Folliculogenesis is the process in which a recruited primordial follicle
grows and develops into a specialized graafian follicle with the potential
to either ovulate its egg into the oviduct at mid-cycle to be fertilized
or to die by atresia. In women, the process is long, requiring
almost 1 year for a primordial follicle to grow and develop to the
ovulatory stage. During the course of folliculogenesis, growth is achieved
by cell proliferation and formation of follicular fluid, whereas
development involves cytodifferentiation of all the cells and tissues
in the follicle. Only a few follicles in the human ovary survive to complete
the cytodifferentiation process, with 99.9% dying by a programmed
cell death mechanism called apoptosis. The mechanisms regulating follicle growth and development are under the
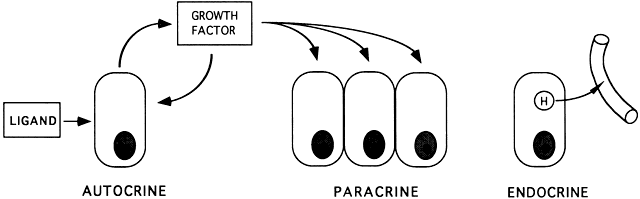
control of changing concentrations of ligands (i.e. hormones and growth factors). At the endocrine level, folliculogenesis
is regulated by a central nervous system, anterior pituitary, and ovary
cascade mechanism. Specialized hypothalamic neurons secrete pulses
of gonadotropin-releasing hormone (GnRH) into the portal blood vessels, which
acts on the gonadotrophs to cause a pulsatile release of follicle-stimulating
hormone (FSH) and luteinizing hormone (LH), which act
on ovarian follicle cells to control folliculogenesis. Although GnRH, FSH, and
LH are critically important in regulating folliculogenesis, hormones
and growth factors, which are themselves products of the follicle, can
act locally to modulate (amplify or attenuate) FSH and LH action. This
is the autocrine/paracrine system of developing follicles. It
is believed that this local regulatory system plays an important role
in the complex mechanisms governing the timing of folliculogenesis and
whether a follicle becomes dominant or atretic. Chronology
The steps and timing of human folliculogenesis are shown in Fig.
2. In women, folliculogenesis is a long process.1,2,3
In each menstrual cycle, the dominant follicle that ovulates its egg originates
from a primordial follicle that was recruited to initiate growth almost
1 year earlier (Fig.
2). In a broad sense, there are two types of follicles (Fig.
2): preantral (primordial, primary, secondary [class 1], tertiary
[class 2]) and antral (graafian, small [class 3, 4, 5], medium
[class 6], large [class 7], preovulatory [class 8]). The development of
preantral and antral follicles is gonadotropin independent and gonadotropin
dependent, respectively.
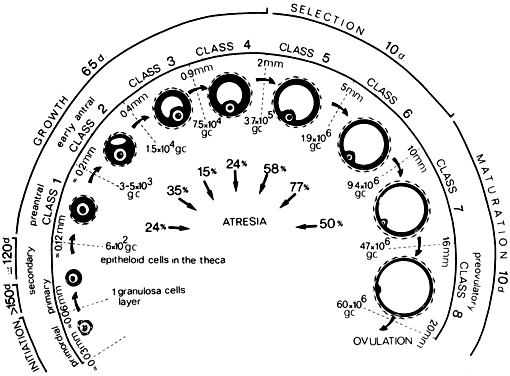 Fig. 2. Chronology of folliculogenesis in human ovaries. Notice the time line at
the periphery. Preantral period: It takes Fig. 2. Chronology of folliculogenesis in human ovaries. Notice the time line at
the periphery. Preantral period: It takes  300 days for a recruited primordial to grow and develop to the class 2/3 (0.4 mm) or
cavitation (early antrum) stage. Atresia can occur in preantral
class 1, 2, and 3 follicles. Antral period: A class 4 (1 to 2 mm) follicle, if selected, requires about 50 days to
grow and develop to the preovulatory stage. The dominant follicle of
the cycle appears to be selected from a cohort of class five follicles, and
it requires about 20 days to develop to the ovulatory stage. Atresia
is common during the antral period. gc, number of granulosa cells; d, days.(From Gougeon A: Dynamics of follicular growth in the human: A model from
preliminary results. Hum Reprod 1:81, 1986.) 300 days for a recruited primordial to grow and develop to the class 2/3 (0.4 mm) or
cavitation (early antrum) stage. Atresia can occur in preantral
class 1, 2, and 3 follicles. Antral period: A class 4 (1 to 2 mm) follicle, if selected, requires about 50 days to
grow and develop to the preovulatory stage. The dominant follicle of
the cycle appears to be selected from a cohort of class five follicles, and
it requires about 20 days to develop to the ovulatory stage. Atresia
is common during the antral period. gc, number of granulosa cells; d, days.(From Gougeon A: Dynamics of follicular growth in the human: A model from
preliminary results. Hum Reprod 1:81, 1986.)
|
The rate of preantral follicle development is slow, requiring about 300
days for a recruited primordial follicle to complete the whole preantral
period (Fig. 2).
A long doubling time (about 10 days) for the granulosa cells is responsible
for the slow growth rate. After antrum formation in the class 3 follicle
(about 0.4 mm in diameter), the rate of growth accelerates (Fig.
2). The time interval between antrum formation and the development
of a 20-mm preovulatory follicle is about 50 days (Fig.
2). The dominant follicle appears to be selected from a cohort of
class 5 follicles at the end of the luteal phase of the menstrual cycle.1,2,3,4
About 15 to 20 days are required for a dominant follicle to grow and develop
to the preovulatory stage (Fig.
2). Atresia can occur in all follicles (preantral and antral) after
the class 1 or secondary follicle stage; however, the highest incidence
is seen in the antral follicles that are more than 2 mm in diameter (i.e.
class 5, 6, and 7) (Fig.
2).
The Process Folliculogenesis occurs within the cortex of the ovary (Fig. 3). The follicles in the cortex are present in a wide range of sizes representing
various stages of folliculogenesis. The goal of folliculogenesis
is to produce a single dominant follicle from a pool of growing follicles. There
are four major regulatory events involved in this process: recruitment, preantral follicle development, selection, and atresia.  Fig. 3. Photomicrograph of an adult primate ovary. Follicular and luteal units
are seen in the cortex and large blood vessels and nerves in the medulla. se, serous
or surface epithelium; ta, tunica albuginea; pf, primary
follicle; sf, secondary follicle; tf, tertiary follicle; gf, graafian
follicle.(From Bloom W, Fawcett DW: A Textbook of Histology. Philadelphia: WB Saunders, 1975.) Fig. 3. Photomicrograph of an adult primate ovary. Follicular and luteal units
are seen in the cortex and large blood vessels and nerves in the medulla. se, serous
or surface epithelium; ta, tunica albuginea; pf, primary
follicle; sf, secondary follicle; tf, tertiary follicle; gf, graafian
follicle.(From Bloom W, Fawcett DW: A Textbook of Histology. Philadelphia: WB Saunders, 1975.)
|
THE PRIMORDIAL FOLLICLE. All primordial follicles are composed of a small primary oocyte (about 25 μm
in diameter) arrested in the diplotene (or dictyate) stage of
meiosis, a single layer of flattened (squamous) granulosa cells, and
a basal lamina (Fig. 4). The mean diameter of the human primordial follicle is 29 μm.5 By virtue of the basal lamina, the granulosa and oocyte exist within a
microenvironment in which direct contact with other cells does not occur. The
primordial follicles do not have an independent blood supply.6 It follows that primordial follicles have limited access to the endocrine
system. 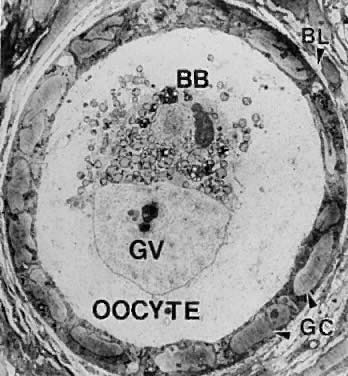 Fig. 4. Electron micrograph of a human primordial follicle shows the flattened
granulosa cells (GC), the oocyte with its germinal vesicle (GV) or nucleus, the
Balbiani body (BB), with all the oocyte organelles gathered
at one pole of the GV, and basal lamina (BL).(From Erickson GF: The ovary: Basic principles and concepts. In Felig P, Baxter
JD, Frohman L (eds): Endocrinology and Metabolism. New York: McGraw-Hill, 1995.) Fig. 4. Electron micrograph of a human primordial follicle shows the flattened
granulosa cells (GC), the oocyte with its germinal vesicle (GV) or nucleus, the
Balbiani body (BB), with all the oocyte organelles gathered
at one pole of the GV, and basal lamina (BL).(From Erickson GF: The ovary: Basic principles and concepts. In Felig P, Baxter
JD, Frohman L (eds): Endocrinology and Metabolism. New York: McGraw-Hill, 1995.)
|
Recruitment. The first major event in folliculogenesis is recruitment. Recruitment
is the process by which an arrested primordial follicle is triggered to
reinitiate development and enter the pool of growing follicles. All
primordial follicles (oocytes) present in the human ovaries are formed
in the fetus between the sixth and the ninth month of gestation. Because
the entire stock of oocytes in primordial follicles is in meiotic
prophase, none is capable of dividing mitotically. All oocytes (primordial
follicles) capable of participating in reproduction during a woman's
life are present in the ovaries at birth (Fig. 5). The total number of primordial follicles in the ovaries at any given
moment of time is called the ovary reserve (OR).7 The process of recruitment begins soon after the formation of the primordial
follicles in the fetus,8 and it continues throughout the life of the female until the pool of primordial
follicles is exhausted at the menopause (Fig. 5). There is a bi-exponential decrease in OR during aging7,9,10 (Fig. 6). The number of primordial follicles falls steadily for more than three
decades, but when the OR reaches a critical number of about 25,000 at 37.5 ± 1.2 years
of age, the rate of loss of primordial follicles
accelerates about twofold (Fig. 6). This change in OR is associated in an age-related decrease in fecundity, perhaps
causal to the age-related increase in FSH that occurs in
women after 36 years of age.7 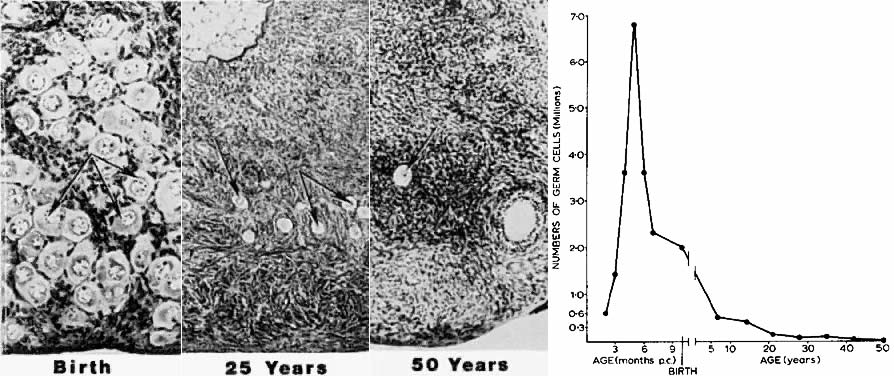 Fig. 5. Age-dependent changes in the number of primordial follicles (oocytes) in
human ovaries. Left panel: The number of eggs decreases from 6 months of gestation to 50 years of
age. (From Baker TG: Radiosensitivity of mammalian oocytes with particular
reference to the human female. Am J Obstet Gynecol 110:746, 1971.) Right panel: Photomicrographs illustrating the age-dependent decrease in primordial
follicles ( arrows) in human ovaries.(From Erickson GF: Analysis of follicle development and ovum maturation. Semin
Reprod Endocrinol 4:233, 1986.) Fig. 5. Age-dependent changes in the number of primordial follicles (oocytes) in
human ovaries. Left panel: The number of eggs decreases from 6 months of gestation to 50 years of
age. (From Baker TG: Radiosensitivity of mammalian oocytes with particular
reference to the human female. Am J Obstet Gynecol 110:746, 1971.) Right panel: Photomicrographs illustrating the age-dependent decrease in primordial
follicles ( arrows) in human ovaries.(From Erickson GF: Analysis of follicle development and ovum maturation. Semin
Reprod Endocrinol 4:233, 1986.)
|
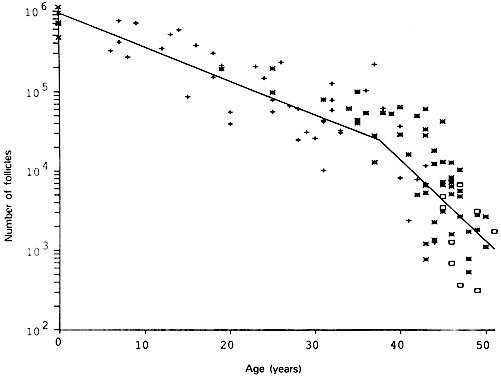 Fig. 6. The age-related decrease in the number of primordial follicles (PF) within
both human ovaries from birth to the menopause. As a consequence of
recruitment, the PF number decreases progressively from about 1,000,000 at
birth to about 24,000 at 37 years. At 37, the rate of recruitment
accelerates approximately twofold, and the number of PF declines to
about 1000 at 51 years ( i.e. the mean age for the onset of menopause).(From Faddy MJ, Gosden RG, Gougeon A et al: Accelerated disappearance of
ovarian follicles in mid-life: Implications for forecasting menopause. Hum
Reprod 7:1342, 1992.) Fig. 6. The age-related decrease in the number of primordial follicles (PF) within
both human ovaries from birth to the menopause. As a consequence of
recruitment, the PF number decreases progressively from about 1,000,000 at
birth to about 24,000 at 37 years. At 37, the rate of recruitment
accelerates approximately twofold, and the number of PF declines to
about 1000 at 51 years ( i.e. the mean age for the onset of menopause).(From Faddy MJ, Gosden RG, Gougeon A et al: Accelerated disappearance of
ovarian follicles in mid-life: Implications for forecasting menopause. Hum
Reprod 7:1342, 1992.)
