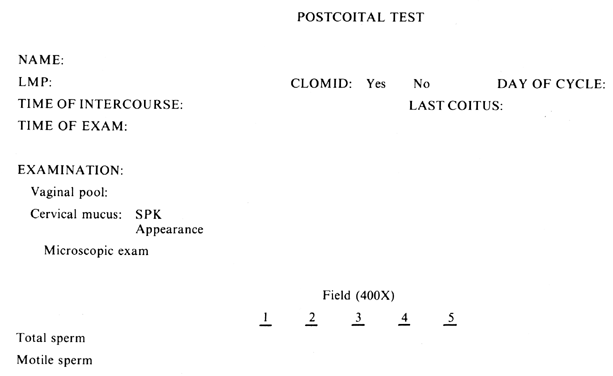
With the basis and method of evaluation for the PCT established. it is
important to distinguish the different factors that can be assessed during
the clinical evaluation. The first problem to be solved is to distinguish
among nonovulatory cervical mucus. altered sperm deposition. altered
sperm penetration (sperm defect). and altered sperm transport (mucus
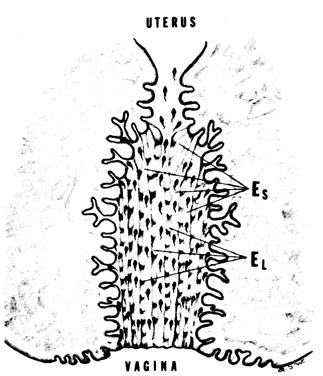
defect). Nonovulatory Cervical Mucus One of the most prevalent etiologic factors for poor cervical mucus is
improper time of collection due to nonovulatory cycles or to incorrect
time of the cycle. Ovulation must be documented before the cervical mucus
is sampled. and correct interpretations can be based only on preovulatory
cervical mucus.2 It should be remembered that cervical mucus deficiencies with consequent
impedance of sperm penetration are quite frequently the result of nonovulation
and insufficient estrogen stimulation. Ovulation can be easily
documented by measuring urinary luteinizing hormone (LH) levels collected every morning, and in some situations, in the late
afternoon as well. This is determined by a monoclonal antibody technique.16,17 In addition, the use of ultrasound monitoring to document ovulation has
increased the prognostic value of the PCT. 17,18 Once ovulation is secured, then the other factors can be determined from
the PCT. Altered Sperm Deposition When the cervical mucus is normal, the PCT can determine difficulties with
sperm deposition. Premature ejaculation, hypospadias, oligospermia, azoospermia, decreased
semen volume, and impotence are conditions that
can be identified. In conditions such as these, there will also be
a decreased or absent number of sperm in the vaginal pool. The various
etiologic factors must be considered. and once appropriate counseling
is accomplished, artificial insemination can be a viable method of conception
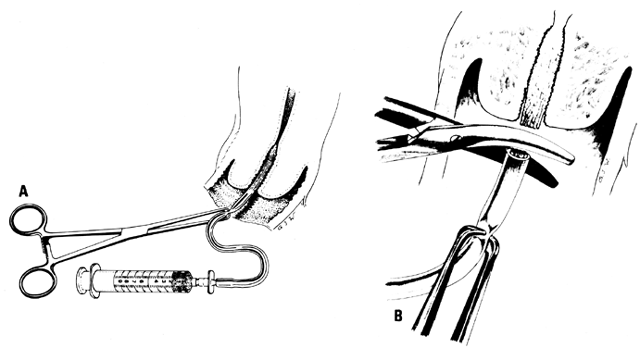
in many cases. Homologous insemination (or artificial insemination, husband [AIH]) can
be done by intracervical or cup (Milex Products, Chicago, IL) insemination.1,2 The volume of the semen usually determines the method. If there is only 1 mL
or less, intracervical insemination is usually done; a cup is used
for larger quantities. It is important that the PCT be repeated after
the insemination. Adequate sperm exposure to the cervical mucus should
result in a normal PCT after the insemination. From the cervical
mucus-sperm transport studies. it has been noted that by 15 minutes the
total number of sperm in cervical mucus remains fairly constant for 24 hours: however. a
decline in the number of sperm starts 2 1/2 hours
after insemination.11,12 The cervical cup is left in place for 20 to 30 minutes and then removed: the
portio vaginalis cervicis is cleansed, and the PCT is performed. Conditions
applicable to this form of therapy result in a success rate
of 30% to 50%.19 If the PCT is abnormal after the cervical insemination. intrauterine insemination (IUI) can
be performed with washed motile sperm. 19,20 Altered Sperm Penetration Poor PCT results often are associated with abnormal semen analysis. Poor
motility can be noted when varicoceles and infection are present. If
a varicocele is confirmed by physical examination and semen analysis, high
ligation of the internal spermatic vein results in an improvement
of motility and a 40% to 55% pregnancy rate.21 Occasionally, the PCT is abnormal because of sperm immobilization. Nonspecific
prostatitis can be treated successfully with trimethoprim-sulfamethoxazole (1 tablet
by mouth two times a day for 1 to 2 months) or, if
ureaplasma or Chlamydia infection is suspected, with doxycycline (206 mg by mouth the first day, then 100 mg
by mouth four times a day for 9 days).22 Although ureaplasma and Chlamydia infections have been proposed as factors contributing to poor PCT, recent
studies have found no correlation.23,24 Immunologic immobilization of sperm in the endocervical canal can occur, but
this diagnosis must be confirmed by immunologic tests.1,2,17,25,26 I also have seen a few instances of poor sperm penetration in patients
with grossly normal-appearing cervical mucus. Immunologic test results
were negative, but when the pH of the mucus was determined, it was very
acidic. In vitro, this mucus can be dialyzed to a normal pH, allowing
normal sperm penetration. The activity of the cervical mucus can be
improved by an alkaline douche27 or by high-dose estrogen therapy (discussed below). Altered Cervical Mucus Preovulatory cervical mucus has the following characteristics: (1) it is
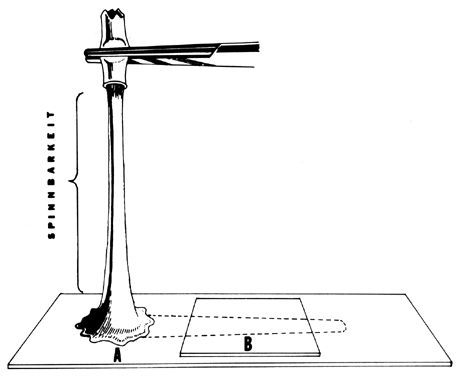
