Definition and Extent of the Problem
U.S. data from the 1995 National Survey of Family Growth (NSFG) revealed that 28% of all women of reproductive age had ever experienced an unintended birth.1 About 20% of these women indicated that the birth had been unwanted, while 80% stated that it was mistimed. Estimates from earlier NSFG data cycles factoring in pregnancies that ended in abortion placed the percentage of unintended pregnancies (as opposed to births) as greater than 50%.2 An equal proportion of unintended pregnancies end in abortion (44%) compared with birth (43%).3 About one half of unintended pregnancies occurred among the 10% of women using no method of contraception. The remaining half (1.7 million pregnancies in 1988) occurred in women who were using a method of contraception, which demonstrates that all methods of contraception can fail to prevent pregnancy.2
Contraceptive Efficacy
Contraceptive efficacy can be calculated from population-based surveys such as the NSFG or from clinical trials and investigations. In general, failure rates derived from population-based surveys are higher than those from clinical trials, in part because women who participate in clinical trials are likely different from those who do not and may be more motivated or have incentives to use a given method of contraception correctly.4 A precise definition of contraceptive effectiveness is the proportional reduction in the monthly probability of conception, a value which is neither observable nor accurately estimated.4 Thus, contraceptive efficacy is usually assessed by measuring the number of pregnancies that occur during a specified interval of exposure to a given contraceptive method and is reported using either the Pearl index or life table techniques. The Pearl index is widely used and is required by the U.S. Food and Drug Administration (FDA) for approval of a new method of contraception. It is defined as the number of pregnancies per 100 woman-years of exposure. Because failure rates for most methods of contraception decline over time as women gain experience in using the method or those most likely to have a failure get pregnant sooner, the life table analysis calculates a separate failure rate for each month of use. When failure rates using the life table method are reported, 12 months is frequently given as a reference period.
Contraceptive effectiveness depends on both the inherent effectiveness of the method itself and correct or perfect usage of the method. The inherent or theoretical efficacy of a method is difficult if not impossible to ascertain, and even perfect use will not result in zero failures. Failure rates among couples who used a contraceptive method perfectly (both consistently and correctly) have been estimated to range from 0.1% to 10%.5 The failure rates of contraceptive methods during actual use have been shown to be higher than the estimated rates for perfect use.
The failure rate of a method during actual use depends on a number of factors, such as age, experience in using the method, and motivation to prevent pregnancy. These factors result in variations in the percentage of users who use a given method incorrectly or inconsistently. Estimates of failure rates among typical U.S. married women are included in Table 1, along with the lowest expected failure rates.6 Jones and Forrest addressed the issue of underreporting of abortions in the NSFG and arrived at corrected failure rates by method of contraception, age, marital status, and race.7 These corrected failure rates are much higher than the estimated failure rates for perfect use and vary widely depending on the characteristics of the user. For example, the failure rate of white users of oral contraceptive pills aged 35 to 44 years was 2%, whereas nonwhite oral contraceptive pill users younger than 20 years of age experienced an 18% failure rate. In general, failure rates declined with increasing age.
| Percentage of Women Experiencing an Unintended | Percentage | |
of Women |
|
| |
| Pregnancy Within the First Year of Use | Continuing | |
Use |
|
|
|
Method | Typical Usea | Perfect Useb | at One Yearc |
Chanced | 85 | 85 |
|
Spermicidese | 26 | 6 | 40 |
Periodic abstinence | 25 |
| 63 |
Calendar |
| 9 |
|
Ovulation method |
| 3 |
|
Symptothermalf |
| 2 |
|
Postovulation |
| 1 |
|
Capg |
|
|
|
Parous women | 40 | 26 | 42 |
Nulliparous women | 20 | 9 | 56 |
Sponge |
|
|
|
Parous women | 40 | 20 | 42 |
Nulliparous women | 20 | 9 | 56 |
Diaphragmg | 20 | 6 | 56 |
Withdrawal | 19 | 4 |
|
Condomh |
|
|
|
Female (Reality) | 21 | 5 | 56 |
Male | 14 | 3 | 61 |
Pill | 5 |
| 71 |
Progestin only |
| 0.5 |
|
Combined |
| 0.1 |
|
IUD |
|
|
|
2.0 | 1.5 | 81 | |
Copper T 380A | 0.8 | 0.6 | 78 |
LNg 20 | 0.1 | 0.1 | 81 |
0.3 | 0.3 | 70 | |
Norplant and Norplant-2 | 0.05 | 0.05 | 88 |
Female sterilization | 0.5 | 0.5 | 100 |
Male sterilization | 0.15 | 0.10 | 100 |
Emergency Contraceptive Pills: Treatment initiated within 72 hours after unprotected intercourse reduces the risk of pregnancy by at least 75%.i | |||
Lactational Amenorrhea Method: LAM is a highly effective, temporary method of contraception.j | |||
a Among typical couples who initiate use of a method (not necessarily for the first time), the percentage who experience an accidental pregnancy during the first year if they do not stop use for any other reason.
