, 73% resumed intercourse and had no sexual complaints. Of the 27% who stopped intercourse, it seemed that 38% of them reported loss of desire, 17% had orgasmic dysfunction, 24% had dyspareunia, and 19% no longer had a sexual partner. The length of the time to follow-up was not reported.
The only report on psychological or sexual adjustment of African women comes from Adelusi,130 who prospectively studied 101 women treated for stage I through III cervical cancer at the University Hospital in Ibadan, Nigeria. The report is remarkable for its large sample; longitudinal assessment (pretreatment and 12 to 18 months’ post-treatment); and specific data on intercourse frequency, causes of difficulty, and marital and relationship status. Despite the modest statistical analyses, important findings indicated significant negative effects: 27% of the patients became separated or divorced after treatment, and 75% became sexually inactive. Reasons noted for sexual difficulty included dyspareunia, vaginal bleeding (36%), and fear of cancer or recurrence (18%). This magnitude of problems may be due to racial, cultural, or other related factors; however, this is difficult to discern because of the absence of other studies from Africa.
LONGITUDINAL STUDIES WITH COMPARISON GROUPS AND DATA.
Andersen and colleagues70,131 examined the nature and timing of sexual difficulties for women with early-stage disease and other quality-of-life outcomes. A total of 47 women with stage I or II cervical or endometrial disease were assessed before treatment and at 4, 8, and 12 months after treatment. Two matched comparison groups, women diagnosed and treated for benign disease (e.g., uterine fibroid treated with simple hysterectomy) and gynecologically healthy women, also were followed longitudinally. The former provided an estimate of sexual disruption resulting from disease in and treatment to the pelvis, and the latter estimated the base rate of sexual difficulties resulting from normal life circumstances.
Analyses indicated that the primary sexual behavior disrupted by the disease and treatment process for women with malignant or benign disease was the frequency of intercourse, declining from an average of 9.5 occasions per month to 6 to 7 occasions per month during the post-treatment period. The absence of change in other sexual behavior variables (e.g., range of current sexual activities) indicated that when couples engaged in intercourse, albeit less often, the women reported the same sexual activities (e.g., body caressing, oral-genital stimulation) as having occurred. There were no significant differences between groups in the percentage of women becoming sexually inactive, with the estimates ranging from 5% to 15% across the assessments. Half of the sexually inactive cases in the cancer group (i.e., two of four women) were due to disease-related causes, however. Considering the data on the sexual response cycle, difficulty with sexual excitement for both disease groups was substantial. A likely reason for the arousal deficits was the co-occurrence of significant disrupters (e.g., dyspareunia, resulting in part from radiation effects or induced menopause). By 12 months’ post-treatment, 30% to 40% of the sample had sexual dysfunctions, including inhibited desire, excitement, or orgasm and dyspareunia.
Weimar Schultz and associates132 studied women with stage Ia through IIIb cervical cancer. Comparison groups included 10 women treated with simple hysterectomy for benign disease, 21 gynecologically healthy women, and 12 women treated 1 year previously for cervical cancer. The benign group was followed longitudinally; however there was only one assessment for the healthy and previously treated cervical patients. Follow-up data were available only for 48% of the sample, resulting in 26 women. Analyses of 12-month post-treatment data indicated that there were no differences between the women with cancer and healthy women in the areas of current sexual activity or motivation for sexual activity; however, there were significant decrements in sexual arousal, disrupting genital sensations during intercourse, and increases in sexual dissatisfaction. However, This pattern of difficulties was similar to sexual disruptions reported by the women treated for benign disease. These outcomes for the women treated for disease are consistent with the data of Andersen and coworkers.131
A report by Kylstra and colleagues133 assessed a predominantly cervical cancer sample (73%; n = 58) with stage I or II disease before treatment and at 6 and 12 months after treatment. Most of the sample (76%) was treated with surgery alone. Neither sexual activity nor sexual satisfaction changed significantly from pretreatment to post-treatment. These positive outcomes may be accounted for by the circumstance of availability of sexuality information from the physician for virtually all (92%) of the women.
