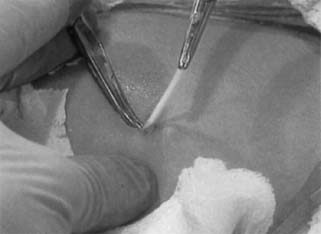
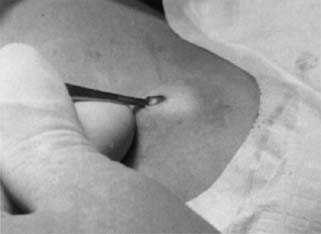
Insertion Contraceptive implants may be inserted at any time during the menstrual
cycle, immediately postpartum, or after an abortion. Pregnancy should
always be excluded by menses, contraceptive history, or a negative pregnancy
test. Practice with the “model arm” and coaching
by an experienced clinician will result in symmetric subdermal placement
that will enable easy removal of the implants. A description of both
Norplant and Implanon insertion follows. After providing counseling and obtaining written consent, position the
woman comfortably in the supine position, with the shoulder extended to 90 degrees
and the elbow flexed to expose the inner aspect of the upper
arm. Select the site for placement 8 to 12 cm proximal to the medial
epicondyle of the humerus in an area that is on the medial aspect of
the arm (on the triceps, not the biceps) and unlikely to be visible
when wearing short-sleeved shirts. When inserting six Norplant capsules, use
a template as a guide to mark the skin over the site for the incision
and points for the distal tips of the implants. Clean the area
around the insertion with antiseptic solution and create a sterile field
with a drape. Infiltrate the skin for a Norplant insertion with a total
of about 6 mL 1% lidocaine with 1:100,000 epinephrine buffered
with 1 mEq sodium bicarbonate per 10 mL lidocaine. When infiltrating
the anesthetic, make sure it is placed just beneath the dermis in
a fan shape, creating six channels to aid in placement of the implants. To
insert a single implant like Implanon, simply raise a subdermal wheal
of lidocaine with a tuberculin syringe. For Norplant, use the No. 10 trocar with the obturator in place (or the
scalpel, if the skin is tough), to pierce the skin and enter the subdermal
space. The trocar has two marks along the length of the barrel to
guide the placement of the implants. The first mark is close to the
bevel and indicates how far the trocar should be retracted before redirecting
it for placement of the subsequent implants. The second mark is
close to the hub and indicates the distance the trocar must be inserted
under the dermis before loading the implants for placement (Fig. 3). 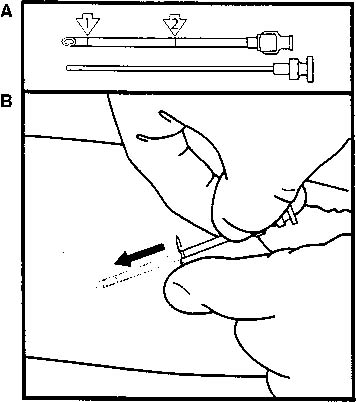 Fig. 3. A. No. 10 trocar illustrating first (1) and second (2) markings to guide placement of implants. B. After infiltrating with local anesthetic, advance the trocar and obturator
under the skin to the second mark nearer the hub.(Speroff L, Darney P: Long-acting steroid methods. In Fisher M [ed]: A
Clinical Guide for Contraception. Baltimore, Williams & Wilkins, 1992) Fig. 3. A. No. 10 trocar illustrating first (1) and second (2) markings to guide placement of implants. B. After infiltrating with local anesthetic, advance the trocar and obturator
under the skin to the second mark nearer the hub.(Speroff L, Darney P: Long-acting steroid methods. In Fisher M [ed]: A
Clinical Guide for Contraception. Baltimore, Williams & Wilkins, 1992)
|
Insert the Norplant trocar with the obturator in place up to the second
mark. Remove the obturator and load the implant into the trocar, then
gently replace the obturator until resistance is felt. Retract the trocar
to the first mark while holding the obturator stationary to deposit
the implant in the subdermal space the proper distance from the insertion