b Among couples who initiate use of a method (not necessarily for the first time) and who use it perfectly (both consistently and correctly), the percentage who experience an accidental pregnancy during the first year if they do not stop use for any other reason.
c Among couples attempting to avoid pregnancy, the percentage who continue to use a method for 1 year.
d The percentages becoming pregnant in the two middle columns are based on data from populations in which contraception is not used and from women who cease using contraception to become pregnant. Among such populations, about 89% become pregnant within 1 year. This estimate was lowered slightly (to 85%) to represent the percentages who would become pregnant within 1 year among women now relying on reversible methods of contraception if they abandoned contraception altogether.
e Foams, creams, gels, vaginal suppositories, and vaginal film.
f Cervical mucus (ovulation) method supplemented by calendar in the preovulatory and basal body temperature in the post-ovulatory phases.
g With spermicidal cream or jelly.
h Without spermicides.
i The treatment schedule is one dose within 72 hours after unprotected intercourse and a second dose 12 hours after the first dose. The U.S. Food and Drug Administration has declared the following brands of oral contraceptives to be safe and effective for emergency contraception: Ovral (1 dose is 2 pills), Alesse (1 dose is 5 pills), Nordette or Levlen (1 dose is 4 pills), Lo/Ovral (1 dose is 4 pills), Triphasil or Tri-Levlen (1 dose is 4 pills).
j However, to maintain effective protection against pregnancy, another method of contraception must be used as soon as menstruation resumes, the frequency or duration of breastfeeds is reduced, bottle feedings are introduced, or the baby reaches 6 months of age.
(From Trussell J, Kowal D: The essentials of contraception. In: Hatcher RA, Trussell J, et al, eds. The Essentials of Contraception: Efficacy, Safety, and Removal Considerations in Contraceptive Technology, 17th ed, pp 216---217. New York: Ardent Media, Inc., 1998.)
A number of authors have commented on the imprecision with which the terms contraceptive efficacy, effectiveness, failure rate, and pregnancy rate are used and the extent to which characteristics of the user influence the determination of these measures.4,8,9 The inherent level of protection of the method, differences in fecundity by age, frequency of intercourse, and exposure to the risk of pregnancy influence these measures.8 In addition to these factors, the gap between the lowest expected failure rate of a given contraceptive method and the failure rate in typical use is due to differences in the correct and consistent use of the method (i.e. compliance).
Compliance
DEFINITION.
Compliance has been defined as “the extent to which a person's behavior … coincides with medical or health advice.”10 Compliance in contraception refers to “the use of a contraceptive method in an ongoing and consistent manner for the prevention of pregnancy.”11 Thus both continuation and correct use are required. Although some have argued that the term compliance has paternalistic and pejorative connotations, it is widely used in contraception literature. The terms adherence or just simply correct or successful use have been suggested, although they have not yet been widely adopted.