Predictors of Sexual Morbidity Risk
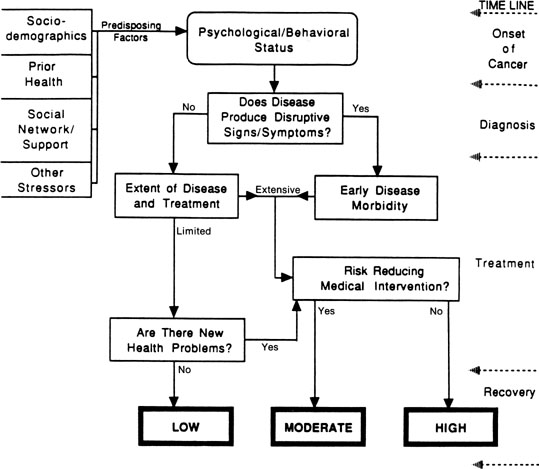
Across studies, we have been reminded of the significant role that medical factors, such as the extent of disease and magnitude of treatment, seem to play in determining sexual outcomes for women with gynecologic cancer. We have formulated a model of disease and treatment pathways that can yield differential levels of risk for sexual morbidity (Fig. 1). Variables are introduced according to a disease time line, from cancer appearance to the immediate post-treatment recovery period, as disease-relevant events provide a meaningful framework to understand psychological adjustment to cancer.134 Turning to the model, at the onset of cancer, we consider the occurrence of disruptive signs and symptoms. When the disease is detected and produces sexual disruption, this is the first point of psychological and behavioral morbidity. This early change is included because of its role in increasing a woman’s emotional distress and alerting her to the potential for subsequent life changes.
The model indicates that the extent of disease and treatment are major determinants of risk. As indicated, the disease and treatment status is summarized into routes of limited versus extensive; these alternatives should have supporting medical end point data and stage and magnitude of treatment information. The limited/extensive distinction is based in part on the classification of disease stage—localized versus regional or metastatic—and its direct relationship to survival in gynecologic cancer (i.e., across sites, 5-year survival rates are 89%, 54%, and 20% for localized, regional, and distant gynecologic disease).135 The model goes one step further and suggests that the extent of disease be considered in the context of the magnitude of treatment that is received. Women with localized or regional vulvar cancer often receive the same disfiguring surgical treatment—radical vulvectomy—and might differ only in the provision of radiotherapy for the groin nodes in the case of regional disease.
For women whose therapy cannot be modified, the model next considers the availability of risk-reducing medical interventions. For women with extensive disease and treatment, the availability of such interventions might reduce the level of risk from high to moderate. Examples of rehabilitative medical efforts include vaginal reconstruction for pelvic exenteration patients or labia preservation for vulvar cancer patients. For patients who have had such interventions, psychological, behavioral, and sexual outcomes are significantly better, although the interventions are not panaceas109 (see Berek and Andersen for a review136). For patients with limited disease and treatment, the availability of medical interventions could reduce their risk from low to approximately that of healthy individuals undergoing related experiences (i.e., the base rate). Some women experiencing a surgical menopause after treatment for localized cervical cancer can be treated with hormonal replacement therapy, similar to healthy women who experience a natural menopause with its attendant difficulties.137
The final contributor to risk is new health problems (e.g., hormonal changes or continuing stressors from the disease and treatment). Consideration of hormonal changes includes two issues: induced menopause and infertility. Most women who were premenopausal before gynecologic cancer become postmenopausal afterward either because of ovary removal or because of the inability to be treated with estrogens. Menopausal changes produce significant sexual effects.138 Also, ovary removal or sterilization ends childbearing—a potential stressor for a young woman with cancer. Continuing stressors can be heterogeneous. Examples include chronic fatigue or nausea and vomiting from toxic or lengthy chemotherapy regimens or late morbidities from treatments (e.g., a bowel fistula after pelvic irradiation). Should such problems arise, the model considers the availability of effective treatments. As can be seen, multiple pathways can lead to high or moderate risk for morbidity, but only patients who have limited disease and treatment and who have no new or continuing problems are hypothesized to have the lowest risk.
