In 1993, the first results of the clinical application of PGD for aneuploidy in combination with IVF treatment cycles were reported.9 Following ovarian stimulation and IVF, embryos underwent blastomere biopsy and chromosomal analysis, with the aim of selecting the embryos for transfer on the basis of their chromosomal condition. The original idea was based on the observation that women in advanced reproductive age experience a decline in implantation. Although several factors could be implicated, the main cause is represented by an increased risk of mitotic errors at oogenesis whose immediate clinical consequences are spontaneous abortion and implantation failure. Therefore, it was postulated that the identification of euploid embryos could reverse the age effect, and PGD for aneuploidy has been applied not only to reduce the incidence of trisomic pregnancies but also to improve the ongoing implantation rate by decreasing the occurrence of spontaneous abortion. The results obtained have confirmed that the tendency to develop aneuploid embryos increases proportionally to maternal age.10,11,12 Data have also shown that the incidence of chromosomal abnormalities in preimplantation embryos was notably higher than that reported in spontaneous abortions, indicating a strong selection against aneuploidy at implantation. This is confirmed by the finding that monosomies, with the exception of monosomy X and 21, are undetected in clinical pregnancy, although they occur at the same frequency as trisomies. It is conceivable that, in agreement with animal studies and in vitro culture of human embryos, monosomies arrest developing following human genome activation.13,14 Accordingly, the rate of blastocyst formation has been reported to be dependent on maternal age, with a decrease at older ages.15
All these data provided the molecular support to the already known decline in the reproductive performance depending on age. Impulse was given to the application of PGD for aneuploidy in couples with a poor prognosis of pregnancy, and categories of patients were identified who yield high proportions of chromosomally abnormal embryos.12,16,17 In some of them, the selection of embryos for transfer based on PGD demonstrated an increased implantation rate and a concomitant reduction in the incidence of spontaneous abortions owing to the highest viability associated with a normal chromosomal complement.17,18
The Technique
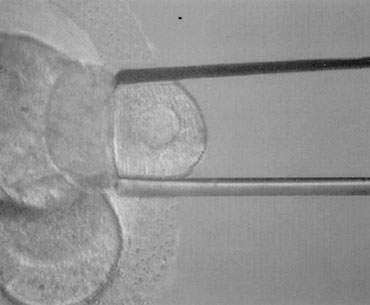
PGD is performed on blastomeres biopsied from regularly developing day 3 embryos by removing one or two cells. The effects of biopsy on embryo viability had been studied at different stages in the mouse. Embryo damage as the result of the procedure itself was negligible, and the highest live birth rates derived from biopsy done at the eight-cell stage compared with earlier stages, possibly due to the reduction in the embryonic mass (1/8 instead of 1/4). Embryo biopsy is generally carried out at 62 to 64 hours after insemination. At this time, compaction starts to take place, and cellular damage is very likely to occur if the biopsy is performed when cell-cell interactions and junctions begin to assemble. Alternatively, embryos can be shortly incubated in a divalent cation–deficient medium. The biopsy procedure entails an opening in the zona pellucida of approximately 20 to 25 μm diameter, which is performed chemically, mechanically, or using contact laser. The position of the embryo is selected to have a nucleated blastomere at the three o’clock position that is removed by using a polished glass needle and gently released in the medium (Fig. 1). Extreme attention is taken to avoid rupture of the cell membrane and damage to the surrounding blastomeres. The biopsied embryo is removed from the biopsy disk, washed, and incubated in fresh medium, whereas the blastomere undergos genetic analysis.
