Thyroid hormone influences a wide range of processes, including growth, development, maturation
of the nervous system, reproduction, metabolism, and
muscle function.2 The primary target organs include the brain, liver, skeletal muscle, heart, and
bone. The thyroidal secretion consists of thyroxine (T4) and triiodothyronine (T3). T3 is the active form of thyroid hormone and combines with a specific nuclear
receptor that binds to the regulatory region of genes and modifies
expression.3,4 Thyroid hormone synthesis and secretion is regulated by TSH secreted from
the anterior pituitary, which in turn is stimulated by thyrotropin-releasing
hormone (TRH) from the hypothalamus. Both TSH and TRH are regulated
in a negative feedback loop by circulating T4 and T3. Iodine, a trace element, and selenium (see later discussion) are essential
for normal thyroid hormone metabolism.5 The mechanisms that regulate thyroid hormone metabolism allow for continuous
production of thyroid hormone despite variation in the supply of
dietary iodine. There are also maternal and fetal modifications of thyroid
hormone metabolism associated with various stages of pregnancy
that maintain a euthyroid state for mother and fetus.6 Iodine Transport The majority, 70% to 80%, of the 15 to 20 mg of total body iodine is contained
in the thyroid.5 To produce the daily requirement of thyroid hormone, the thyroid must
trap approximately 60 mg of iodine from the circulation each day. The
iodine that is not trapped by the thyroid is excreted in the urine and
closely matches dietary intake. Iodine uptake in the thyroid is an active
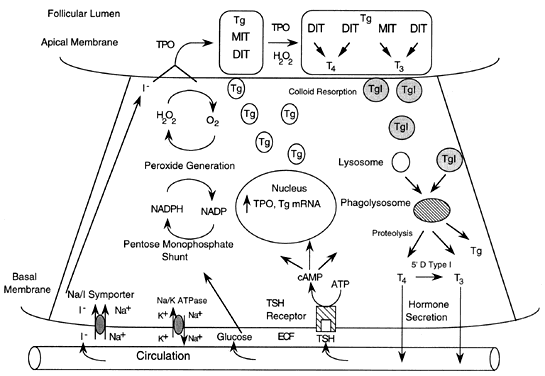
process, driven by the Na+ gradient generated by Na+/K+ adenosine triphosphatase (see Fig. 1). The recently cloned Na+/I- symporter is a membrane-bound protein located in the basolateral portion
of the thyroid follicular cell. The symporter passively transports
two Na+ and one I- down the Na+ ion gradient, producing an iodine gradient from the thyroid cell to the
extracellular fluid of 100:1. In conditions of iodine deficiency, a
gradient as high as 400:1 can be generated. Other tissues that concentrate
iodine include the salivary gland, gastric mucosa, lactating mammary
gland, ciliary body of the eye, skin, placenta, and choroid plexus. Iodine
transport is stimulated by TSH via cyclic adenosine monophosphate (cAMP) and
can be competitively inhibited. Inhibitors of iodine transport
include natural dietary “goitrogens,” such as the
cyanogenic glucosides found in cassava, a staple in many parts of Asia
and Africa, as well as perchlorate and high concentrations of iodine. The primary source of iodine is in the diet, about 500 mg daily in the
United States, but there are a number of areas worldwide, especially mountainous
regions of Africa, Asia, and some parts of Europe, with dietary
iodine deficiency.7 The offspring of iodine-deficient woman have a high incidence of cretinism
with severe neurologic deficit and growth retardation. Even in areas
of marginal iodine insufficiency, such as Belgium, there is a reduction
in maternal circulating thyroid hormones and an elevation in serum
TSH concentration compared to pregnant women from iodine-sufficient
areas.6 The influence of these changes on fetal development are unknown. Thyroid Hormone Synthesis Thyroid hormone synthesis requires a number of components, including iodide, thyroid