site (Fig. 4). Redirect the trocar at an angle 15 degrees from the previous implant
and repeat this procedure for placement of all six capsules (Fig. 5). After removing the trocar, place a finger over the incision and gently
palpate the implants to document their location and ensure that their
tips are at least 5 mm from the incision site. Reapproximate the skin
with an adhesive strip and apply a pressure dressing to reduce bruising. The
woman may remove the dressing after 24 hours. A diagram should
be placed in the chart documenting the location of the implants as
determined by palpation after the procedure. An accurate drawing will
help locate the implants for future removal. The Implanon inserter is
preloaded with the single contraceptive rod, easily pierces skin, and
employs a withdrawal technique to deposit the rod subdermally (Fig. 6). 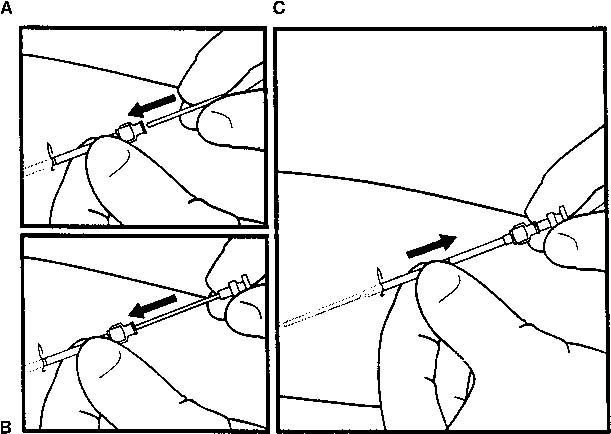 Fig. 4. Implant insertion. A. Once the trocar is inserted to the second mark, remove the obturator and
place an implant in the trocar. B. Advance the obturator to the implant (to a point of gentle resistance). C. Withdraw the trocar to the mark nearer the tip, leaving the implant behind.(Speroff L, Darney P: Long-acting steroid methods. In Fisher M [ed]: A
Clinical Guide for Contraception. Baltimore, Williams & Wilkins, 1992) Fig. 4. Implant insertion. A. Once the trocar is inserted to the second mark, remove the obturator and
place an implant in the trocar. B. Advance the obturator to the implant (to a point of gentle resistance). C. Withdraw the trocar to the mark nearer the tip, leaving the implant behind.(Speroff L, Darney P: Long-acting steroid methods. In Fisher M [ed]: A
Clinical Guide for Contraception. Baltimore, Williams & Wilkins, 1992)
|
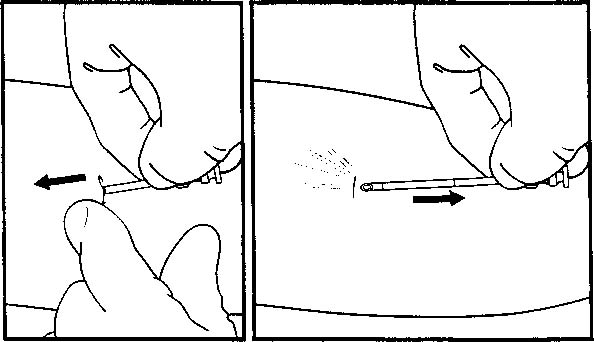 Fig. 5. Implant insertion. Redirect and advance the trocar and obturator while
pushing the previously placed implant away from the trocar.(Speroff L, Darney P: Long-acting steroid methods. In Fisher M [ed]: A
Clinical Guide for Contraception. Baltimore, Williams & Wilkins, 1992) Fig. 5. Implant insertion. Redirect and advance the trocar and obturator while
pushing the previously placed implant away from the trocar.(Speroff L, Darney P: Long-acting steroid methods. In Fisher M [ed]: A
Clinical Guide for Contraception. Baltimore, Williams & Wilkins, 1992)
|
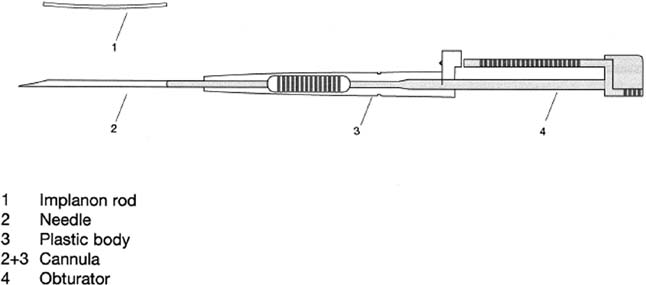 Fig. 6. Implanon inserter/trocar.(Herjan JT, Coelingh Bennink HJ: Introduction; Presentation of clinical
data on Implanon. Contraception 58[Suppl 1]:75S, 1998) Fig. 6. Implanon inserter/trocar.(Herjan JT, Coelingh Bennink HJ: Introduction; Presentation of clinical
data on Implanon. Contraception 58[Suppl 1]:75S, 1998)
|
COMPLICATIONS OF INSERTION. Complications are uncommon and rarely serious. They include infection (0.8%), expulsion (0.4%), hematoma formation (<1.0%), and
local irritation (temporary) (4.7%).138 Infection at the site usually occurs within the week after insertion but
may occur up to 4 to 5 months later. These infections may be treated
with oral antibiotics (e.g. dicloxacillin 250 mg, four times daily for 5 days) and
local heat with the implants in place. However, one third
of these infections are unresponsive to antibiotic therapy and require
subsequent implant removal.138 If expulsion of one or more implants occurs, it usually happens shortly
after insertion and is rare after the first few months of use. The primary