Compliance has been studied in a number of different medical settings, and although there are important differences between complying with a 10-day prescription of antibiotics and the use of oral contraceptives on a daily basis over a number of years to prevent pregnancy, rates of compliance with contraception differ little from rates of compliance with other medical treatments.12 With the antibiotic regimen, compliance is enhanced by a decrease in symptoms; with oral contraceptives, pregnancy is avoided, although this does not provide the same ongoing reinforcement for compliance. The treatment of symptoms is generally an unequivocally positive result, whereas the decision to avoid pregnancy can be associated with ambivalence. The prescription of an antibiotic by a physician involves little choice from the patient, whereas there are a number of patient-chosen options for contraception. The antibiotic regimen is time-limited, whereas the use of oral contraceptives is ongoing. Finally, contraceptive decisions involve complex interactions between sexual partners in a social milieu. For all of these reasons, the study of compliance with contraceptive regimens is complex compared with the study of compliance with other medical regimens.13 However, Cramer has concluded that the study of medical disorders has shown that “no consequence is so severe that all patients can be assumed to comply with the prescribed treatment plan.”12
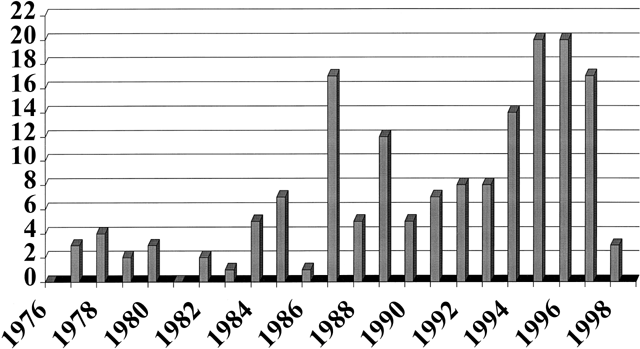
The issues of contraceptive compliance have received increasing attention and focus in the medical literature. The term, concepts of compliance, and practical problems which contribute to less successful contraception are now familiar to most clinicians. A search of the medical literature using the terms “contraception” and “compliance” revealed no such references prior to 1978; in 1988, there was a marked increase in the number of such citations, and a plateau in citations has occurred since that time (Fig. 1).
CONTINUATION.
In general, research on contraceptive compliance has focused on continuation rather than correct usage.14,15,16,17,18,19 Discontinuation of a method of contraception may be followed by continued sexual activity and the choice of an alternative method of contraception (either reversible or permanent); continued sexual activity and the choice not to use a method of birth control; the decision to be celibate; or the choice of sexual activity that does not entail a risk of pregnancy.
Women who discontinue one method of contraception and who continue to be sexually active while wishing to avoid pregnancy must be encouraged to use another method immediately without any gap in protection. The most effective method for an individual woman is one that she has chosen to use consistently and that suits her individual needs. Pratt and Bachrach noted that 8% of women who discontinued oral contraceptive use did not adopt any method of contraception, continued to be sexually active, and were not seeking pregnancy.20
Nearly one half of unintended pregnancies end in induced abortion.21 It has been estimated that as many as 70% of all abortions could be avoided if all women at risk for unintended pregnancy used the most effective methods of contraception.22 Up to 75% of women undergoing abortion report that they were practicing contraception during the month in which they conceived, representing individuals who experienced a contraceptive failure due to user or method factors.23,24,25 Nearly one half of prior users had discontinued a method of contraception within 3 months prior to conception, representing individuals who were caught in the gap between contraceptive methods.23 Thus, induced abortion is a significant outcome of many unintended pregnancies that result from problems with contraceptive compliance.