|
Mechanism. The first visible sign (Fig. 7) that a primordial follicle is being recruited is that some granulosa
cells begin to change from a squamous to a cuboidal shape.5 The first cuboidal cell is seen when the primordial follicle contains 8 granulosa
cells, and the process is complete when the granulosa number
reaches 19 (Fig. 8). The shape change is followed by the onset, albeit slow, of DNA synthesis
and mitosis in the granulosa cells.8 A change in shape and acquisition of mitotic potential in the granulosa
cells are the hallmarks of recruitment. Such observations suggest that
the mechanisms governing recruitment may involve a regulatory response
at the level of the granulosa cell. Recruitment is pituitary independent, and
it probably is controlled by autocrine/paracrine mechanisms. Whether
it is effected by a stimulator or the loss of an inhibitor
is uncertain; however, primordial follicles undergo rapid recruitment
when removed from the ovary and cultured in vitro.11 These observations support the inhibitor idea. 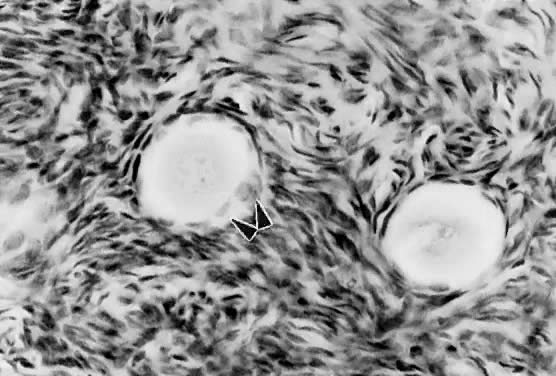 Fig. 7. Photomicrograph of nongrowing primordial and a newly recruited (growing) follicle
in the human ovary. Notice the cuboidal granulosa cells ( arrowheads) in the newly recruited primordial follicle. Fig. 7. Photomicrograph of nongrowing primordial and a newly recruited (growing) follicle
in the human ovary. Notice the cuboidal granulosa cells ( arrowheads) in the newly recruited primordial follicle.
|
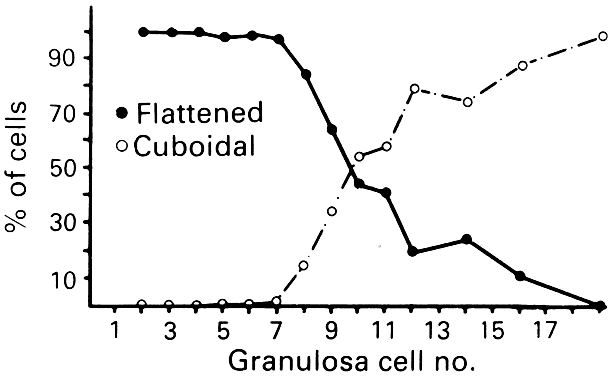 Fig. 8. Relation between granulosa number in the largest cross section of the follicle
and the distribution of flattened and cuboidal cells.(From Gougeon A, Chainy GBN: Morphometric studies of small follicles in
ovaries of women at different ages. J Reprod Fertil 81:433, 1987.) Fig. 8. Relation between granulosa number in the largest cross section of the follicle
and the distribution of flattened and cuboidal cells.(From Gougeon A, Chainy GBN: Morphometric studies of small follicles in
ovaries of women at different ages. J Reprod Fertil 81:433, 1987.)
|
Several different hypotheses have been put forth to explain the mechanism
of recruitment. First, the process appears to occur in primordial follicles
nearest the medulla where blood vessels are prominent. This supports
the hypothesis that exposure to nutrients or blood-borne regulatory
molecules could play a role in the control of recruitment. Second, an
internal oocyte clock mechanism has been proposed to control recruitment.12 In this hypothesis, the clock is related to the time that the oocyte initiates
meiosis in the embryo. It is noteworthy that recruitment can
be modulated.8 In rodents, the rate of recruitment can be attenuated by removing the
neonatal thymus gland, starvation, or treatment with exogenous opioid
peptides. These are important observations, because they argue that ligand-receptor
signaling pathways are likely to regulate recruitment. Understanding
the regulatory mechanisms underlying recruitment remains
a major task in reproductive biology. THE PREANTRAL FOLLICLE. The early stages of folliculogenesis can be divided into three classes
based on the number of layers of granulosa cells, the development of
theca tissue, and the expression of a small cavity or antrum. The classes
are the primary, secondary, and early tertiary follicles (Fig. 9). As the morphologic complexity increases, important cellular and physiologic
changes occur in the follicle that render it competent to respond
to gonadotropins. The following sections examine the structure and
function changes that accompany preantral follicle growth and development. 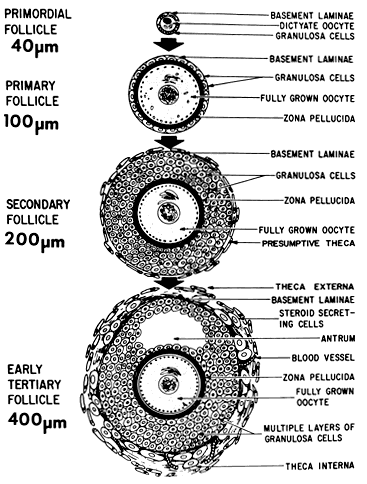 Fig. 9. Diagram illustrating the size and histologic organization of early developing
human follicles during the gonadotropin-independent period of folliculogenesis.(Erickson GF: The ovary: Basic principles and concepts. In Felig P, Baxter
JD, Frohman L (eds): Endocrinology and Metabolism. New York: McGraw-Hill, 1995.) Fig. 9. Diagram illustrating the size and histologic organization of early developing
human follicles during the gonadotropin-independent period of folliculogenesis.(Erickson GF: The ovary: Basic principles and concepts. In Felig P, Baxter
JD, Frohman L (eds): Endocrinology and Metabolism. New York: McGraw-Hill, 1995.)
|
Primary Follicle. A primary follicle consists of one or more cuboidal granulosa cells that
are arranged in a single layer surrounding the oocyte (Fig. 10). Simultaneous with the shape change and mitotic activities that accompany
recruitment (Figs. 7 and 10), the cuboidal granulosa cells begin to express FSH receptors.13,14 The mechanism underlying this critical event in folliculogenesis remains
uncertain, but there is evidence in rodents15 that granulosa-derived activin may play an important role in the expression
of FSH receptor by autocrine/paracrine mechanisms (Fig. 11). Although the granulosa cells express FSH receptors at this very early
stage in folliculogenesis, it is believed that the physiologic levels
of plasma FSH during the normal menstrual cycle do not influence granulosa
responses because primary follicles lack an independent vascular
system. Nevertheless, because there are blood vessels in the vicinity (Fig. 10), FSH-induced changes in primary follicle function may occur in response
to abnormally high levels of plasma FSH, such as those that occur during
ovulation induction or aging. 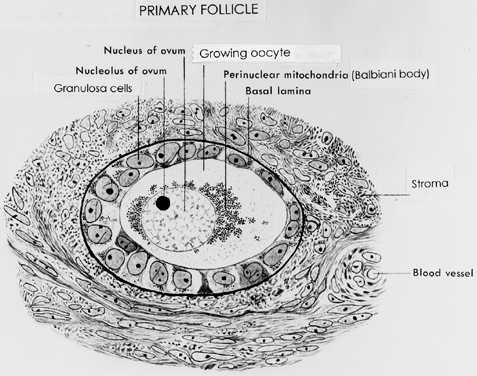 Fig. 10. Drawing of a developing primary follicle embedded in the connective tissue
or stroma of the ovary cortex. A nucleolus and meiotic chromosomes
are evident in the oocyte nucleus. The mitochondria appear aggregated
at one pole of the oocyte nucleus ( i.e. Balbinni body). A total of 19 cuboidal granulosa cells are seen, one of
which is giving rise to a second layer of cells.(From Bloom W, Fawcett DW: A Textbook of Histology. Philadelphia: WB Saunders, 1975.) Fig. 10. Drawing of a developing primary follicle embedded in the connective tissue
or stroma of the ovary cortex. A nucleolus and meiotic chromosomes
are evident in the oocyte nucleus. The mitochondria appear aggregated
at one pole of the oocyte nucleus ( i.e. Balbinni body). A total of 19 cuboidal granulosa cells are seen, one of
which is giving rise to a second layer of cells.(From Bloom W, Fawcett DW: A Textbook of Histology. Philadelphia: WB Saunders, 1975.)
|
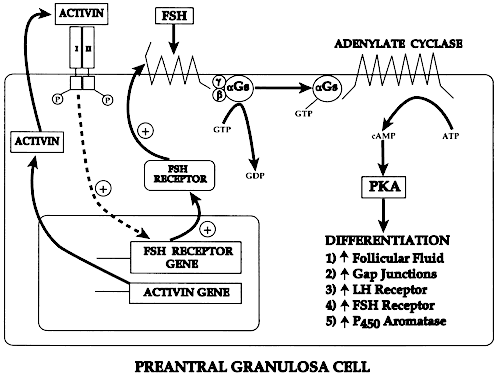 Fig. 11. Diagram of the proposed mechanism for the autocrine control of follicle-stimulating
hormone receptor expression in granulosa cells of preantral
follicles.(From Erickson GF: Dissociation of endocrine and gametogenic ovarian function. In
Lobo R (ed): Perimenopause. New York: Springer-Verlag, 1997.) Fig. 11. Diagram of the proposed mechanism for the autocrine control of follicle-stimulating
hormone receptor expression in granulosa cells of preantral
follicles.(From Erickson GF: Dissociation of endocrine and gametogenic ovarian function. In
Lobo R (ed): Perimenopause. New York: Springer-Verlag, 1997.)
|
Beginning approximately at the time of recruitment, the oocyte begins to
grow and differentiate. This period is marked by a progressive increase
in the level of oocyte RNA synthesis.16 A number of important oocyte genes are turned on at this time. For example, the
genes encoding the zona pellucida (ZP) proteins (i.e. ZP-1, ZP-2, and ZP-3) are transcribed and translated.17 The secreted ZP proteins begin to polymerize near the oocyte surface, forming
an extracellular matrix coat (the zona pellucida) that eventually
encapsulates the egg. The importance of the zona pellucida is emphasized
by the fact that the carbohydrate moiety of ZP-3 is the species-specific
sperm-binding molecule.18 It is responsible for initiating the acrosome reaction in capacitated
sperm.19 During primary follicle development, the granulosa cells send processes
through the zona layer, where they form gap junctions with the oocyte
cell membrane, or oolemma (Fig. 12). Gap junctions are intercellular channels composed of proteins called
connexins.20,21 There are at least 13 members of the connexin family that directly couple
adjacent cells to allow the diffusion of ions, metabolites, and other
low-molecular-weight signaling molecules such as cAMP and calcium.20,21 Connexin 37 (C×37) is an oocyte-derived connexin that forms gap
junctions between the oocyte and surrounding granulosa cells.22 Evidence from C×37-deficient mice assigns C×37 an obligatory
role for folliculogenesis, ovulation, and fertility.22 Large gap junctions are also present between the granulosa cells themselves (Fig. 12). C×43 is a major gap junction protein expressed in the granulosa
cells.23 As a consequence of gap junctions, the primary follicle becomes a metabolically
and electrically coupled unit. This communication between the
granulosa and oocyte remains throughout folliculogenesis and is responsible
for the synchronous expression of important activities (positive
and negative). 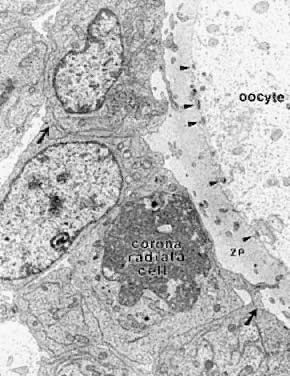 Fig. 12. Electron micrograph of the oocyte-corona radiata granulosa cells in a preantral
follicle. The granulosa cell processes traversing the zona pellucida (ZP) make
small gap junctions ( arrowheads) with the oocyte plasma membrane. Larger gap junctions (arrows) are evident between corona radiata cells.(Gilula NB, Epstein ML, Beers WH: Cell-to-cell communication and ovulation: A
study of the cumulus-oocyte complex. J Cell Biol 78:58, 1978, reproduced
with permission from the Rockefeller University Press.) Fig. 12. Electron micrograph of the oocyte-corona radiata granulosa cells in a preantral
follicle. The granulosa cell processes traversing the zona pellucida (ZP) make
small gap junctions ( arrowheads) with the oocyte plasma membrane. Larger gap junctions (arrows) are evident between corona radiata cells.(Gilula NB, Epstein ML, Beers WH: Cell-to-cell communication and ovulation: A
study of the cumulus-oocyte complex. J Cell Biol 78:58, 1978, reproduced
with permission from the Rockefeller University Press.)