causes of expulsion are concurrent infection and placement of the
implants too close to the insertion site. If one implant is expelled
without signs of infection, a new one can be inserted promptly. If infection
is present, wait for resolution. Removal When a woman decides to have her implants removed and does not intend to
conceive, plans must be made for another method of contraception before
the removal because she may be susceptible to unwanted pregnancy within
hours after the removal. Removal of implants is done with the patient
under local anesthesia and may take from 2 to 60 minutes, depending
on the number of implants, the skill of the practitioner, the depth
of the implants, and the thickness of the surrounding fibrous sheaths. Several techniques are available for removal of contraceptive implants. The
original technique is described in the package insert for Norplant
and uses a mosquito forceps to grasp the implant through the incision
and a scalpel or second forceps to incise the fibrous sheath. In the
U technique, a modified vasectomy clamp is used to grasp the implants, followed
by scalpel incision of the fibrous sheath. The “fingers
only” method, particularly appropriate for Implanon, uses finger
manipulation and extrusion of the implant through a small incision, followed
by scalpel incision of the sheath.139,140,141,142 Other methods have been reported; no one method is ideal for all situations.143,144 Some research suggests that the U technique may be preferable to the standard
technique because of decreased complication rates, decreased removal
times, and speedier training.140,145,146 The “fingers only” technique is less painful and produces
a smaller scar but is applicable only when the implants are easily palpable. STANDARD REMOVAL TECHNIQUE. Position the woman as for insertion of the implants, clean the skin with
antiseptic, and create a sterile field. Press down on the tips of the
implants closest to the axilla to raise the distal (closest to the elbow) tips
of the implants. This will identify the incision site over
the implant that is most centrally located and equidistant from the other
tips. Inject 1 to 2 mL buffered 1% lidocaine with 1:100,000 epinephrine
under the tips of the implants at the incision site (Fig. 7). There is no need to inject additional anesthetic along the length of
the capsules. Too much anesthetic makes palpation difficult. Make a 3- to 5-mm
incision at the distal tip of the middle implant. 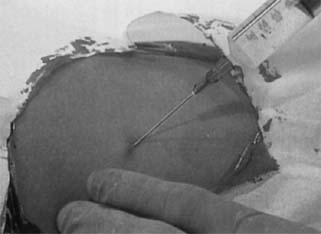 Fig. 7. Implant removal. Inject local anesthetic under the tips of the implants
to be removed.(Norplant System: Levonorgestrel Implants. Vol. 2, Standard Removal Technique, p 7. Philadelphia, Wyeth-Ayerst Laboratories, 1995) Fig. 7. Implant removal. Inject local anesthetic under the tips of the implants
to be removed.(Norplant System: Levonorgestrel Implants. Vol. 2, Standard Removal Technique, p 7. Philadelphia, Wyeth-Ayerst Laboratories, 1995)
|
To remove the implants, the fibrous sheath encasing each capsule must be
opened, preferably at the tip of each implant, where the implant wall
is thickest. To extract the implants with instruments, open the subdermal
space with the curved mosquito clamp. Grasp the tip of the implant
closest to the incision and pull it into direct view at the incision (Fig. 8). Then, using a finger covered with an open gauze sponge, a straight mosquito