peroxidase, thyroglobulin, and hydrogen peroxide (H2O2).1 Iodine is transported into the thyroid in the inorganic form, is oxidized
by the thyroid peroxidase-H2O2 system and then used to iodinate tyrosyl residues in thyroglobulin (see Fig. 1). T4 and T3 are produced by coupling of iodinated tyrosyl intermediates, which are
then hydrolyzed and secreted into the circulation. The function of this
system requires that these processes are closely linked, and defects
in any of the components can lead to impairment of thyroid hormone production
or secretion. Thyroglobulin, a large dimeric glycoprotein (660 kilodaltons [kd]), is
the major iodine-containing protein of the thyroid.8 It serves as a storage form of accessible thyroid hormone for the circulation. A
small amount of thyroglobulin can be detected in the serum
and is, in general, proportional to the thyroid mass. The generation of
H2O2 is essential for the iodination of thyroglobulin and subsequent coupling
reactions. Reduction of molecular oxygen to H2O2 requires reduced adenosine nucleotide, nicotinamide adenine dinucleotide
phosphate (NADPH), and NADPH oxidase. Thyroglobulin serves as a substrate
for coupling of monoiodotyrosine (MIT) and diiodotyrosine (DIT) by
the thyroid peroxidase-H2O2 system. A tyrosyl phenolic ring is cleaved and joined to an iodinated
tyrosine by an ether linkage. Coupling of two DIT moieties forms T4, and coupling of MIT and DIT forms T3. The thyroidal secretion usually contains approximately 80% T4 and 20% T3, but this ratio can be altered by a number of conditions, including the
extent of TSH stimulation and iodine status. The TSH receptor-stimulating
IgG of Graves' disease increases the fraction of T3 in the thyroidal secretion by preferential MIT/DIT coupling. Additionally, intrathyroidal
type I 5´-deiodinase accelerates conversion
of T4 to T3 (see later discussion). Thyroid hormone secretion begins with resorption of colloid at the apical
membrane. Pinocytosis produces multiple vesicles containing colloid, which
fuse with lysosomes to form phagolysosomes. The resulting proteolysis
in these vesicles releases iodothyronines, which diffuse across
the cell and into the cytoplasm. Thyroid peroxidase is a membrane-bound glycoprotein that catalyzes the
oxidation of iodine, iodination of tyrosine residues, and coupling of
iodothyronines.9 The human thyroid peroxidase gene is 150 kilobases (kb) and is located
on chromosome 2. The predicted protein consists of 933 amino acids and
has transmembrane domains at the carboxy terminus. TTF1 and TTF2 regulate
thyroid peroxidase gene expression. Circulating IgG antibodies to
thyroid peroxidase are pathogenic in a number of thyroid diseases, including
Hashimoto's disease and postpartum thyroiditis. These antibodies
can damage the thyroid follicular cells directly by activating
the complement cascade. Thyroid Hormone Transport Thyroid hormones are hydrophobic, and the vast majority of hormone circulates
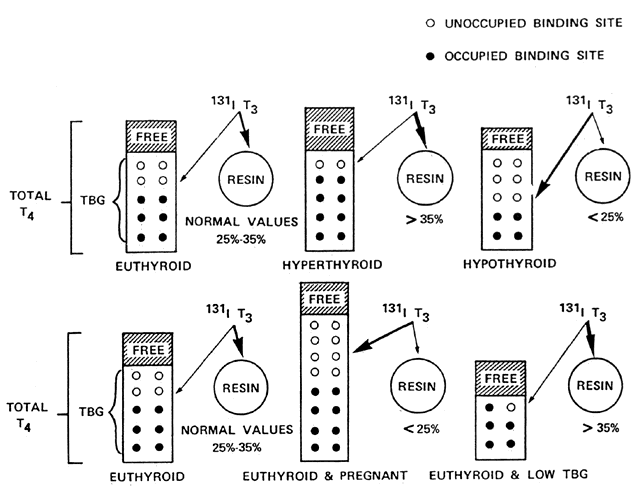
bound to serum proteins with only a small free fraction of T4 (0.02%) and T3 (0.30%).10 The free hormone is the only metabolically active fraction of the total
hormone concentration. The predominant thyroid hormone binding serum
protein is T4-binding globulin (TBG), which carries approximately 70% of serum T4 and the majority of T3. Transthyretin (previously called T4-binding prealbumin) binds approximately 20% of T4 and also transports retinol by forming a complex with retinol-binding