|
Secondary Follicle. A secondary follicle is a preantral follicle with 2 to 10 layers of cuboidal
or low columnar cells that form a stratified epithelium (Fig. 13). As seen in Figure 10, the transition from a primary to a secondary follicle involves the acquisition
of a second layer of granulosa cells. This transition is accomplished
by the continuing division of the granulosa cells. The mechanisms
regulating granulosa mitosis are poorly understood. However, exciting
research in rodents has provided compelling evidence for the involvement
of an oocytederived growth factor, called growth differentiation
factor-9 (GDF-9). GDF-9 is a novel member of the transforming growth
factor-β (TGF-β) superfamily.24 GDF-9 is strongly expressed in the ovary; it is localized only in oocytes
of recruited follicles.25 In GDF-9 deficient mice, follicle growth and development stop at the primary
stage; consequently no dominant follicles form, and the females
are infertile.26 Accordingly, GDF-9 is obligatory for folliculogenesis after the primary
stage, presumably because it is an obligatory mitogen for granulosa
cells. A fundamental concept that emerges from this work is that the oocyte
plays a pivotal role in regulating folliculogenesis through its
ability to produce novel regulatory ligands (e.g. GDF-9), which are crucial for folliculogenesis. 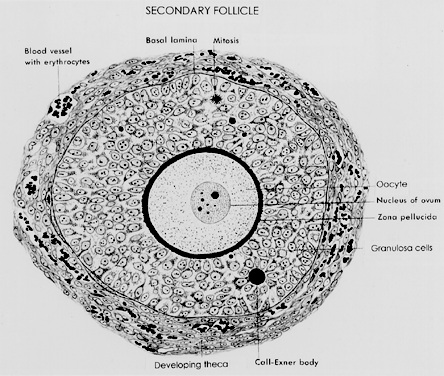 Fig. 13. A typical healthy secondary follicle contains a fully grown oocyte surrounded
by the zona pellucida, five to eight layers of granulosa cells, a
basal lamina, and developing theca tissue with numerous blood vessels.(From Bloom W, Fawcett DW: A Textbook of Histology. Philadelphia: WB Saunders, 1975, with permission from Arnold Ltd.) Fig. 13. A typical healthy secondary follicle contains a fully grown oocyte surrounded
by the zona pellucida, five to eight layers of granulosa cells, a
basal lamina, and developing theca tissue with numerous blood vessels.(From Bloom W, Fawcett DW: A Textbook of Histology. Philadelphia: WB Saunders, 1975, with permission from Arnold Ltd.)
|
One of the most important changes that occur in the development of a secondary
follicle is the acquisition of a theca layer. This tissue, which
consists of a layer of stroma-like cells around the basal lamina, subsequently
differentiates into the inner theca interna and outer theca
externa (Fig. 13). Theca development is accompanied by the neoformation of numerous small
vessels, presumably through angiogenesis (Fig. 13). This is a critical event because blood circulates around the follicle, bringing
nutrients and hormones (e.g. FSH, LH) to and waste and secretory products from the secondary follicle. In
this regard, some stromal cells in the inner layer express LH receptors.27 These cells subsequently differentiate into steroidogenic cells called
theca interstitial cells (TICs), most likely in response to the plasma
LH delivered by the theca vascular system.27 All the granulosa cells in secondary follicles express FSH receptors.13 It seems likely that diffusion of plasma FSH into the secondary follicle
may evoke FSH-dependent granulosa responses. The outer layer of stroma
cells subsequently differentiates into smooth muscle cells called
the theca externa. These smooth muscle cells are innervated by the autonomic
nervous system.27 In the secondary follicle, the oocyte completes its growth. When the follicle
is about 200 μm in diameter, the oocyte has attained its maximum
size and grows no more, despite the fact that the human follicle
enlarges to a diameter of 2 cm or more (Fig. 14). It is well documented in rodents that granulosa cells play an obligatory
role in the growth and differentiation of the oocyte.28,29 An important differentiation event that occurs when the oocyte completes
its growth is acquisition of the capacity to resume meiosis.30 Oocytes normally do not resume meiosis during folliculogenesis, and a
mechanism must operate to inhibit this process (i.e. germinal vesicle breakdown [GVBD]) and the resumption of meiosis. The
underlying mechanism for the inhibition remains unknown; however, there
is evidence to support the concept that granulosa derived
cAMP may play an important role in inhibiting the resumption of meiosis.30 In such a mechanism, FSH-induces cAMP in the granulosa cells, which diffuses
into the oocyte through the C×37 gap junction, where it proceeds
to inhibit GVBD (Fig. 15). 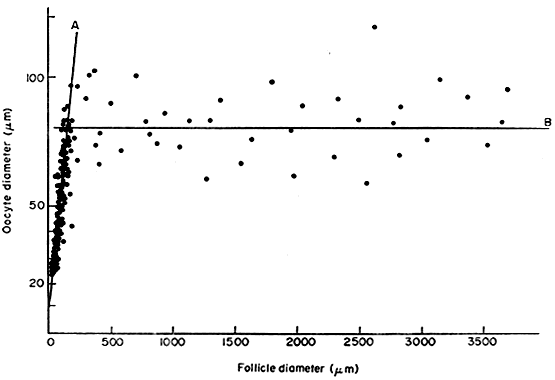 Fig. 14. Diagram showing the relation between the size of the oocyte and the size
of the follicles in the human infant ovary.(From Mandl AM, Zuckerman S: The growth of the oocyte and follicle in the
adult rat. J Endocrinol 8:126, 1952, reproduced by permission from
the Society for Endocrinology.) Fig. 14. Diagram showing the relation between the size of the oocyte and the size
of the follicles in the human infant ovary.(From Mandl AM, Zuckerman S: The growth of the oocyte and follicle in the
adult rat. J Endocrinol 8:126, 1952, reproduced by permission from
the Society for Endocrinology.)
|
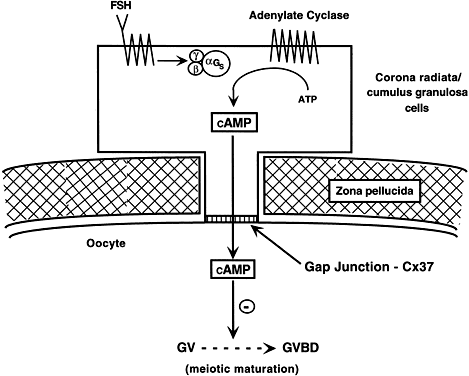 Fig. 15. A diagram of the hypothetical mechanism for the cyclic AMP (cAMP) inhibition
of germinal vesicle breakdown (GVBD) or resumption of meoisis. Follicle-stimulating
hormone (FSH) receptor signal transduction in the
granulosa cells leads to increased cAMP production. The cAMP can diffuse
through the granulosa-oocyte connexin-37 (C×37) gap junctions, where
it accumulates at high levels in the ooplasm to inhibit the breakdown (BD) of
the germinal vesicle (GV) ( i.e. inhibits the resumption of meiosis or GVBD). Fig. 15. A diagram of the hypothetical mechanism for the cyclic AMP (cAMP) inhibition
of germinal vesicle breakdown (GVBD) or resumption of meoisis. Follicle-stimulating
hormone (FSH) receptor signal transduction in the
granulosa cells leads to increased cAMP production. The cAMP can diffuse
through the granulosa-oocyte connexin-37 (C×37) gap junctions, where
it accumulates at high levels in the ooplasm to inhibit the breakdown (BD) of
the germinal vesicle (GV) ( i.e. inhibits the resumption of meiosis or GVBD).
|
Tertiary Follicle. When a preantral follicle completes the secondary stage in development, it
contains five distinct structural units: a fully grown oocyte surrounded
by a zona pellucida, six to nine layers of granulosa cells, a
basal lamina, a theca interna, and a theca externa (Fig. 13). The first indication of the onset of tertiary follicle development is
the appearance of a cavity in the granulosa cells.31 In response to an intrinsic stimulus, a cavity begins to form at one pole
of the oocyte. This process, called cavitation or beginning antrum
formation, is characterized by the accumulation of fluid between the
granulosa cells that in time results in the formation of an internal cavity (Fig. 16). At completion of cavitation, the basic plan of the graafian follicle
is established, and all the various cell types are in their proper position
awaiting the stimuli that will shift them along paths of differentiation
and proliferation (Fig. 16). Based on evidence from polyoocyte follicles, the specification mechanism
of cavitation probably is tightly regulated (Fig. 17). 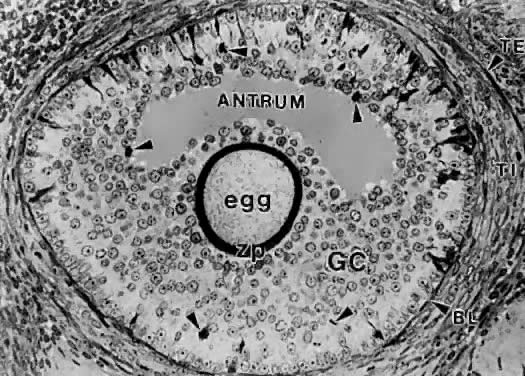 Fig. 16. Photomicrograph of an early tertiary follicle (0.4 mm in diameter) at the
cavitation of early antrum stage. ZP, zona pellucida; GC, granulosa
cells; BL, basal lamina; TI, theca interna; TE, theca externa; arrowheads, granulosa mitosis.(From Bloom W, Fawcett DW: A Textbook of Histology. Philadelphia: WB Saunders, 1975, with permission from Arnold Ltd.) Fig. 16. Photomicrograph of an early tertiary follicle (0.4 mm in diameter) at the
cavitation of early antrum stage. ZP, zona pellucida; GC, granulosa
cells; BL, basal lamina; TI, theca interna; TE, theca externa; arrowheads, granulosa mitosis.(From Bloom W, Fawcett DW: A Textbook of Histology. Philadelphia: WB Saunders, 1975, with permission from Arnold Ltd.)
|
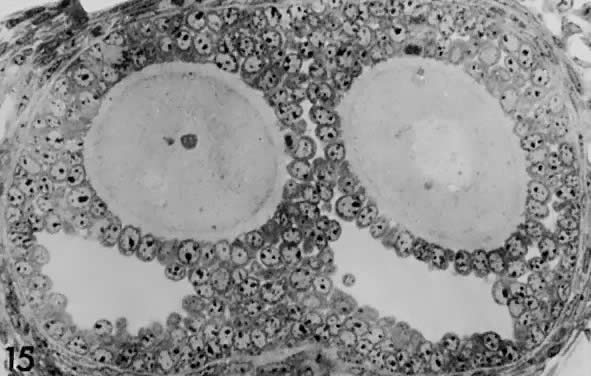 Fig. 17. Photomicrograph of a polyovular follicle at the early tertiary stage shows
the sites of cavitation or early antrum formation ( clear spaces) just above oocytes ( asterisk ). This event, which is under intraovarian control, seems to arise in
a specific synchronized manner and establishes the polarity of the follicle.(From Zamboni L: Comparative studies on the ultra-structure of mammalian
oocytes. In Biggers JD and Schultz AW (eds): Oogenesis. Baltimore: University Park Press, 19972.) Fig. 17. Photomicrograph of a polyovular follicle at the early tertiary stage shows
the sites of cavitation or early antrum formation ( clear spaces) just above oocytes ( asterisk ). This event, which is under intraovarian control, seems to arise in
a specific synchronized manner and establishes the polarity of the follicle.(From Zamboni L: Comparative studies on the ultra-structure of mammalian
oocytes. In Biggers JD and Schultz AW (eds): Oogenesis. Baltimore: University Park Press, 19972.)
|
What controls cavitation or early antrum formation? It is well known that
cavitation occurs in hypophysectomized animals, demonstrating that
pituitary hormones such as FSH are not required for this morphogenetic
event.32 Consistent with this concept is the observation that cavitation occurs
in FSH-β-deficient mice.33,34 It seems reasonable to conclude that cavitation is controlled by autocrine/paracrine
mechanisms. Two growth factors expressed in the follicle
itself have been implicated in cavitation: activin and KIT ligand. Treating
cultured granulosa cells with activin causes morphogenetic changes
that result in the formation of a histologic unit with an antrum-like
cavity.35 Blocking the action of the KIT ligand in the ovary prevents the formation
of antral follicles; consequently, there are no ovulations, and the
female is infertile.36 In this regard, evidence supports the concept that the oocyte gap junctions
are also important for cavitation. Gap junctions are intercellular
channels composed of proteins called connexins.20,21 There are at least 13 members of the connexin family that directly couple
adjacent cells, allowing diffusion of ions, metabolites, and other
low-molecular-weight signaling molecules such as cAMP.20,21 C×37 appears to be an oocyte-derived connexin that forms gap junctions
between the oocyte and surrounding granulosa cells. Evidence from
C×37-deficient mice assigns to C×37 an obligatory role
in graafian follicle formation, ovulation, and fertility.22 Collectively, all this evidence suggests that follicle-derived activin, KIT, and
C×37 are involved in the autocrine/paracrine mechanisms
that control cavitation. THE GRAAFIAN FOLLICLE. A graafian follicle can be defined structurally as a heterogeneous family
of relatively large follicles (0.4 to 23 mm) characterized by a cavity
or antrum containing a fluid called follicular fluid or liquor folliculi. The
characteristic structural unit of all graafian follicle is
the antrum. For this reason, the term antral follicle is used correctly
as a synonym for graafian follicle. The follicular fluid is the medium
in which the granulosa cells and oocyte are found and through which
regulatory molecules must pass on their way to and from this microenvironment.37 Surprisingly, we know almost nothing about the physiologic significance
of the antrum and follicular fluid in folliculogenesis. It is clear
that follicle development and ovulation occur in birds and amphibians
despite the absence of an antrum and follicular fluid. Nonetheless, its
presence in all mammalian species testifies to its physiologic importance. Structure. A graafian follicle is a three-dimensional structure with a central antrum
surrounded by a variety of different cell types (Fig. 18). There are six distinct histologic components in the graafian follicle, including
the theca externa, theca interna, basal lamina, granulosa
cells, oocyte, and follicular fluid (Fig. 18). A graafian follicle does not change its morphologic complexity as growth
proceeds. All graafian follicles have this same basic architecture; even
though there are dramatic changes in graafian follicle size, their
appearance remains more or less the same. 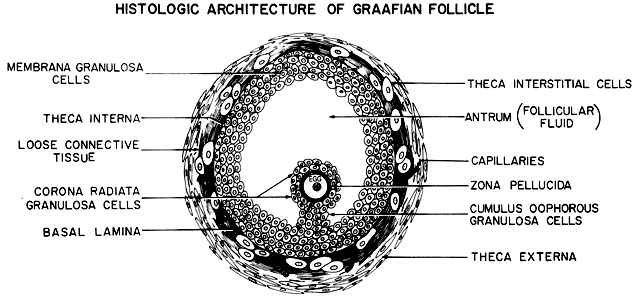 Fig. 18. Diagram of the architecture of a typical class 5 graafian follicle.(From Erickson GF: The ovary: Basic principles and concepts. In Felig P, Baxter
JD, Broadus AE, Froman LA, (eds): Endocrinology and Metabolism. 3rd ed. New York: McGraw-Hill, 1987.) Fig. 18. Diagram of the architecture of a typical class 5 graafian follicle.(From Erickson GF: The ovary: Basic principles and concepts. In Felig P, Baxter
JD, Broadus AE, Froman LA, (eds): Endocrinology and Metabolism. 3rd ed. New York: McGraw-Hill, 1987.)