|
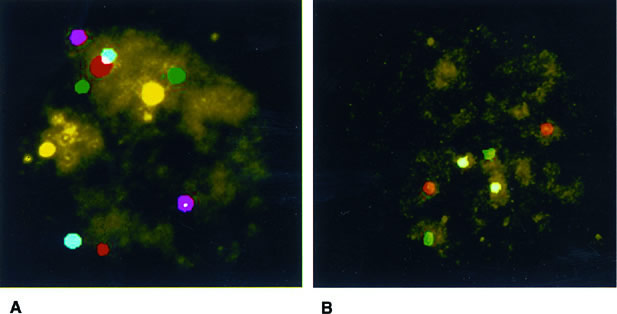
Conventional karyotyping is not applicable to PGD owing to the low metaphase yield and the long time necessary for all the steps involved. The technique of fluorescence in situ hybridization (FISH) permits chromosome enumeration to be performed in interphase nuclei and, with the use of multiple probes, provides the diagnosis of different chromosomes that can be completed by subsequent rounds with additional probes (Fig. 2). In this way, diagnosis of nine or more chromosomes is currently available, including those that are more frequently associated with abnormalities in clinical pregnancies: XY, 13, 15, 16, 18, 21, and 22.19,20,21 This is done in a time frame compatible with IVF, and embryo transfer is normally performed on day 4 or day 5 with the purpose of (1) having enough time to complete the genetic analysis; (2) selecting embryos on the basis of morphology and PGD results; and (3) reducing the risk of losing blastomeres through the breach opened in the zona pellucida during the transfer procedure owing to more advanced compaction.22
The high risk of developing aneuploid embryos as well as the error rate inherent in one-cell analysis should prompt the physician to advise pregnant patients to undergo conventional prenatal diagnosis to confirm and complete the results obtained by PGD.23
Fluorescence In Situ Hybridization: Scoring Criteria
The analysis performed on a single cell makes scoring criteria extremely important to enable a correct diagnosis. Basically, the distance between two signals specific for the same chromosomes must be at least two domains (a domain is represented by the diameter of a signal); if the distance is shorter, the pattern is considered to belong to split signals.24 Valuable information on FISH accuracy and for the formulation of scoring criteria derived from the analysis of all the blastomeres in nontransferred embryos.23,25 As represented in Table 1, 711 embryos were spread and their nuclei were fixed and analyzed with the same probes used for PGD. PGD results were confirmed in 91%; misdiagnosis occurred in 62 embryos but with a clinical relevance in only 31 (4%): 27 were erroneously classified as abnormal and 4 as normal; the remaining 31 carried an abnormality different from that diagnosed by PGD.
TABLE 1. Embryo Spreading and Fluorescence In Situ Hybridization Reanalysis of All Blastomeres*
Reanalysis† | ||||
PGD | Embryos (n) | Confirmed | Normal | Other Abnormality |
Normal |
137 |
133 (97) |
— |
4 (3) |
Monosomic |
193 |
164 |
9 |
20 |
|
One chromosome |
135 |
118 |
5 |
12 |
|
Two chromosomes |
45 |
40 |
3 |
2 |
|
Three chromosomes |
13 |
6 |
1 |
6 |
Trisomic |
184 |
164 |
14 |
6 |
|
One chromosome |
166 |
151 |
12 |
3 |
|
Two chromosomes |
15 |
11 |
1 |
3 |
|
Three chromosomes |
3 |
2 |
1 |
0 |
Haploid |
19 |
17 |
— |
2 |
Polyploid |
34 |
29 |
2 |
3 |
Complex abnormalities |
16 |
16 |
— |
— |
Total |
711 |
449 (91) |
27 (4) |
35 (5) |
PGD, preimplantation genetic diagnosis.
* Results are related to those obtained by PGD on one cell.
† Values in parentheses are percentages.
Different sources of FISH error have been identified: mosaicism, signal overlaps or splitting, failed hybridization, and loss of micronuclei during fixation. Mosaicism affects approximately 30% of in vitro–generated embryos, and the consequences for further development are diverse, depending on the type of mosaic and the proportion of abnormal cells.26 This condition is not efficiently diagnosed at PGD; however, the use of multiple probes enables the detection of complex abnormalities.
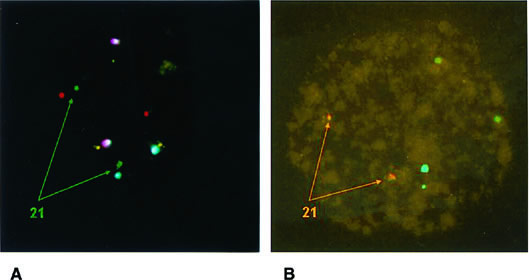
According to the data presented in Table 1, the highest source of error was represented by false monosomies. Consequently, adequate fixation protocols for the formation of good spreads have been proposed to minimize errors during this step owing to loss of micronuclei and signal overlapping.24,27 Improvement can also be derived by the use of a computerized image analysis system. A special strategy aimed at verifying failed hybridization and signal overlapping is based on the use of two probes for the same chromosome that target different loci. This is routinely done for chromosome 21, whose clinical relevance makes the use of two different probes a safety measure to prevent misdiagnosis (Fig. 3).28
Preimplantation Genetic Diagnosis on Polar Body
PGD relies on the analysis of both polar bodies to deduce the oocyte chromosomal status.29 The main advantage of the technique is the maintenance of embryo integrity owing to the fact that just meiosis by-products are used to infer the oocyte condition. However, the impossibility of diagnosing paternally derived defects and those originated after fertilization or the first cleavage events imposes notable limits to the application of this approach compared with embryo biopsy.