thick, opaque, and cellular: (2) it has a poor spinnbarkheit: and (3) it
is found in approximately 60% of abnormal PCTs. Repeated cervical
bleeding during mucus collection may indicate anatomic defects or cervicitis. Colposcopic
examination sometimes reveals a poorly developed
endocervical canal with thin, columnar epithelium: this has been successfully
treated with cryosurgery. A very cellular cervical mucus often
represents cervicitis, which should be treated with antibiotic therapy
after appropriate cultures are obtained. If the infection is not resolved
by systemic antibiotic therapy, cryosurgery can be employed. In most of these patients. poor cervical mucus is not secondary to infection
but represents a partial or total lack of response of the cervical
crypts and glands to estrogen stimulation. The impaired function in
these cases may be a late sequela of an inflammatory process or a consequence
of surgical procedures such as conization, amputation, or electrocoagulation (loop
electrical excision procedure [LEEP]) of
the cervix. In many cases, however, no etiology can be determined. TREATMENT. Treatment of poor cervical mucus is best accomplished with low-dose estrogen (0.1 mg
diethylstilbestrol, 0.625 mg conjugated estrogen. or 50 μg
ethinyl estradiol) for the 14 days before ovulation. In a 28-day
menstrual cycle, this would be from day I to day 14. There is no advantage
to continuing the hormonal therapy during the luteal phase. This
treatment markedly improves the quality of cervical mucus in approximately 40% of
patients.2 After the quality of the cervical mucus is corrected, the PCT should be
repeated. It should be mentioned that in some patients receiving clomiphene (Clomid) therapy
there is a persistent antiestrogen effect on the cervical
mucus. Once the ovulatory dose of clomiphene is achieved, low-dose estrogen
therapy is given for 7 days following the 5 days of clomiphene. Another
antiestrogen, tamoxifen (Nolvadex), has been recommended in place
of clomiphene for patients in whom clomiphene produces a persistent
antiestrogen effect on the cervical mucus.28 In some patients, human menopausal gonadotropin-human chorionic gonadotropin (HMG-HCG) therapy
has been successfully used to induce higher endogenous
estrogen levels. This results in greater cervical mucus production
and is successful, but it requires close monitoring.20, 29 Relative Dysmucorrhea. Lunenfeld and Insler3 have interpreted these problems as either relative or absolute dysmucorrhea, depending on whether or not the cervical mucus is responsive to high doses
of estrogen therapy. respectively. In patients who do not respond
to low-dose estrogen therapy, I have used an altered version of their
therapy (Fig. 6). as follows: - For the first week, ethinyl estradiol (50 μg/day) is given: a PCT is
done on day 12 of the cycle.
- The dosage is increased for another week (150 μg/day. and the PCT is
repeated. Abundant cervical mucus should be obtained.
- A progestin is then added to accomplish withdrawal bleeding.
This cycle is of course lost for conceptive purposes because of the high-dose
estrogen therapy. The purpose is to see whether the cervical crypts
and glands will respond to this therapy. Lunenfeld and Insler3 originally described this as a diagnostic procedure, but I he found it
useful therapeutically. This therapy is successful in a majority of patients. After
the one cycle of therapy. the pre-ovulatory cervical mucus
is assessed again by PCT during the subsequent spontaneous cycle. Most
patients continue to have good cervical mucus production after this
therapy cycle. Full scientific evaluation of this therapeutic mode
requires the collection of a larger amount of clinical data. Absolute Dysmucorrhea. The only therapy available to overcome the barrier of unresponsive thick
cervical mucus is IUI.1,2,19,20 In fact during the past few years, IUI techniques have essentially replaced
both estrogen therapy and cervical insemination as a means of treating
the cervical factor in infertility.19,20 A sterile polyethylene tube (No. 16 intercatheter). a Jones in vitro fertilization (IVF) catheter. or a Tefcat catheter (Monoject. Shearwood
Medical, St. Louis, MO) attached to a tuberculin syringe is threaded
through the cervix. In the past, only 0.2 mL of whole semen was used because
a larger quantity could produce an anaphylactic-type reaction and
strong uterine contractions. Recently, the use of washed motile sperm
instead of whole sperm has been proposed.2,19,20,31 In performing the IUI, the spermatozoa can be prepared for insemination
by different washed sperm and sperm-selection techniques.2,19,20,31 |