protein. The remaining serum T4 and T3 are bound by albumin. All three of these proteins are made in the liver, and
their levels—as well as levels of total thyroid hormone—are
influenced by liver disease (elevated in hepatitis, reduced in
chronic liver insufficiency) and renal disease (excessive loss in the
urine in nephrotic syndrome). TBG consists of approximately 20% carbohydrates
by weight, and the extent of sialylation influences the circulating
serum half-life (range, 15 minutes to 3 days). Estrogen increases
the extent of sialylation and increases serum TBG concentration by
prolonging the circulating half-life.11 Mutations of the TBG gene, located on the X chromosome, can result in
partial or complete deficiency. Affected persons have reduced total thyroid
hormone concentration but normal levels of free hormone and are
clinically euthyroid. Thyroid Hormone Metabolism The thyroidal secretion is primarily the prohormone T4, which is metabolically inactive and must be converted to the active hormone
T3 by removal of the 5´ phenolic (outer) ring iodine by 5´-deiodinase (Fig. 2).12,13 Approximately 20% of circulating T3 is derived from thyroidal secretion and 80% from peripheral conversion
of T4, primarily in the liver, muscle, and kidney. T4 can also be converted to reverse T3 (rT3), which is metabolically inactive; however, when elevated in the serum, it
is usually associated with reduced T4 to T3 conversion (e.g., in severe nonthyroidal illnesses). Degradation products (containing 3 to 0 iodides) undergo
sulfation and glucuronide formation for excretion
in urine and bile. 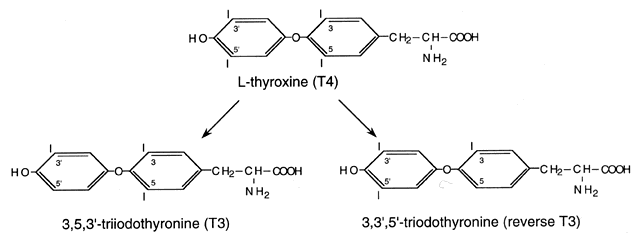 Fig. 2. Structure of L-thyroxine (T4) and its major metabolites, triiodothyronine (T3) and reverse triiodothyronine (rT3).(Brent GA: Thyroid Hormones [T4, T3]. In Conn PM, Melmed S [eds]: Endocrinology: Basic and Clinical Principles, p 291. Totowa, NJ, Humana
Press, 1996) Fig. 2. Structure of L-thyroxine (T4) and its major metabolites, triiodothyronine (T3) and reverse triiodothyronine (rT3).(Brent GA: Thyroid Hormones [T4, T3]. In Conn PM, Melmed S [eds]: Endocrinology: Basic and Clinical Principles, p 291. Totowa, NJ, Humana
Press, 1996)
|
The deiodinases can be separated into outer ring (phenolic) 5´-deiodinases (types
I and II) and inner ring (tyrosyl) 5-deiodinases.13 All deiodinases have a characteristic pattern of expression during development
and in adult tissues, substrate preference, direction of T3 regulation, and sensitivity to inhibitors (Table 1). All deiodinases share the property of being selenoproteins that contain
the amino acid selenocysteine, essential for full catalytic activity.12 The trace element selenium is required for normal thyroid hormone metabolism, and
abnormal thyroid function is seen in areas of selenium deficiency, such
as China and Africa. TABLE 1. Properties of Iodothyronine Deiodinases
| Type I 5'-Deiodinase | Type II 5'-Deiodinase | 5-Deiodinase (Type III) |
Tissue distribution | Thyroid, liver, kidney | Brain, pituitary, brown fat, thyroid | Placenta, developing brain, skin |
Preferred substrate | rT3>>T4> T3 | T4> T3 | T3(sulfate) > T4 |
Target | Outer ring | Outer ring | Inner ring |
Response to T4 | Increase | Decrease | Increase |
Inhibition by PTU | Yes | No | No |
Inhibition by iodine | Yes | Yes | Yes |
contrast agents |
Physiologic role | Extracellular T3production | Intracellular T3production | Inactivation of T4 and T3 |