In addition to medical contributors, we have focused on identifying sexually relevant psychological factors in predicting risk for sexual morbidity. Before turning to this discussion, we note that the Figure 1 model begins with “baseline” sexual status. In addition to the individual difference variable described subsequently, straightforward predictors include such factors as age, sexual status (active or inactive), and prior frequency of important sexual activities (e.g., intercourse). These factors have emerged as important predictors of sexual activity in the earliest research on sexuality.139 These variables are important predictors in studies of healthy individuals, and their predictive utility is found in studies of individuals with chronic conditions and illnesses.140 We note these variables in preface to the subsequent discussion.
In the effort to identify a sexually relevant individual difference variable, we explored the concept of body image because it has been hypothesized as relevant to sexuality for healthy women141 and women with cancer.142 Our studies found, however, weak theoretical notions of the construct and poor operationalizations (i.e., measures of poor reliability and validity).143 In empirical tests, we found that measures could not predict outcome either among breast cancer patients144 or in multiple samples of women with gynecologic cancer.143 This finding led us away from considering a view of the body per se to a more central perspective—a woman’s view of herself as a sexual person.
We have proposed that sexual self-schema (self-concept) is a cognitive view about sexual aspects of the self; it is derived from past experience, it is manifest in current experience, and it guides the processing of domain-relevant social information.145 The concept includes two positive aspects—an inclination to experience romantic or passionate emotions and a behavioral openness to sexual experiences and relationships—and a negative aspect—embarrassment or conservatism, which seems to be a deterrent to sexual expression. Having developed a valid and reliable measure of sexual schema and tested it in the prediction of sexual behavior, attitudes, and responsiveness in healthy women, we wished to examine its predictive power in the context of risk for sexual morbidity after gynecologic cancer.
Consistent with our definition of the construct, we that found that women “low or negative” in sexual self-concept, in contrast to women with a “high or positive” sexual self-concept, are at greatest risk for sexual difficulty. We anticipated that women with more negative sexual schemata would have more difficulties because they are, in general, less romantic or passionate in their emotions, less open to sexual experiences, and more likely to have negative feelings about their sexuality. In the context of cancer, with disease or treatment factors causing direct changes to the sexual body or sexual responses, we anticipated that women with low sexual self-schemata would be at greater risk. Women with negative self views of their sexuality might find that their sexual arousability would worsen; they might be less apt to try new sexual activities as a way to cope with their sexual difficulties; or they may have more negative cognitions or feelings, such as embarrassment, about any body changes.
We tested these hypotheses with gynecologic146 and breast cancer patients.147 For the women with gynecologic cancer, 62 women who were currently disease-free but who had received treatment 6 months to 5 years previously for stage I or II disease were assessed. Comparison subjects included 68 women seeking routine gynecologic care. Analysis of the quality-of-life data replicated earlier prospective longitudinal findings70,131—specifically, sexuality is the major life area of disruption for the survivor. There were no differences between the groups in the areas of mental health (emotional distress, depression) or social functioning. The survivors did report, however, slightly more negative evaluations of their physical functioning and their perceptions of their health, yet there were no differences in reports of physical symptoms.
In contrast, a comparison between the samples in terms of current sexual functioning found significant differences, with the cancer sample reporting lower levels of sexual behavior, sexual responsiveness, and global evaluations. We tested the risk for morbidity model134 and the utility of the schema construct with the cancer sample in the prediction of sexual responsiveness (i.e., desire, excitement, orgasm, and resolution) and sexual behavior (frequency of intercourse) with regression analyses. Sexual self-schema accounted for a significant and large portion of the variance (26%) in the prediction of current sexual responsiveness. These general effects replicated in predicting sexuality and body change stress for women with breast cancer147 provide important, basic information on the processes of female sexuality and the specific relevance of the schema construct in understanding sexuality after gynecologic cancer.