|
The theca externa (Fig. 19) is characterized by the presence of smooth muscle cells,38,39 which are innervated by autonomic nerves.27 Although the physiologic significance of the theca externa remains unclear, there
is evidence that it contracts during ovulation and atresia.40,41 Changes in the contractile activity of the theca externa may be involved
in atresia and ovulation; however, this has not been rigorously proved. The
corpus luteum retains a theca externa throughout its life,42 but the significance during luteinization and luteolysis is not known.  Fig. 19. Drawing of the wall of a graafian follicle.(From Bloom W, Fawcett DW: A Textbook of Histology. Philadelphia: WB Saunders, 1975.) Fig. 19. Drawing of the wall of a graafian follicle.(From Bloom W, Fawcett DW: A Textbook of Histology. Philadelphia: WB Saunders, 1975.)
|
The theca interna is composed of differentiated TICs located within a matrix
of loose connective tissue and blood vessels (Fig. 19). In all graafian follicle, LH is a key regulatory hormone for TIC function, and
its importance in regulating TIC androgen production in vivo and in vitro has been established.27 Beginning at the very early stages of graafian follicle development, the
TICs express their differentiated state as androgen (i.e. androstenedione-producing cells).27 The theca interna is richly vascularized and serves to deliver hormones (e.g. FSH, LH), nutrient molecules, vitamins, and cofactors required for the
growth and differentiation of the oocyte and granulosa cells. We know little about the regulatory elements that control the theca vasculature. A
functional link between the vasculature and graafian follicle
development is suggested by the evidence43 that all monkey graafian follicles express high levels of FSH and LH receptor
regardless of size, but when 125I-human chorionic gonadotropin (hCG) is injected systemically, only the
dominant graafian follicle appears capable of accumulating 125I-hCG in the theca interna. These results suggest that the dominant graafian
follicle expresses increased vascularization, which plays an important
role in its selected maturation. In this regard, follicle-derived
vascular endothelial growth factor44,45 and other angiogenic factors such as endothelin46 are being intensively investigated. The theca compartments (i.e. theca externa and interna) express their differentiated functions at the
beginning of graafian follicle development (at cavitation) and appear
to constitutively express a mature phenotype throughout the life and
death of the graafian follicle. In a broad sense, there is little or
no evidence that major changes occur in the theca layers during the various
stages of graafian follicle development beyond those related to
vascular and proliferative activities. This could imply that it is the
granulosa cells (and perhaps the oocyte) that are variable and therefore
responsible for graafian follicle diversity. In the graafian follicle, the granulosa cells and oocyte exist as a mass
of precisely shaped and precisely positioned cells (Fig. 18). The spatial variation creates at least four different granulosa cell
layers or domains: the outermost domain is the membrana granulosa, the
inner most domain is the periantral, the intermediate domain is the
cumulus oophorus, and the domain juxtaposed to the oocyte is the corona
radiata (Fig. 20). A characteristic histologic property of the membrana domain is that
it is composed of a pseudostratified epithelium of tall columnar granulosa
cells, all of which are anchored to the basal lamina. 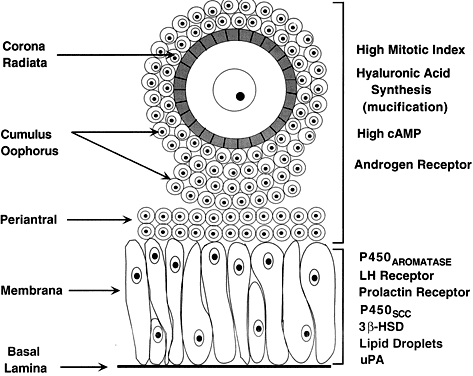 Fig. 20. Diagram of the structure and function heterogeneity of the granulosa cells
in a healthy graafian follicle. The relative position of a granulosa
cell in the cellular mass determines its proliferation and differentiation
potential.(From Erickson GF: The graafian follicle: A functional definition. In Adashi
EY (ed): Ovulation: Evolving Scientific and Clinical Concepts. New York: Springer-Verlag, 2000.) Fig. 20. Diagram of the structure and function heterogeneity of the granulosa cells
in a healthy graafian follicle. The relative position of a granulosa
cell in the cellular mass determines its proliferation and differentiation
potential.(From Erickson GF: The graafian follicle: A functional definition. In Adashi
EY (ed): Ovulation: Evolving Scientific and Clinical Concepts. New York: Springer-Verlag, 2000.)
|
The differentiation of a granulosa cell can be traced to its position within
the cellular mass (Fig. 20). For example, cells in the membrana domain stop proliferating before
those in central domain.47,48 The ability of the granulosa cells in the inner domains to continue dividing
throughout graafian follicle development suggests they may be precursor
cells. The cessation of mitosis in the membrana domain is characterized
by the progressive expression of overt differentiation in which
they assume the functional phenotype of fully differentiated cells. This
process requires the temporal and coordinate expression of genes
that form the basis of granulosa cytodifferentiation. The mechanisms
by which this occurs involves ligand-dependent signaling pathways that
are coupled to the activation and inhibition of specific genes. For
example, normal differentiation of the membrana granulosa cells requires
the activation of specific genes, including those for cytochrome P450 aromatase (P450arom)49 and the LH receptor,50 and the inhibition of structural genes in the apoptotic pathway. In contrast, the
granulosa cells in the periantral, cumulus, and corona radiata
domains proliferate, but they fail to express the genes that are
involved in a terminal differentiation (Fig. 20). What controls granulosa heterogeneity? All the granulosa cells in the healthy
graafian follicle express FSH receptor,13,51,52 and it has been shown that murine granulosa cells in the membrana and
cumulus domains produce cAMP in response to FSH stimulation.53 These observations argue that post cAMP regulatory events are involved
in the aspects of granulosa heterogeneity. The idea that the oocyte plays
a key role in causing the different patterns of granulosa cytodifferentiation
during graafian follicle development is supported by studies
in rodents.54 A dialogue takes place between the oocyte and granulosa cells that has
a great impact on folliculogenesis. In developing murine graafian follicles, the
differential pattern of proliferation and differentiation
between the granulosa in the membrana and cumulus domains are under the
control of secreted oocyte morphogens.54 A novel TGF-β family member, GDF-9, was discovered in the mouse.24,25 Definitive evidence that GDF-9 is obligatory for folliculogenesis came
from studies of GDF-9-deficient mice.26 In these animals, the absence of GDF-9 resulted in the arrest of follicle
growth and development at primary stage and the females are infertile. These
data support the idea that GDF-9 secreted by the egg is obligatory
for graafian follicle development, granulosa cytodifferentiation
and proliferation, and female fertility. The clinical relevance of
this new concept is demonstrated by the presence of GDF-9 mRNA in the
human ovary.25 The current challenges are to elucidate the mechanisms controlling GDF-9 expression
and to identify the target cells for GDF-9 and the biologic
processes that GDF-9 regulates. The concept that oocyte-derived growth
factors control folliculogenesis and fertility could have important
implications for human physiology and pathophysiology. Classification. All graafian follicles can be divided broadly into two groups: healthy
and atretic (Fig. 21). The main difference between these two groups is whether apoptosis is
occurring in the granulosa cells. The development of a graafian follicle (healthy
or atretic) follows a progressive course over time. This
implies that variability or heterogeneity is a normal consequence of folliculogenesis. A
healthy graafian follicle becomes progressively more
differentiated with increasing time until it attains the preovulatory
stage (Fig. 22). The time for this process (Fig. 2) is about 2 months in women.3 As this occurs, there is a temporal and spatial pattern of expression
of large numbers of genes. In healthy follicles, these genes direct cytodifferentiation, proliferation, and follicular fluid formation. In atretic
follicles, the time-dependent changes in gene expression cause
the cessation of mitosis and the expression of apoptosis (i.e. follicle atresia). During atresia, the oocyte and granulosa cells become
committed to express genes that lead to apoptosis.55 In healthy and atretic graafian follicles, the control mechanisms involve
ligand-dependent signaling pathways that inhibit or stimulate the
expression of differentiation and apoptosis (Fig. 22). Understanding the molecular mechanisms and cellular consequences of
the ligand-receptor signaling pathways that control graafian follicle
fate is a major goal of reproductive research. 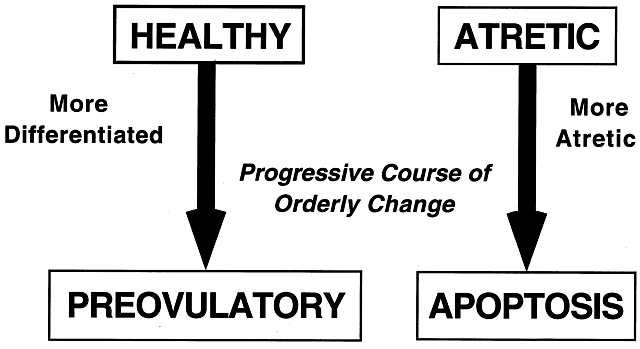 Fig. 21. The two major classes of graafian follicles: healthy and atretic. Each
undergoes a regulated course of progressive change that results in ovulation
or apoptosis.(From Erickson GF: The graafian follicle: A functional definition. In Adashi
EY (ed): Ovulation: Evolving Scientific and Clinical Concepts. New York: Springer-Verlag, 2000.) Fig. 21. The two major classes of graafian follicles: healthy and atretic. Each
undergoes a regulated course of progressive change that results in ovulation
or apoptosis.(From Erickson GF: The graafian follicle: A functional definition. In Adashi
EY (ed): Ovulation: Evolving Scientific and Clinical Concepts. New York: Springer-Verlag, 2000.)
|
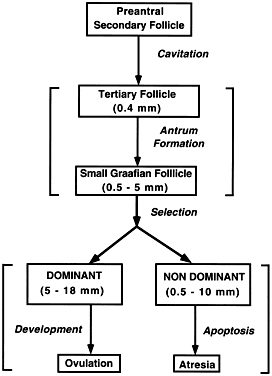 Fig. 22. Diagram of the life cycle of graafian follicles in human ovaries.(From Erickson GF: The graafian follicle: A functional definition. In Adashi
EY (ed): Ovulation: Evolving Scientific and Clinical Concepts. New York: Springer-Verlag, 2000.) Fig. 22. Diagram of the life cycle of graafian follicles in human ovaries.(From Erickson GF: The graafian follicle: A functional definition. In Adashi
EY (ed): Ovulation: Evolving Scientific and Clinical Concepts. New York: Springer-Verlag, 2000.)
|
The process of graafian follicle growth and development can be arbitrarily
divided into several stages based on follicle size (Figs. 2 and 22). It is convenient and important for clinicians and researchers to identify
the physiologic function of various types or classes of follicles
over the cycle. The healthy human graafian follicle has a destiny to
complete the transition from the small (1 to 6 mm), medium (7 to 11 mm), and
large (12 to 17 mm) to the fully differentiated preovulatory state (18 to 23 mm). The
atretic graafian follicle has a destiny to complete
the transition from the small to the medium stage (1 to 10 mm) but
appears incapable of growing to the large size under normal physiologic
conditions.56 Because the process of graafian follicle development is asynchronous, it
produces a large, heterogeneous population of graafian follicles in
the ovaries at any moment in time (Fig. 3). Each of these morphologically distinct graafian follicles is a dynamic
structure undergoing a flow or progression of developmental change
on its way to becoming more differentiated or more atretic (Fig. 22). It should be kept in mind that this results in the presence of an extremely
heterogeneous pool of graafian follicles. It is the heterogeneity
that makes it difficult to come to grips with a simple functional
definition for the graafian follicle. The size of a graafian follicle is determined largely by the size of the
antrum, which is determined by the volume of follicular fluid, which
is determined by the bioavailability of FSH in the fluid.57 FSH is obligatory for graafian follicle development, and no other ligand
by itself has the ability to induce follicular fluid formation. In
the absence of FSH, follicular fluid is not produced, and no graafian
follicles develop. The proliferation of the follicle cells also contributes
to graafian follicle growth; in healthy follicles, the granulosa
and theca cells proliferate extensively (as much as 100-fold), concomitant
with the antrum becoming filled with follicular fluid (Fig. 23). These events (i.e. increased follicular fluid accumulation and cell proliferation) are responsible
for the tremendous growth of healthy graafian follicles.3,58 In contrast, it is the cessation of mitosis and follicular fluid formation
that determines the size of the atretic graafian follicle. 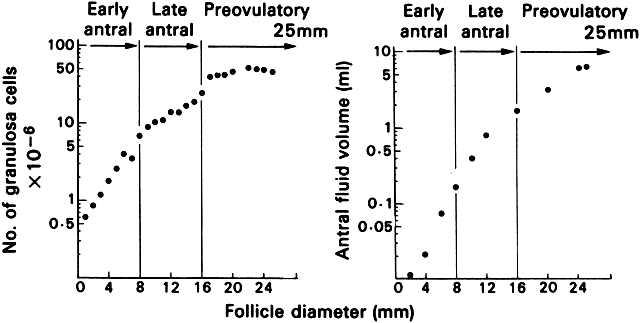 Fig. 23. Changes in the number of granulosa cells and volume of follicular fluid
in human graafian follicles throughout the course of folliculogenesis. The
dominant follicle at ovulation is about 25 mm in diameter and contains
about 50 million granulosa cells and 7 ml of follicular fluid.(From McNatty KP: Hormonal correlates of follicular development in the
human ovary. Aust J Biol Sci 34:249, 1981.) Fig. 23. Changes in the number of granulosa cells and volume of follicular fluid
in human graafian follicles throughout the course of folliculogenesis. The
dominant follicle at ovulation is about 25 mm in diameter and contains
about 50 million granulosa cells and 7 ml of follicular fluid.(From McNatty KP: Hormonal correlates of follicular development in the
human ovary. Aust J Biol Sci 34:249, 1981.)
|
Selection of the Dominant Follicle.