T4 = thyroxine; T3 = triiodothyronine; rT3 = reverse T3; PTU = propylthiouracil
Type I 5´-deiodinase is found predominantly in liver, kidney, skeletal
muscle, and the thyroid and converts T4 to T3. It requires reduced thiol as a cofactor and is sensitive to inhibition
by propylthiouracil, starvation, illness, glucocorticoids, and propranolol. The
type I gene is positively regulated by T3, and its activity increases in hyperthyroidism. Type II 5´-deiodinase is predominantly found in the pituitary, brain, brown
fat, and human thyroid. The brain, unlike the periphery, derives
the majority of T3 locally from T4 by the action of type II deiodinase. In hypothyroid states, type II 5´deiodinase
activity increases, presumably to sustain a T3 supply to the brain. Type II 5´-deiodinase is insensitive to propylthiouracil
but is inhibited by iodine-containing contrast agents, such
as iopanoic acid. Type III 5-deiodinase is found in the placenta, developing brain, and skin. It
inactivates T4 and T3 by removal of a tyrosyl ring iodide. Like type I, the type III gene is
positively regulated by T3. This enzyme is expressed very early in development and may be important
in regulating maternal and fetal thyroid hormone metabolism. The Hypothalamic-Pituitary Axis The primary regulator of thyroid hormone production is TSH. TSH is a heterodimeric
protein that shares a common alpha subunit with the other
anterior pituitary glycoproteins (follicle-stimulating hormone, luteinizing
hormone [LH], and human chorionic gonadotropin [hCG]), but
has a unique beta subunit.14 The TSH alpha and beta subunit genes are both transcriptionally regulated
by T3, and sequences in the 5´-flanking region have been identified that
bind thyroid hormone receptor and confer these effects. Although elevated
circulating T3 levels alone will suppress TSH, circulating T4, via intrapituitary type II 5´-deiodinase, is thought to be the
primary regulator of TSH. T4 and T3 also provide feedback to TRH. TRH stimulates TSH as well as prolactin, and
T3 inhibits expression of the TRH gene. TSH acts via the membrane-bound TSH receptor.15 TSH is a classic seven-transmembrane structure G-protein coupled receptor. Stimulation
of the TSH receptor promotes protein iodination as well
as thyroid hormone synthesis and secretion. The majority of these effects
are the result of enhanced intracellular cAMP accumulation. Point
mutations in the TSH receptor gene have been reported that inactivate
as well as those that constituitively activate the receptor, resulting
in clinically significant hypothyroidism and hyperthyroidism, respectively. Acquired
activating mutations of the TSH receptor gene have
been reported in a high fraction of autonomously functioning thyroid adenomas. TSH
is mitogenic and promotes thyroid growth, as is seen in Graves' disease
and some goiters. The similarity of the TSH receptor to
the LH/hCG receptor is thought to be the basis for thyroid simulation
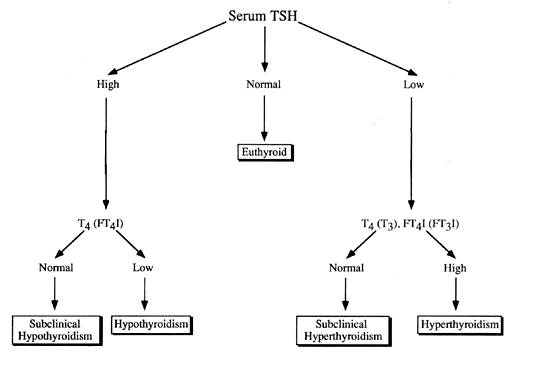
in high hCG states, such as hydatidiform mole and hyperemesis gravidarum.16 There is a log-linear relationship between serum TSH and T4 concentrations. This is an important principle to remember when adjusting
T4 replacement or following thyroid function tests over time. A small change
in serum T4 can produce a large change in TSH. This relationship is likely designed
to achieve tight control of serum thyroid hormone levels around physiologic
levels. |