Data from the 1982 NSFG were analyzed to address the issue of contraceptive discontinuation.14 About one third of married women discontinued using a method of contraception in the first year of use. Discontinuation rates ranged from 16% for periodic abstinence to 45% for spermicide use. Twenty-six percent stopped using oral contraceptives, 29% discontinued use of condoms, 30% discontinued using a diaphragm, and 21% requested intra-uterine device (IUD) removal. Although some women switched from one method of contraception to another method, about one half continued to be at risk for an unintended pregnancy because they did not use another method of birth control.14
Discontinuation rates vary depending on characteristics of the populations studied. Trussell and associates (see Table 1) list the percentage of women estimated to be continuing use of a given method of contraception at the end of 1 year.26 Two alternative assumptions could be made: that no one becomes pregnant, or that the percentage of women who continue to use the method excludes the women who experienced an accidental pregnancy in the course of the year. Adolescents who begin using a method of contraception are more likely than older women to discontinue its use. The problems of compliance with contraception in adolescents have been widely addressed in the literature.15,16,27,28,29,30 In one study, up to two thirds of oral contraceptive pill users were no longer using the pill at the end of 1 year.31
Factors that have been associated with contraceptive compliance in adolescents include a number of social, developmental, and behavioral variables.32 These variables include the perceived risk of pregnancy, frequency of intercourse, degree of intimacy with a sexual partner, acknowledgment of sexuality, feelings of ambivalence about sexual behavior, religious commitment, emotional and sexual maturity, support by significant others, and experience with contraception.11 Health beliefs about contraceptive methods also relate to both the intention to use contraception and the actual use of contraception.33 Some adolescents who fail to return for follow-up appointments for contraception are not sexually active and thus feel that they do not need ongoing contraception. However, the pattern of adolescent sexual activity tends to be serial monogamy. The individual adolescent who is not currently sexually active may resume sexual activity in a new relationship. She may or may not use contraception effectively with a new partner.
Issues related to contraceptive discontinuation in women beyond the age of adolescence have not been addressed as thoroughly. Why do older women discontinue using a given method of contraception? The answer is complex, but there are a number of psychological factors that relate to satisfaction with the method. Miller has stated that “a woman's contraceptive vigilance … frequently depends upon … the internal balance between her positive and negative feelings toward getting pregnant and between her positive and negative feelings about her current contraceptive method.”34
In addition to psychological traits and attitudes, a variety of other factors affect contraceptive use and compliance. Demographic variables such as age, education, religion, socioeconomic status, marital status, and number of children impact on the effective use of contraception. Older, better-educated women of higher socioeconomic status and income have been shown in some studies to have fewer accidental pregnancies. The motivation to use contraception effectively varies depending on the intent of contraception: to postpone pregnancy, to space births, or to avoid pregnancy.35
Factors related to the specific method of contraception may effect compliance. Methods that are coitally related may be perceived as inconvenient or a barrier to spontaneity, and may thus be used less effectively. Cultural factors also play a part; in some societies, injections are viewed as powerful and effective medicines that may make compliance more acceptable with this mode of administration.
The experience of side-effects is the primary reason that many women give for discontinuing a method of contraception, particularly the most popular reversible method, oral contraception.17,19,20,36,37,38,39 Although some of the studies addressing this issue were performed at a time when oral contraceptive pill formulations contained higher doses of steroid hormones and were thus more likely to be associated with side-effects, the experience of side-effects or even the fear of side-effects remain important factors in contraceptive discontinuation. Continuation rates have been shown to be better with 35-μg estrogen-containing pills than with 50-μg pills.40
Correct Use
It has been stated that the “accurate measurement of compliance is not easy; easy measurements of compliance are not accurate.”10 Methods of measuring compliance include direct methods, which involve biologic assays of serum, urine, or saliva for the level of the prescribed drug or the presence of a marker substance. These methods have been used with oral contraceptives on a very limited basis and are expensive and time consuming for larger populations. Indirect methods of measuring compliance include physician estimates, self-reports or diaries, and measurements of outcome; in the case of contraceptives, the measurement of contraceptive failure is pregnancy.30 Many studies of contraceptive compliance relied on patient self-reporting, although issues surrounding sexuality, privacy, or the personal choice of pregnancy termination may make it more difficult for an individual to admit to less than perfect use of a contraceptive method or to admit to an unintended pregnancy. Innovative ways of recording oral contraceptive pill use electronically have been studied41 and may have future practical uses, such as triggering a reminder alarm. Measurements of outcome (i.e. pregnancy rates) are an inaccurate reflection of compliance, because not all noncompliance results in pregnancy. Pregnancy does, however, represent one measure—the “bottom line”—of contraceptive compliance.