In each menstrual cycle, the ovaries normally produce a single dominant
follicle that participates in a single ovulation. Morphometric analysis
of normal human ovaries (Figs.
2 and 3) indicates
that the dominant follicle that will ovulate in the subsequent cycle is
selected from a cohort of healthy, class 5 follicles measuring 4.7 ±
0.7 mm in diameter at the end of the luteal phase of the menstrual cycle.1,2,3,59
At the time of selection, each cohort follicle contains a fully grown
oocyte, about 1 million granulosa cells, a theca interna containing several
layers of TICs, and theca externa composed of smooth muscle cells (Figs.
3 and 23).
A characteristic feature of a dominant follicle is a high rate of mitosis
in the granulosa cells. The evidence suggests that shortly after the
mid-luteal phase, the rate of granulosa mitosis increases sharply (about
twofold) in the granulosa cells within all cohort follicles.2,56,60 This suggests that luteolysis may be accompanied by a burst of mitosis
in the granulosa of the cohort of class 5 follicles. The first indication
that one follicle has been selected appears to be that the granulosa
cells in the chosen follicle continue dividing at a relatively fast
rate while proliferation slows in the granulosa of the other cohort
follicles. Because this difference becomes apparent at the end of the
luteal phase, it has been argued that selection occurs at the late luteal
phase of the menstrual cycle. As a consequence of increased mitosis, the
dominant follicle continues to grow rapidly3,4 during the follicular phase, reaching 6.9 ± 0.5 mm at days 1 to 5, 13.7 ± 1.2 mm
at days 6 to 10, and 18.8 ± 0.5 mm at
days 11 to 14. Conversely, growth proceeds more slowly in the cohort follicles, and
with time, atresia becomes increasingly more evident in
the nondominant cohort follicles, presumably because of the expression
of specific genes in the apoptotic pathway.56 Rarely does an atretic follicle reach more than 10 mm in diameter, regardless
of the stage in the cycle.4,56,60 The Process. There is compelling evidence from laboratory animal61 and primate experiments,62 that a secondary rise in plasma FSH must be attained for a follicle to
achieve dominance. As shown in Figure 24, the secondary FSH rise in women begins a few days before the progesterone
levels fall to basal levels at the end of luteal phase, and the FSH
levels remain elevated during the first week of the follicular phase
of the cycle.63 Experiments using monkeys have demonstrated that the dominant follicle
undergoes atresia if the secondary rise in FSH is prevented by treatment
with exogenous estradiol.64 An important concept in reproductive biology is that an increase in bioactive
FSH is obligatory for follicle selection and fertility.33,65 It appears that decreased estradiol production by the corpus luteum is
the principal cause for the secondary rise in FSH66 rather than the fall in corpus luteum-derived inhibin A (Fig. 24). 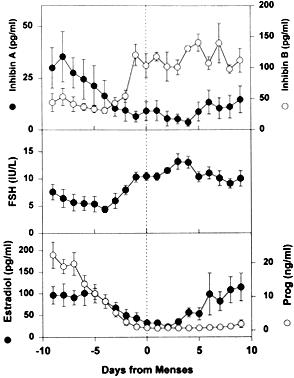 Fig. 24. The luteal-follicular transition in women. Data are mean (±SEM) for
daily inhibin A, inhibin B, FSH, estradiol, and progesterone levels
in the luteal-follicular transition of normal cycling women ( n = 5). Data are centered to the day of menses in cycle 2.(From Welt CK, Martin KA, Taylor AE et al: Frequency modulation of follicle-stimulating
hormone (FSH) during the luteal-follicular transition: Evidence
for FSH control of inhibin B in normal women. J Clin Endocrinol
Metab 82:2645, 1997, with permission from The Endocrine Society.) Fig. 24. The luteal-follicular transition in women. Data are mean (±SEM) for
daily inhibin A, inhibin B, FSH, estradiol, and progesterone levels
in the luteal-follicular transition of normal cycling women ( n = 5). Data are centered to the day of menses in cycle 2.(From Welt CK, Martin KA, Taylor AE et al: Frequency modulation of follicle-stimulating
hormone (FSH) during the luteal-follicular transition: Evidence
for FSH control of inhibin B in normal women. J Clin Endocrinol
Metab 82:2645, 1997, with permission from The Endocrine Society.)
|
How does the secondary rise in FSH control selection? The results from
studies of human follicular fluid support the conclusion that the rise
in plasma FSH leads to a progressive accumulation of relatively high
concentrations of FSH in the microenvironment of one follicle in the cohort; this
follicle is destined to become dominant (Fig. 25). In developing healthy (dominant) follicles (class 5 to 8 follicles), the
mean concentration of follicular fluid FSH increases from about 1.3 mIU/ml (about 58 ng/ml) to about 3.2 mIU/ml (about 143 ng/ml) through
the follicular phase.4,67 In contrast,4,67 the levels of FSH are low or undetectable in the microenvironment of the
nondominant cohort follicles (Fig. 25). 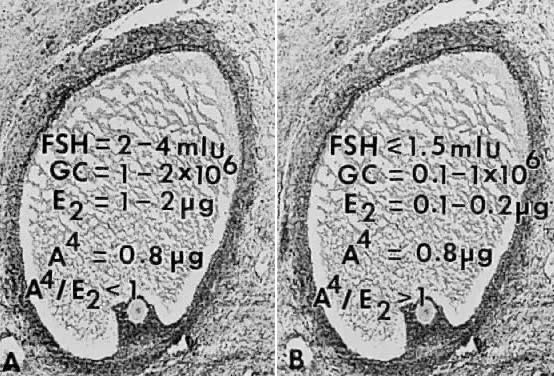 Fig. 25. Illustration of the concept that the dominant follicle contains relatively
high levels of follicle-stimulating hormone (FSH) in the follicular
fluid, whereas FSH levels are low or undetectable in cohort follicles
destined for atresia. A. In dominant follicles, FSH in follicular fluid induces P450arom activity that metabolizes androgen substrate to estradiol (E2 ). In such follicles, E2 and androstenedione (A4) accumulate in very high concentrations in the follicular fluid. B. In nondominant follicles, the low levels of FSH lead to a paucity of granulosa
cells (GC) and low concentrations of estradiol, despite the high
levels of A4.(From Erickson GF, Yen SSC: New data on follicle cells in polycystic ovaries: A
proposed mechanism for the genesis of cystic follicles. Semin
Reprod Endocrinol 2:231, 1984.) Fig. 25. Illustration of the concept that the dominant follicle contains relatively
high levels of follicle-stimulating hormone (FSH) in the follicular
fluid, whereas FSH levels are low or undetectable in cohort follicles
destined for atresia. A. In dominant follicles, FSH in follicular fluid induces P450arom activity that metabolizes androgen substrate to estradiol (E2 ). In such follicles, E2 and androstenedione (A4) accumulate in very high concentrations in the follicular fluid. B. In nondominant follicles, the low levels of FSH lead to a paucity of granulosa
cells (GC) and low concentrations of estradiol, despite the high
levels of A4.(From Erickson GF, Yen SSC: New data on follicle cells in polycystic ovaries: A
proposed mechanism for the genesis of cystic follicles. Semin
Reprod Endocrinol 2:231, 1984.)
|
The entry of FSH into follicular fluid at cavitation is believed to provide
the induction stimulus that initiates the process of graafian follicle
growth and development. At the cellular level, it is the FSH receptor
on the granulosa cell that is the fundamental player in this process. When
an appropriate high FSH threshold is reached in one graafian
follicle, it is selected to become dominant.31 In contrast, the small graafian follicles in the cohort with subthreshold
levels of FSH become nondominant (Figs. 22 and 25). The mechanism whereby one small graafian follicle in a cohort is able
to concentrate high levels of FSH in its microenvironment remains one
of the mysteries in ovary physiology. An important point is that estradiol
produced by the dominant follicle inhibits the secondary rise in
FSH by a negative feedback mechanism (Figs. 24 and 26). This is believed to ensure a subthreshold level of FSH in the nondominant
cohort follicles, which then leads to atresia. Mitosis in granulosa
cells of atretic cohort follicles can be stimulated by treatment with
human menopausal gonadotropin (hMG) during the early follicular phase.59 If FSH levels are increased to threshold levels within the microenvironment, then
nondominant follicles may be rescued from atresia. This phenomenon
could have implications for the way in which exogenous FSH or
hMG triggers the formation of multiple dominant follicles in women undergoing
ovulation induction. 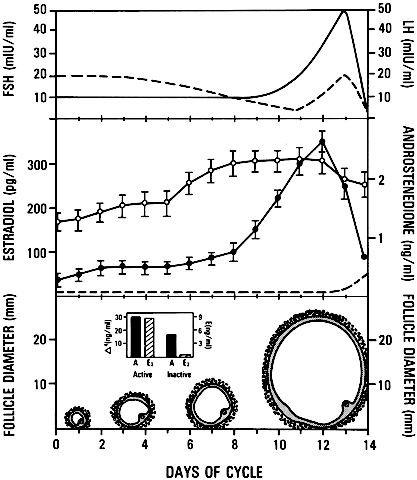 Fig. 26. Diagram illustrating the important consequences of the increased levels
of follicle-stimulating hormone(FSH) in the early follicular phase of
the human menstrual cycle on the growth and development of the dominant
follicle. Estradiol produced by the dominant follicle is physiologically
coupled to the mechanisms governing suppressor of FSH at the mid-follicular
phase and for the stimulation of luteinizing hormone (LH) secretion
at mid-cycle.(From Erickson GF: Analysis of follicle development and ovum maturation. Semin
Reprod Endocrinol 4:233, 1986.) Fig. 26. Diagram illustrating the important consequences of the increased levels
of follicle-stimulating hormone(FSH) in the early follicular phase of
the human menstrual cycle on the growth and development of the dominant
follicle. Estradiol produced by the dominant follicle is physiologically
coupled to the mechanisms governing suppressor of FSH at the mid-follicular
phase and for the stimulation of luteinizing hormone (LH) secretion
at mid-cycle.(From Erickson GF: Analysis of follicle development and ovum maturation. Semin
Reprod Endocrinol 4:233, 1986.)
|
The Mechanism. In the ovary (Fig. 27), the granulosa cells are the only cells that express FSH receptors.68 It follows that the primary mechanism by which FSH evokes dominant follicle
formation is by stimulating FSH-dependent signaling pathways in
the granulosa cells. How does this occur? 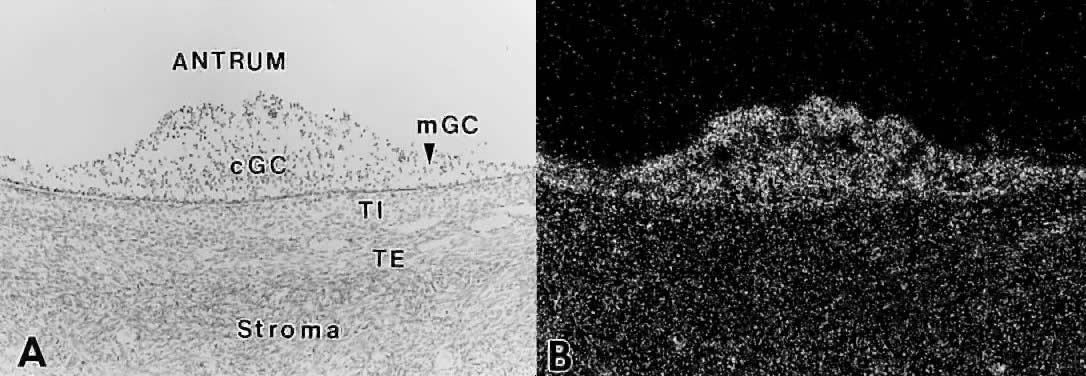 Fig. 27. In situ hybridization analysis of follicle-stimulating hormone (FSH) receptor
mRNA in developing human follicles. A. Bright-field photomicrograph after hybridization with 35 S-labeled hFSH receptor cRNA probe. cGC, cumulus granulosa cells; mGC, membrana
granulosa cells; TI, theca interna; TE, theca externa. B. Dark-field photomicrograph of the same subject as in A. The hybridization signal appears as white grains. All the granulosa cells
express FSH receptor mRNA.(GF Erickson and S Shimasaki, unpublished data.) Fig. 27. In situ hybridization analysis of follicle-stimulating hormone (FSH) receptor
mRNA in developing human follicles. A. Bright-field photomicrograph after hybridization with 35 S-labeled hFSH receptor cRNA probe. cGC, cumulus granulosa cells; mGC, membrana
granulosa cells; TI, theca interna; TE, theca externa. B. Dark-field photomicrograph of the same subject as in A. The hybridization signal appears as white grains. All the granulosa cells
express FSH receptor mRNA.(GF Erickson and S Shimasaki, unpublished data.)