clamp, or a scalpel, dissect away the fibrous sheath (Fig. 9). If the sheath is too dense to enter with the sponge or clamp, dissect
it cautiously with the scalpel directly over the tip of the implant. Do
not cut across the implant because you may inadvertently open or transect
the capsule. Once the sheath is opened and the tip of the first
implant is exposed, regrasp the exposed tip with the straight clamp. Release
the first clamp and pull the implant out from the surrounding
fibrous tissue (Fig. 10). If the implant cannot be brought into the incision with direct traction, redirecting
the mosquito clamp anteriorly will help expose the implant. Repeat
this procedure to remove all six implants, and show all
the capsules to the woman to assure her they have all been removed. Close
the incision with an adhesive strip and cover with a small dressing. If
dissection was extensive, reduce bruising with a pressure dressing
and an ice pack. 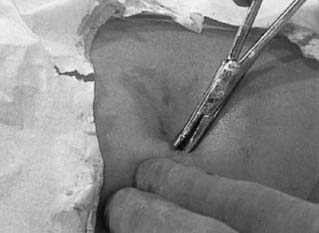 Fig. 8. Implant removal. Push an implant toward the incision and grasp its tip
with the curved mosquito clamp.(Norplant System: Levonorgestrel Implants. Vol. 2, Standard Removal Technique, p 7. Philadelphia, Wyeth-Ayerst Laboratories, 1995) Fig. 8. Implant removal. Push an implant toward the incision and grasp its tip
with the curved mosquito clamp.(Norplant System: Levonorgestrel Implants. Vol. 2, Standard Removal Technique, p 7. Philadelphia, Wyeth-Ayerst Laboratories, 1995)
|
 Fig. 9. Implant removal. Lift up on the clamp and clean the fibrous sheath from
the implant using a finger covered with an open sponge or gently with
a scalpel.(Norplant System: Levonorgestrel Implants. Vol. 2, Standard Removal Technique, p 8. Philadelphia, Wyeth-Ayerst Laboratories, 1995) Fig. 9. Implant removal. Lift up on the clamp and clean the fibrous sheath from
the implant using a finger covered with an open sponge or gently with
a scalpel.(Norplant System: Levonorgestrel Implants. Vol. 2, Standard Removal Technique, p 8. Philadelphia, Wyeth-Ayerst Laboratories, 1995)
|
THE U TECHNIQUE. Prepare the field and inject the anesthetic over the planned incision site
parallel to and between the third and the fourth implants for Norplant
and adjacent to a single implant. Make a 4-mm incision parallel to
the implant and use the vasectomy clamp to grasp the nearest implant. Holding
a fingertip on the skin surface against the implant can help
guide it into the vasectomy clamp. Pull the implant into the incision
by moving the handle of the clamp toward the patient’s head (Fig. 11) and clean the fibrous sheath with an open sponge or incise it with a
scalpel to free the implant for removal (Fig. 12). Repeat this procedure to remove the other implants. Close the incision
as described previously. 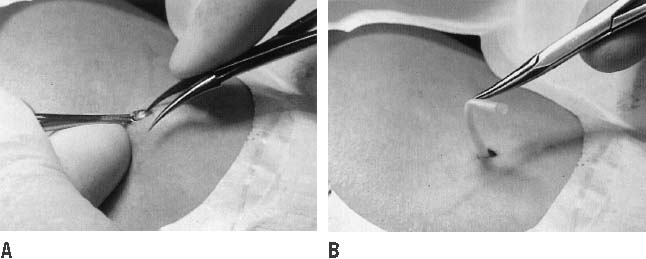 Fig. 12. Removal of the capsule using the U technique. A. With the jaws of the dissecting forceps open, impale the capsule shaft
with one of the pointed forceps tips. B. Rotate the hand holding the dissecting forceps palm upward, so the forceps
tips are facing upward with the implant still impaled. Close the
dissecting forceps gently—just enough to hold the implant without
cutting it—then release the ring forceps, allowing the implant