|
Granulosa Cells: Follicle-Stimulating Hormone Receptor Signaling. The human FSH receptor is part of a large family of transmembrane receptors
that regulate the heterotrimetric G proteins.69,70 The mature FSH receptor contains 678 amino acids (Mr 76,465) that are organized into three domains: - A long 359-residue extracellular NH2-terminal ligand binding domain with six potential N-linked glycosylation sites and a cluster of cysteines at the junction
between the extracellular and transmembrane domains
- A transmembrane spanning domain composed of seven hydrophobic a helices
that anchor the receptor to the plasma membrane
- An intracellular COOH-terminal domain with a relatively high proportion
of serine and threonine residues; if these amino acids are phosphorylated, they
could play a role in desensitization and down regulation of
the FSH receptor.69
Truncated isoforms of the FSH receptor corresponding to the extracellular
binding domain have been identified.71 The physiologic or pathophysiologic role of the FSH receptor isoforms
is unknown. The FSH signaling cascade is illustrated in Fig. 28: FSH interacts with its receptor with high affinity; the binding event
initiates a conformational change in the receptor that activates the
G proteins; GDP bound to the αGs subunit is exchanged for GTP, and the newly active αGs-GTP dissociates from the βγ complex; free αGs-GTP interacts with the adenylate cyclase to generate cAMP; cAMP binds
to the regulatory (R) subunits of PKA, causing the complex to dissociate
into an R2 dimer and two free catalytic (C) subunits; the C subunits can phosphorylate
serine and threonine residues of the CREB and CREM proteins.72 After phosphorylation, these proteins can bind to upstream DNA regulatory
elements called cAMP response elements (CRE), where they stimulate
or inhibit gene transcription.72 These events are critical for the expression of the developmental program
that generates a dominant preovulatory follicle. The FSH interactions
within a dominant follicle induce three major responses in the granulosa
cells: stimulation of division, acquisition of steroidogenic potential, and
induction of LH receptors. 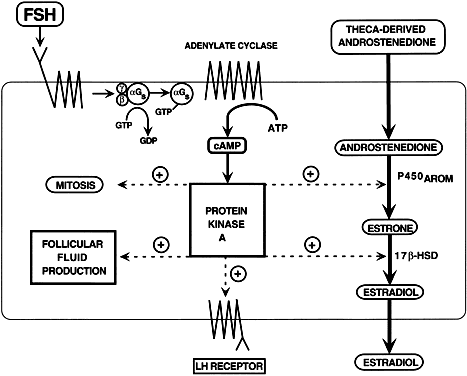 Fig. 28. Diagram of the follicle-stimulating hormone (FSH) signal transduction pathway
in granulosa cells of a dominant follicle. FSH interacts with a
receptor protein that has seven transmembrane-spanning domains. The binding
event is transduced into an intracellular signal by the heterotrimeric
G proteins. The active αGstimulating (αGs -GTP) protein interacts with its effector protein, adenylate cyclase, to
initiate cAMP formation. cAMP binds to and activates protein kinase
A, which phosphorylates substrate proteins that stimulate transcription
of the genes encoding P450AROM, 17β-hydroxysteroid dehydrogenase, and the luteinizing hormone and
that activate mitosis and follicular fluid formation.(Adapted from Erickson GF: Polycystic ovary syndrome: Normal and abnormal
steroidogenesis. In Schats R, Schoemaker J (eds): Ovarian Endocrinopathies: Proceedings of the 8th Reinier deGraaf Symposium. Park Ridge, NJ: Parthenon Publishing, 1994.) Fig. 28. Diagram of the follicle-stimulating hormone (FSH) signal transduction pathway
in granulosa cells of a dominant follicle. FSH interacts with a
receptor protein that has seven transmembrane-spanning domains. The binding
event is transduced into an intracellular signal by the heterotrimeric
G proteins. The active αGstimulating (αGs -GTP) protein interacts with its effector protein, adenylate cyclase, to
initiate cAMP formation. cAMP binds to and activates protein kinase
A, which phosphorylates substrate proteins that stimulate transcription
of the genes encoding P450AROM, 17β-hydroxysteroid dehydrogenase, and the luteinizing hormone and
that activate mitosis and follicular fluid formation.(Adapted from Erickson GF: Polycystic ovary syndrome: Normal and abnormal
steroidogenesis. In Schats R, Schoemaker J (eds): Ovarian Endocrinopathies: Proceedings of the 8th Reinier deGraaf Symposium. Park Ridge, NJ: Parthenon Publishing, 1994.)
|
The granulosa cells in the dominant follicle have the ability to divide
at a relatively rapid rate throughout the follicular phase of the cycle,2,5 increasing from about 1 × 106 cells in the class 5 follicle at selection to more than 50 × 106 cells when it reaches the preovulatory stage (Fig. 23). FSH has been shown to be an effective stimulator of primate granulosa
proliferation in vivo2,73 and in vitro.73,74 Precisely how the FSH signaling mechanism controls the rate of mitosis
is not understood. A variety of growth factors, including insulin-like
growth factor-I (IGF-I),75 fibroblast growth factor,76 and epidermal growth factor,76 are also effective stimulators of mitosis in human granulosa cells grown in vitro. It is possible that these growth factors may serve as important stimulators
of granulosa proliferation in the dominant follicle by autocrine/paracrine
mechanisms. This proposition is supported by direct evidence
that follicular fluid contains these growth factors and that human granulosa
cells express receptors for and respond to these ligands. Granulosa
cell division continues at a high rate until the end of the follicular
phase, when the preovulatory LH surge shuts off granulosa mitosis
in the dominant follicle.77
FSH plays an important role in the control mechanisms governing estradiol
biosynthesis and in determining the potential for luteinization and progesterone
synthesis by the granulosa cells during the development of the dominant
follicle.32,78
The underlying mechanism involves the expression (or the potential to
express) specific genes that encode the enzymes in the estradiol and progesterone
biosynthetic pathways (Fig.
28). In the estrogen pathway, FSH induces the expression of the P450arom
(the CYP19 gene).79 The type I 17β-hydroxysteroid
dehydrogenase (17β-HSD) appears to be constitutively expressed in
the granulosa cells in follicles from the primary to the preovulatory
stage.80,81,82
The control mechanisms that regulate 17β-HSD in human ovaries are
poorly understood. The first time P450arom is detectable in
the granulosa cells appears to occur when a follicle reaches about 1 mm
in diameter, or the class 2 stage.83 It
is observed in only one follicle, the putative dominant follicle.84
The levels of P450arom activity increase progressively,83,84,85
reaching very high amounts in the granulosa cells of the preovulatory
follicle in the late follicular phase (Fig.
29). By virtue of the expression of P450arom and 17β-HSD,
the granulosa cells have the capacity to metabolize theca-derived androstenedione
to estradiol. A progressive increase in P450arom gene expression
in the dominant follicle is reflected physiologically in the progressive
increase in estradiol in the peripheral plasma during days 7 to 12 of
the menstrual cycle (Fig.
26).
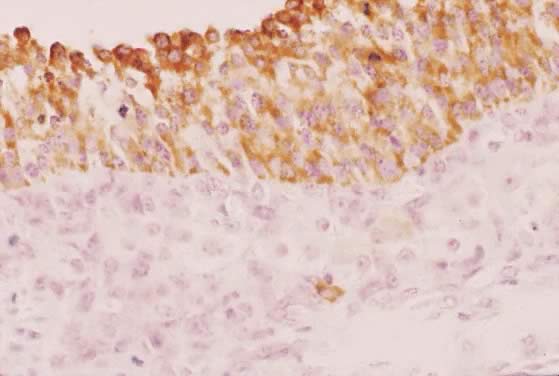 Fig. 29. Immunohistochemistry of P450AROM in a putative dominant small graafian follicle in a normal human ovary
at the late luteal phase of the menstrual cycle. Notice the intense immunostaining
in the dividing and nondividing granulosa cells. An occasional
theca cell exhibits anti-P450AROM immunoreactivity.(GF Erickson and RJ Chang, unpublished data.) Fig. 29. Immunohistochemistry of P450AROM in a putative dominant small graafian follicle in a normal human ovary
at the late luteal phase of the menstrual cycle. Notice the intense immunostaining
in the dividing and nondividing granulosa cells. An occasional
theca cell exhibits anti-P450AROM immunoreactivity.(GF Erickson and RJ Chang, unpublished data.)
|
Granulosa cytodifferentiation is also accompanied by the acquisition of
the potential to express the enzymes in the progesterone pathway, including
steroid acute regulatory protein (StAR),86 the P450 side chain cleavage enzyme (P450SCC),87 and 3βhydroxysteroid dehydrogenase (3β-HSD) genes.88 Developmentally, these enzymes do not become detectable in granulosa cells
of the dominant follicle until very late in the follicular phase
of the menstrual cycle.78,85 This evidence suggests a putative luteinization inhibitor exists in the
follicular fluid of developing graafian follicles. Although the nature
of the luteinization inhibitor is unknown, work in rodents suggests
it may involve an intrinsic bone morphogenetic protein system.89 It appears that FSH controls the potential of the granulosa cells to express
StAR, P450SCC, and 3β-HSD, while the ovulatory LH surge may induce the expression
of these enzymes by virtue of its ability to suppress the activity
of the luteinization inhibitor.78 Further work is required to determine precisely how FSH and LH control
the expression of the progesterone potential of the granulosa cells during
the growth of the dominant follicle in human ovaries.
Granulosa cells in a dominant follicle (Fig.
28) express a relatively high concentration of LH receptors.90
This FSH-dependent event91,92,93
essentially occurs at the end of the follicular phase, when a dominant
follicle reaches the class 8 stage (Fig.
2). As with P450SCC and 3β-HSD, the increase in LH
receptor in the granulosa layer remains suppressed until the very end
of folliculogenesis, when it appears in the membrana granulosa cells.
The physiologic significance of the induction of LH receptor in the late
follicular phase is that it endows the dominant follicle with the unique
ability to respond to the ovulatory surge of LH/hCG by undergoing ovulation.
Acquisition of LH receptors implies that, when the LH ligand enters the
microenvironment of the dominant follicle in the late follicular phase,67
it can act on the granulosa cells to regulate their function. In this
regard, LH has been shown to act on the granulosa cells of developing
follicles to stimulate estradiol production.94
LH in the microenvironment may facilitate estradiol production by the
dominant follicle in the late follicular phase, possibly replacing FSH
as the principal regulator of granulosa P450arom activity.
The importance of estradiol in regulating granulosa cytodifferentiation
in rodent ovaries is clear,90 but whether this concept is true for humans remains uncertain. Some studies
have shown that human granulosa cells strongly and selectively express
estrogen receptor-β (ERβ),95 suggesting that estradiol may play an autocrine/paracrine role in human
folliculogenesis. Further work is necessary to identify what specific
biologic functions might be regulated by estradiol-ERβ signaling
pathways in human granulosa cells. Theca Interstitial Cytodifferentiation. The TICs begin to express their differentiated state when the tertiary
follicle undergoes cavitation. This cytodifferentiation process is accompanied
by the differential expression of a battery of specific genes, including
those for LH receptors,13 insulin receptors,96 lipoprotein receptors (high-density [HDL] and low-density lipoprotein [LDL]), StAR,86,97 P450scc, 3β-HSD, and P450c17.84,85,98 By virtue of the expression of these genes, the TICs acquire the capacity
for the regulated production of androstenedione.99,100 LH appears to be the most important effector of TIC cytodifferentiation, but
insulin and lipoproteins can amplify the action of LH in human
TICs.101 Throughout the antral period, the TICs of all graafian follicles (class 1 to 8) express
this differentiated state (Figs. 30 and 31). All antral follicles appear capable of responding to hormone stimulation
with increased androstenedione production throughout their course
of development. This idea is supported by the presence of high levels (about 1 μg/ml) of
androstenedione in follicular fluid in all developing
antral follicles.67 The production of androstenedione (aromatase substrate) by the TICs targets
them as being critically important in the regulation of follicle
estrogen production. Accordingly, the LH-dependent processes that occur
in the theca layer of developing graafian follicles are central to
the process of folliculogenesis and fertility in women. Theca-derived
androgens have been implicated in the mechanisms of atresia in rodents99,102; however, there is little definitive evidence to support this concept
in women. 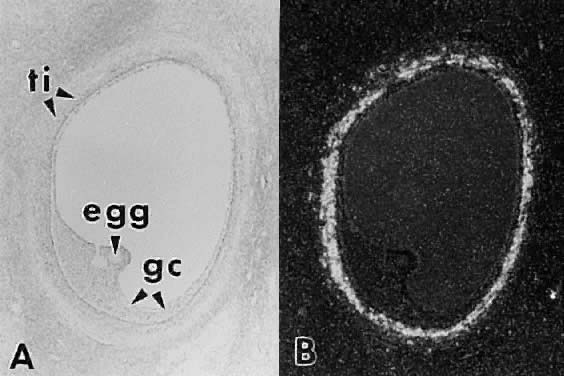 Fig. 30. In situ hybridization of luteinizing hormone (LH) receptor mRNA in a developing
human graafian follicle. A. Bright field. B. Dark-field photomicrograph of the same subject as in A after hybridization with an anti-sense LH receptor cRNA probe.(GF Erickson and S Shimasaki, unpublished data.) Fig. 30. In situ hybridization of luteinizing hormone (LH) receptor mRNA in a developing
human graafian follicle. A. Bright field. B. Dark-field photomicrograph of the same subject as in A after hybridization with an anti-sense LH receptor cRNA probe.(GF Erickson and S Shimasaki, unpublished data.)
|
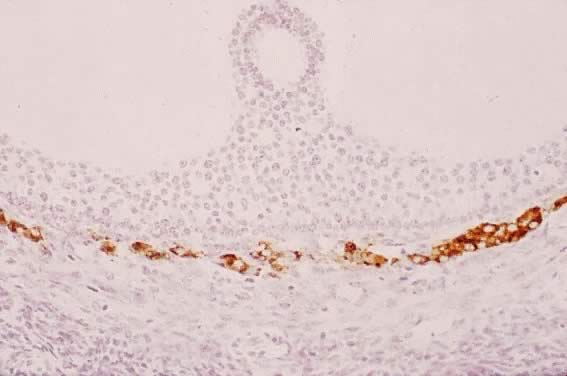 Fig. 31. Immunohistochemistry of P450C17 in a developing human graafian follicle. High levels of immunoreactive
P450C17 are present in theca interstitial cells, whereas none is detectable in
other cell types, including the oocyte and granulosa cells.(The anti-P450C17 antibody was a gift from Dr. Mike Watterman; GF Erickson and RJ Chang, unpublished
data.) Fig. 31. Immunohistochemistry of P450C17 in a developing human graafian follicle. High levels of immunoreactive
P450C17 are present in theca interstitial cells, whereas none is detectable in
other cell types, including the oocyte and granulosa cells.(The anti-P450C17 antibody was a gift from Dr. Mike Watterman; GF Erickson and RJ Chang, unpublished
data.)
|
Despite its importance, little is known about the inducers of theca differentiation. Evidence
is accumulating, however, that growth factors may
be involved.103 Perhaps the most compelling evidence is a report that large graafian follicles
with welldeveloped theca interna (hyperplastic and hypertrophied
TICs) are present in the ovaries of a patient with an LH receptor
inactivating mutation.104 This argues that a yet to be identified regulatory ligand (independent
of LH) can promote theca development, proliferation, and cytodifferentiation. With
respect to mitosis, there occurs a marked increase in the
number of TICs during normal folliculogenesis.2,4 Accordingly, theca mitosis is a critical determinant of the total amount
of androgen produced by the ovaries. An unusually high mitotic rate
could result in an unusually large number of TICs, which could result
in unusually high levels of androgen production in response to hormone
stimulation. Such a mechanism has been proposed to account for ovarian
hyperandrogenism in PCOS patients.105 In rodents, LH is a potent stimulator of theca proliferation102; however, to what extent this concept operates in women is unclear. Given
the importance of theca-derived hyperandrogenism in women, further
studies need to be done to identify the nature of the putative regulatory
factors of TIC mitosis and differentiation and to establish their
physiologic and pathologic significance. Within the past few years, considerable progress has been made in understanding
the mechanisms of LH action. Human LH receptor cDNA has been
cloned and sequenced.106 Like the FSH receptor, it is a part of a large family of transmembrane
receptors that regulate the heterotrimeric G proteins. The mature LH
receptor has a long extracellular NH2-terminal ligand-binding domain, a transmembrane domain containing seven
hydrophobic a helices that integrate the LH receptor to the membrane, and
an intracellular COOH-terminal domain that interacts with residues
of the third intracellular loop (I3) to activate the G proteins. The COOH terminus contains potential phosphorylation
sites which show consensus sites for protein kinase C (PKC) phosphorylation.106 The function of LH receptor phosphorylation is not understood, but in
other receptors in this family, phosphorylation leads to causes desensitization.107 It is possible that phosphorylation of serine and threonine residues in
the I3 and COOH terminus results in desensitization of the LH receptor in the
human ovary. If true, a defect in the desensitization mechanism may cause
continuous activity of the LH receptors, with the constant secretion
of high androgen levels such as that seen in women with hyperandrogenism. Several truncated forms of the LH receptor have been identified in which
the transmembrane domain is absent.106 Hence, the truncated LH receptors may be entirely extracellular, perhaps
being secreted from the cells. We do not know whether these shorter
variants of LH receptor bind LH, but if they do, they could affect the
levels of free LH and thereby modulate cellular responses to LH signals. As with FSH, the signal transduction mechanisms of LH receptors are coupled
to G proteins. As shown in Figure 32, LH interacts with its receptor with high affinity; the binding event
initiates a conformational change in the receptor which activates the
G proteins; GDP bound to the αGS subunit is exchanged for GTP and the αGs-GTP dissociates from the βγ complex; free αGS-GTP interacts with the adenylate cyclase to generate cAMP; cAMP binds
to the regulatory (R) subunits of PKA, causing the complex to dissociate
into an R2 dimer and two free catalytic (C) subunits; the C subunits can phosphorylate
serine and threonine residues of the CREB and CREM proteins that
bind to DNA and modulate gene activity. The second messenger molecules
in the LH signaling pathways are involved in the activation of the genes
in the biochemical pathway that eventually lead to androstenedione
biosynthesis (Fig. 32). 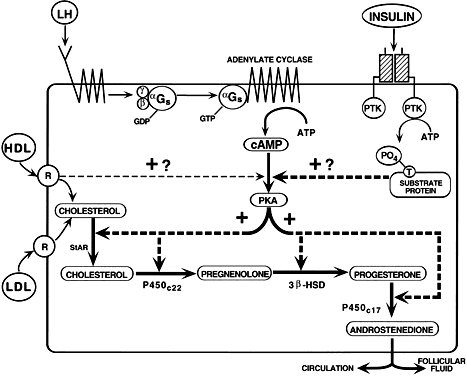 Fig. 32. Diagram showing the regulatory mechanisms of androgen production by theca
interstitial cells. The principal endocrine regulators of androstenedione
production are luteinizing hormone (LH), insulin, and lipoproteins. The
LH receptor cAMP/protein kinase A (PKA) signaling pathway leads
to the induction of specific genes ( broken lines) in the androstenedione biosynthetic pathway. Insulin receptor/protein
tyrosine kinase (PTK) signaling can cause marked increases in this response. Lipoproteins
are potent stimulators of theca androgen production
by virtue of their ability to increase intracellular cholesterol, which
is transferred to P450C22 by means of the steroid acute regulatory protein (StAR).(Adapted from Erickson GF: Normal regulation of ovarian androgen production. Semin
Reprod Endocrinol 11:307, 1993.) Fig. 32. Diagram showing the regulatory mechanisms of androgen production by theca
interstitial cells. The principal endocrine regulators of androstenedione
production are luteinizing hormone (LH), insulin, and lipoproteins. The
LH receptor cAMP/protein kinase A (PKA) signaling pathway leads
to the induction of specific genes ( broken lines) in the androstenedione biosynthetic pathway. Insulin receptor/protein
tyrosine kinase (PTK) signaling can cause marked increases in this response. Lipoproteins
are potent stimulators of theca androgen production
by virtue of their ability to increase intracellular cholesterol, which
is transferred to P450C22 by means of the steroid acute regulatory protein (StAR).(Adapted from Erickson GF: Normal regulation of ovarian androgen production. Semin
Reprod Endocrinol 11:307, 1993.)
|
There appear to be at least three mechanisms that can terminate LH signaling. First, termination
of the G protein signal occurs when bound GTP
is hydrolyzed to GDP. The GTPase activity resides within the αGs molecule itself (i.e. the αGS is an intrinsic GTPase). The inactive αGs-GDP reassociates with the βγ complex and the activity of adenylate
cyclase is shut off. Second, cAMP can be degraded by cyclic nucleotide
phosphodiesterase. Third, a protein serine/threonine phosphatase (phosphatase
type 2A) terminates the activity of the substrate proteins
by dephosphorylating the phosphoserine and phosphothreonine residues. If
a defect existed in the GTPase, phosphodiesterase, or phosphatase
type 2A, ovarian interstitial cells might be expected to produce
high levels of androstenedione continuously. In theory, this could also
be a possible explanation for hyperandrogenism. However, further work
is necessary to investigate this hypothesis. In addition to LH, other ligands can act to control TIC androgen production, including
insulin, lipoprotein, activin, and inhibin. Insulin receptors
with protein tyrosine kinase (PTK) activity have been identified
in human TICs, and the ability of the insulin receptor signal transduction
pathways to stimulate androstenedione production has been demonstrated.101,108,109 The mechanism of insulin-stimulated androgen production is not clear, but
it may involve the activation of a family of PTKs that act and interact
with the LH action downstream of cAMP (Fig. 32). Insulin by itself can increase androstenedione production, and insulin
can synergize with LH to further increase androgen biosynthesis.101,108 The significance of these observations is demonstrated by the evidence
that hyperinsulinemia can result in hyperandrogenism in some women.110 Insulin therefore appears to be an important physiologic stimulus for
androstenedione production by TICs. LDL and HDL also stimulate steroidogenesis
by human TICs, and they can cooperate with LH to effect further
increases.101 Whether the in vitro stimulation of TIC androgen production by LDL or HDL has any physiologic
meaning is not clear, but it may be worth pointing out that HDL is
the most potent stimulator of TIC androgen production known.101 Activin111 and inhibin112 can inhibit and stimulate, respectively, androgen production by human
TICs in vitro. We know nothing about the importance of activin and inhibin in regulating
TIC androgen production in vivo. The Two-Cell, Two-Gonadotropin Concept. To understand the underlying mechanisms of follicle estradiol production, we
need to consider steps that occur in the two-cell, two-gonadotropin
concept (Fig. 33). In response to the LH, which is delivered to the follicle through the
theca vasculature, there occurs an increase in the expression of specific
steroidogenic genes in the TICs, which increases the synthesis of
androstenedione. The level of androgen secretion reflects the presence
within the theca of plus and minus factors, including insulin, lipoproteins, activin, and
inhibin. Some of the TIC-derived androstenedione
diffuses across the basal lamina and enters the follicular fluid and
granulosa cells. In response to the P450arom induced by FSH in the granulosa, the androstenedione is aromatized to
estrone, which then is converted to estradiol by 17β-HSD. These synthetic
activities of the granulosa cells can also be modulated by a
wide variety of regulatory molecules, such as the stimulators insulin113 and IGF-I114 and the inhibitors epidermal growth factor115 and tumor necrosis factor-α.116 The sum total of the ligand controls that operate to determine the activities
of P450arom and 17β-HSD in the granulosa cells determines when and how much estradiol
is produced by the dominant follicle. The estradiol molecules
enter the follicular fluid and then diffuse into the theca vasculature, where
they enter the systemic circulation. 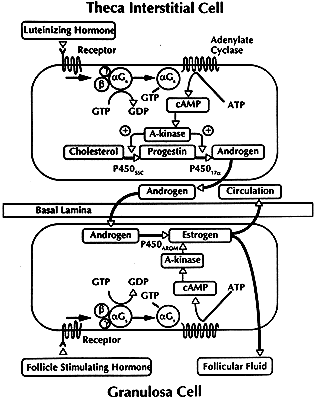 Fig. 33. Diagram showing the two gonadotropin-two cell concept of follicle estrogen
production.(From Kettel M, Erickson GF: Basic and clinical concepts in ovulation induction. In
Rock J, Alverez-Murphy A (eds): Advances in Obstetrics and Gynecology, vol 1. St. Louis: Mosby Year Book, 1994.) Fig. 33. Diagram showing the two gonadotropin-two cell concept of follicle estrogen
production.(From Kettel M, Erickson GF: Basic and clinical concepts in ovulation induction. In
Rock J, Alverez-Murphy A (eds): Advances in Obstetrics and Gynecology, vol 1. St. Louis: Mosby Year Book, 1994.)
|
Atresia Through a process involving a programmed cell-death mechanism called apoptosis, 99.9% of
the ovarian follicles die by atresia.55,117 During atresia, the oocyte and granulosa cells undergo a complex set of
structural and functional changes that ultimately result in the entire
loss of these cells from the follicle (Fig. 34). After some time, the follicular fluid is lost, and the follicle collapses. The
theca exhibit little or no apoptosis. After atresia is complete, the
TICs retain their position in the stroma and begin to carry
out their functional activity as secondary interstitial cells (SICs).27 The SICs retain their capability of secreting androgens in response to
LH and insulin stimulation.108 Accordingly, SICs play a role in determining the levels of androgen produced
by the ovaries. Perhaps the most dramatic example is the production
of abnormally large quantities of androgens in women with so-called “hyperthecosis.” 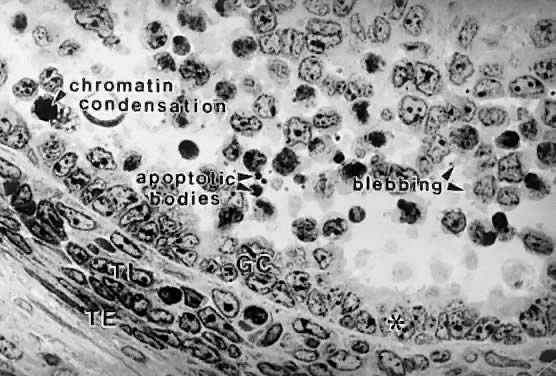 Fig. 34. Photomicrograph showing apoptosis in the granulosa cells of an early tertiary
follicle during atresia. Some granulosa exhibit the cytologic features
of programmed cell death, including chromatin condensation, blebbing, and
release of apoptotic bodies. Some adjacent cells appear healthy. Notice
that theca cells do not undergo apoptosis. asterisk, granulosa cells attached to basal lamina have contracted; TI, theca interna; TE, theca
externa.(From Sadrkhanloo R, Hofeditz C, Erickson GF: Evidence for widespread atresia
in the hypophysectomized estrogen-treated rat. Endocrinology 120:146, 1987, with
permission from The Endocrine Society.) Fig. 34. Photomicrograph showing apoptosis in the granulosa cells of an early tertiary
follicle during atresia. Some granulosa exhibit the cytologic features
of programmed cell death, including chromatin condensation, blebbing, and
release of apoptotic bodies. Some adjacent cells appear healthy. Notice
that theca cells do not undergo apoptosis. asterisk, granulosa cells attached to basal lamina have contracted; TI, theca interna; TE, theca
externa.(From Sadrkhanloo R, Hofeditz C, Erickson GF: Evidence for widespread atresia
in the hypophysectomized estrogen-treated rat. Endocrinology 120:146, 1987, with
permission from The Endocrine Society.)
|
When do follicles become committed to the atretic pathway? Results of morphometric
studies indicate that atresia is a rare event in primordial
and growing preantral follicles. However, once a preantral follicle
proceeds into the graafian stages, apoptosis can occur. Little is known
about the potential for a follicle to undergo apoptosis in any species; however, there
is evidence that oocytederived GDF-9 may play a role
in determining the apoptotic potential of growing follicles.26 If true, the oocyte may ultimately determine when a follicle acquires
the capacity to die by apoptosis.