to emerge from the incision.(Norplant System: Levonorgestrel Implants Vol. 3, Alternative Removal Techniques, pp 8–9. Philadelphia, Wyeth-Ayerst Laboratories, 1995) Fig. 12. Removal of the capsule using the U technique. A. With the jaws of the dissecting forceps open, impale the capsule shaft
with one of the pointed forceps tips. B. Rotate the hand holding the dissecting forceps palm upward, so the forceps
tips are facing upward with the implant still impaled. Close the
dissecting forceps gently—just enough to hold the implant without
cutting it—then release the ring forceps, allowing the implant
to emerge from the incision.(Norplant System: Levonorgestrel Implants Vol. 3, Alternative Removal Techniques, pp 8–9. Philadelphia, Wyeth-Ayerst Laboratories, 1995)
|
THE “FINGERS ONLY” TECHNIQUE. This approach is simple, causes little pain, and is especially applicable
to one-implant (Implanon) and two-implant (Jadelle) systems.147 For six implants, a combination of fingers and instruments is often most
efficient. After positioning the patient’s arm and creating a sterile field
as described previously, inject no more than 0.5 mL of local anesthetic
directly at the tips of the implants. Make a 3-mm incision directly
at the proximal tips of the most centrally located implants. Manipulate
the implant tip into the incision with finger pressure until the fibrous
sheath is visible. Maintaining this finger pressure on the implant
with one hand to keep its tip visible in the incision, nick the sheath
at the tip of the capsule with a No. 11 scalpel (Fig. 13). Once the sheath is opened, simply squeeze out the implant (Fig. 14). Repeat this procedure to remove the remaining implants through the single
incision. 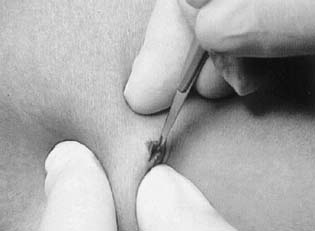 Fig. 13. Incise the fibrous sheath with repeated cuts across the tip of the implant.(Norplant System: Levonorgestrel Implants. Vol. 3, Alternative Removal
Techniques, p 6. Philadelphia, Wyeth-Ayerst Laboratories, 1995) Fig. 13. Incise the fibrous sheath with repeated cuts across the tip of the implant.(Norplant System: Levonorgestrel Implants. Vol. 3, Alternative Removal
Techniques, p 6. Philadelphia, Wyeth-Ayerst Laboratories, 1995)
|
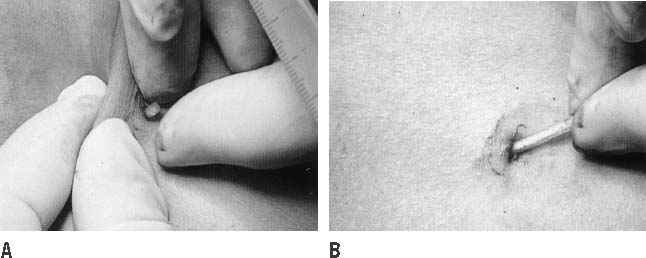 Fig. 14. Push the implant out of its fibrous sheath and remove it.(Norplant System: Levonorgestrel Implants. Vol. 3, Alternative Removal
Techniques, p 6. Philadelphia, Wyeth-Ayerst Laboratories, 1995) Fig. 14. Push the implant out of its fibrous sheath and remove it.(Norplant System: Levonorgestrel Implants. Vol. 3, Alternative Removal
Techniques, p 6. Philadelphia, Wyeth-Ayerst Laboratories, 1995)
|
COMPLICATIONS OF REMOVAL. The most frequent complication of removal is difficulty feeling one or
more of the implants. Impalpable implants can best be located with short-focus, high-frequency (7.5 mHz) ultrasonography or compression radiography (mammography).68,69 We have found sonography to be most efficient. The choice of method depends
on which is most convenient at the institution. Real-time ultrasonography
or fluoroscopy is rarely needed unless removal might pose a
risk to neurovascular structures. Newer implants are packaged in an insertion
trocar that facilitates proper insertion, rendering the problem
of nonpalpable implants a less common concern. If an implant is broken
during attempted removal, it may be necessary to make a second incision
just over the end of the implant to remove the broken portion. It
is better to make a second small incision than to enlarge a poorly situated
one. |