A large-scale effort to study apoptosis has elucidated some of the molecular
mechanisms.118 Anyone interested in exploring
this subject in a comprehensive manner should consult the monograph by
Wyllie et al.119 and the excellent mechanistic
reviews in Science.120,121,122,123,124
At the level of the ovary, the molecular players and events involved in
granulosa and oocyte apoptosis are becoming evident.125
An important concept that emerges from all this work is that apoptosis
is controlled by a dynamic balance of plus (BCL family) and minus (BAX
family) factors124 and that the execution
of apoptosis is brought about by the activation and action of a family
of proteases called caspases.122 Understanding
the molecular basis for the balance between the inhibition and activation
of these factors is a popular research area.
One big question concerns the nature of the effector molecules that cause
apoptosis (i.e. atresia) in the ovaries. FSH withdrawal may be an important part of the
physiologic process. After a follicle has been stimulated by FSH, its
survival depends on continued FSH stimulation. If the concentration
of FSH in the microenvironment falls below threshold, apoptosis is triggered
through an FSH withdrawal mechanism, and the follicle undergoes
atresia.55 In this sense, FSH is a survival factor for the graafian follicle. What is the mechanisms by which FSH withdrawal triggers apoptosis? The
process may be mediated by local regulatory molecules (i.e. steroid hormones and growth factors) which act by autocrine/paracrine
mechanisms55,117; however, further work is required to prove this idea. Inasmuch as the
decision to live or die is one of the most critical events in the life
of a follicle, knowledge of the cellular and molecular mechanism could
have important implications for human fertility and infertility. Ovulation On or about the 15th day of an ideal 28-day cycle, the preovulatory follicle
ruptures, and the eggcumulus complex is released from the ovary
by a process called ovulation (Fig. 1). The preovulatory surges of LH and FSH play a crucial role in the physiologic
mechanism of ovulation (Fig. 35). The major role of the LH surge is to stimulate meiotic maturation and
to coordinate the formation of the stigma or site of follicle rupture. The
major role of the FSH surge is to stimulate cumulus expansion and
to facilitate the production of the protease plasmin. 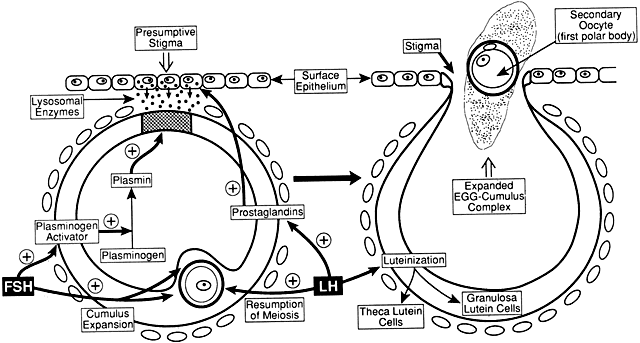 Fig. 35. Diagram of the cellular mechanisms by which the preovulatory surge of follicle-stimulating
hormone (FSH) and luteinizing hormone (LH) causes
ovulation.(From Erickson GF: The ovary: Basic principles and concepts: In Felig P, Baxter
JD, Broadus AE, Froman LA (eds): Endocrinology and Metabolism. 2nd ed. New York: McGraw-Hill, 1995.) Fig. 35. Diagram of the cellular mechanisms by which the preovulatory surge of follicle-stimulating
hormone (FSH) and luteinizing hormone (LH) causes
ovulation.(From Erickson GF: The ovary: Basic principles and concepts: In Felig P, Baxter
JD, Broadus AE, Froman LA (eds): Endocrinology and Metabolism. 2nd ed. New York: McGraw-Hill, 1995.)
|
The oocyte in the preovulatory follicles resumes meiosis in response to
the preovulatory surge of LH and FSH. Unfortunately, the mechanisms by
which this occurs are poorly understood. Based on what we know about
the relation between cAMP and meiosis in vitro (Fig. 15), it appears that the gonadotropin surges in some way cause the level
of cAMP in the oocyte to fall, perhaps in part because of desensitization
and downregulation of the granulosa LH and FSH receptors and because
of activation of specific enzymes that degrade cAMP. The effect of
decreased cAMP levels is to trigger resumption of meiosis.30 In the resulting meiotic division, the oocyte reaches the second meiotic
metaphase or first polar body stage (Fig. 36). The meiotic process proceeds no further unless the ovulated egg is fertilized. Completion
of meiotic maturation is a critical component in
the ovulation process because it is obligatory for normal fertilization. 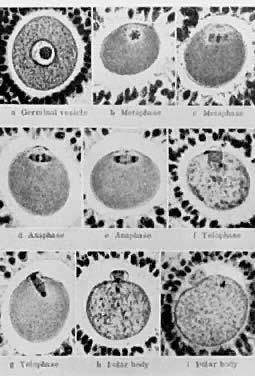 Fig. 36. The process of meiotic maturation from the resumption of meiosis (germinal
vesicle breakdown) through metaphase II (first polar body).(From Witschi E: Development of Vertebrates. Philadelphia: WB Saunders, 1956.) Fig. 36. The process of meiotic maturation from the resumption of meiosis (germinal
vesicle breakdown) through metaphase II (first polar body).(From Witschi E: Development of Vertebrates. Philadelphia: WB Saunders, 1956.)
|
During this process, the cumulus granulosa cells undergo a series of
structural and functional changes called mucification. In response to
the preovulatory surge of FSH, the cumulus cells secrete large quantities
of a newly synthesized glycoprotein mucous substance into the extracellular
spaces.28,29,30
This change results in the dispersal of the cumulus cells and causes the
egg-cumulus complex to expand tremendously (Fig.
37). The process of mucification is physiologically critical for the
pickup and transport of the egg in the fallopian tube.
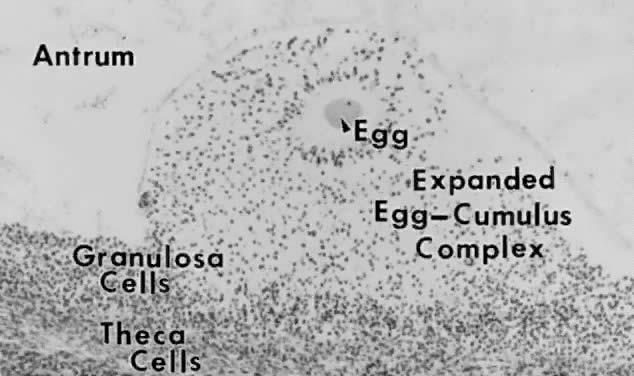 Fig. 37. Photomicrograph showing the effects of the luteinizing hormone (LH)/follicle-stimulating
hormone (FSH) surge on egg-cumulus expansion in situ. The oocyte has resumed meiosis, the corona radiata is radiating from
the zona pellucida, and the cumulus (but not the membrana and periantral) granulosa
cells have lost their attachments and appear as individual
cells dispersed within the proteoglycan matrix.(From Testart J, Thebault A, Frydman R, Papiernik E: Oocyte and cumulus
oophorus changes inside the human follicle cultured with gonadotropins: In
Hafez ESE, Semm K (eds): In Vitro Fertilization and Embryo Transfer. New York: Alan R Liss, 1982.) Fig. 37. Photomicrograph showing the effects of the luteinizing hormone (LH)/follicle-stimulating
hormone (FSH) surge on egg-cumulus expansion in situ. The oocyte has resumed meiosis, the corona radiata is radiating from
the zona pellucida, and the cumulus (but not the membrana and periantral) granulosa
cells have lost their attachments and appear as individual
cells dispersed within the proteoglycan matrix.(From Testart J, Thebault A, Frydman R, Papiernik E: Oocyte and cumulus
oophorus changes inside the human follicle cultured with gonadotropins: In
Hafez ESE, Semm K (eds): In Vitro Fertilization and Embryo Transfer. New York: Alan R Liss, 1982.)
|
Expulsion of the mature egg-cumulus complex depends on the synthesis
and activation of proteases,126,127,128
such as collagenases, that degrade the ovarian tissues in a small, highly
localized area called the macula pellucida or stigma (Figs.
1 and 35).
Although the mechanisms underlying the expression of this proteolytic
activity are still under investigation, some important conclusions have
been reached from animal studies. The concept emerging from this work
is that LH-stimulated progesterone and prostaglandin production by the
follicle wall are required for ovulation.
The most compelling data to support this concept come from gene knockout
experiments, showing that female mice lacking the progesterone receptor (PR) gene129 or the cyclooxygenase (COX) gene130,131 fail to ovulate and are infertile. The PR exists as two isoforms, called
PRA and PRB, that arise from the same gene.129 Mice carrying a null mutation of the PR gene develop mature preovulatory
follicles that undergo cumulus expansion in response to the gonadotropin
surge; however, none ovulates because of the absence of stigma formation.129 This evidence provides strong support for a functional role for the preovulatory
increases in progesterone and PR in stigma formation. Similarly, COX plays a key role in prostaglandin synthesis. It occurs in
two isoforms, the constitutive COX-1 and the inducible COX-2.130,131 The targeted disruption of COX-2 gene produces anovulation and infertility
in female mice.130,131 Anovulation results from the failure of dominant follicles to ovulate
because stigma formation is compromised. Collectively, this evidence supports
the proposition that the LH-induced progesterone and prostaglandin
production are critical determinants of stigma formation, ovulation, and
fertility. Based on all the data, it is possible to propose the following cascade
mechanism. The midcycle surge of LH stimulates the expression of PR and
the production of progesterone; the progesterone ligand interacts with
PR in the follicle cells, which induces COX-2 and prostaglandin production; and
the prostaglandins interact with specific receptors in the
surface epithelial cells of the presumptive stigma and activate a signaling
pathway that leads to the release of lysosomal (proteolytic) enzymes, which
degrades the underlying tissue and causes the surface epithelial
cells to detach from the basement membrane (Fig. 38). This process results in stigma formation and follicle rupture (Fig. 35). 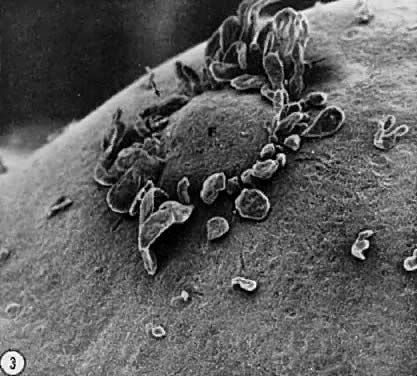 Fig. 38. Scanning electron micrograph of the protruding surface of an ovulatory
follicle(F). Notice the groups of surface epithelial cells in the presumptive
stigma (a flower-like bouquet), which are in the process of being
sloughed off.(From Motta P, Van Blerkon J: A scanning electron microscopic study of
the luteo-follicular complex II events leading to ovulation. Am J Anat 143:241, 1975.) Fig. 38. Scanning electron micrograph of the protruding surface of an ovulatory
follicle(F). Notice the groups of surface epithelial cells in the presumptive
stigma (a flower-like bouquet), which are in the process of being
sloughed off.(From Motta P, Van Blerkon J: A scanning electron microscopic study of
the luteo-follicular complex II events leading to ovulation. Am J Anat 143:241, 1975.)
|
Another active protease relevant to ovulation is plasmin.132 Plasmin is a serine protease derived from plasminogen by enzymatic activation. Two
forms of plasminogen activators have been characterized, urokinase (uPA) and
tissue (tPA) types.126 Both uPA and tPA appear to contribute to ovary plasmin biosynthesis and
ovulation. The follicular fluid contains relatively high levels of the
plasmin precursor, plasminogen. The preovulatory surge of FSH appears
to stimulate granulosa cells to secrete plasminogen activator, which
converts plasminogen to the active protease plasmin (Fig. 35). Plasmin appears to play a role in the degradation of the granulosa cells
and basal lamina in the presumptive stigma. |
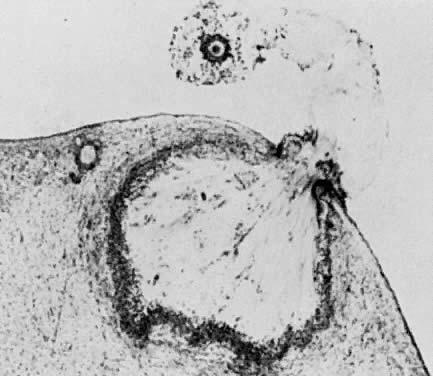

 300 days for a recruited primordial to grow and develop to the class 2/3 (0.4 mm) or
cavitation (early antrum) stage. Atresia can occur in preantral
class 1, 2, and 3 follicles. Antral period: A class 4 (1 to 2 mm) follicle, if selected, requires about 50 days to
grow and develop to the preovulatory stage. The dominant follicle of
the cycle appears to be selected from a cohort of class five follicles, and
it requires about 20 days to develop to the ovulatory stage. Atresia
is common during the antral period. gc, number of granulosa cells; d, days.
300 days for a recruited primordial to grow and develop to the class 2/3 (0.4 mm) or
cavitation (early antrum) stage. Atresia can occur in preantral
class 1, 2, and 3 follicles. Antral period: A class 4 (1 to 2 mm) follicle, if selected, requires about 50 days to
grow and develop to the preovulatory stage. The dominant follicle of
the cycle appears to be selected from a cohort of class five follicles, and
it requires about 20 days to develop to the ovulatory stage. Atresia
is common during the antral period. gc, number of granulosa cells; d, days.