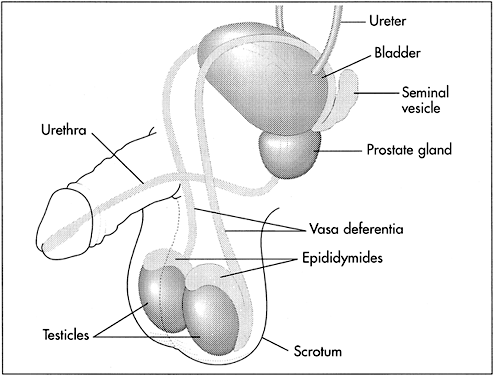
The male reproductive organs include the testicles, the ducts, and the accessory glands (Fig. 1). The testicles produce sperm and the male sex hormone testosterone. After vasectomy, the testes continue to produce both sperm and hormones.
|
The second group of organs is a series of connected ducts: the epididymides, the vasa deferentia, and the urethra. Each of the two epididymides (which begin at and are connected to the testes) are connected to one of the vasa deferentia. Sperm pass through the epididymis to get from the testis to the vas and are also stored in the most distal portion, or tail, of the epididymis. Sperm become motile and acquire the ability to fertilize ova during transport through the epididymides. The vasa begin at the epididymides and end at the base of the prostate, where they come together and are connected to the urethra and the accessory glands. Here, the sperm, which were carried by the vasa deferentia, mix with secretions from the accessory glands. The urethra carries the semen (i.e. sperm contained in the secretions from the accessory glands) out of the body during ejaculation. The urethra also carries urine.
The third group of internal organs are the accessory glands. These include the seminal vesicles, the prostate, and the bulbourethral glands. These glands secrete the seminal fluid that carries sperm through the urethra during ejaculation.
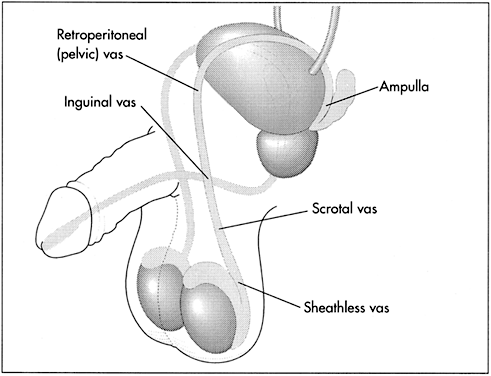
The vas deferens is a firm, tubular structure approximately 3 to 4 mm in diameter and approximately 35 cm in length. It extends from the tail of the epididymis to the prostate, where, together with the duct of the seminal vesicle, it forms the ejaculatory duct.
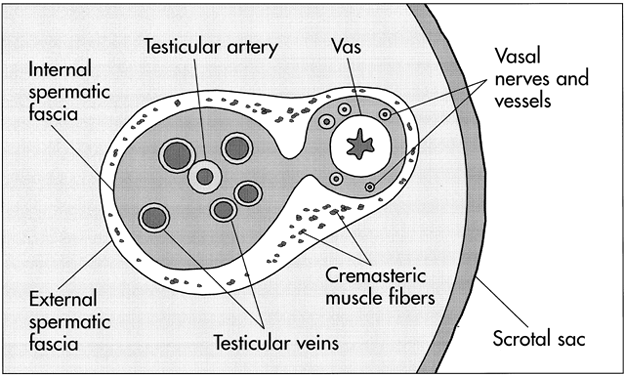
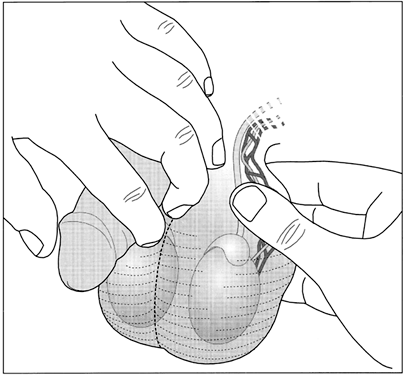
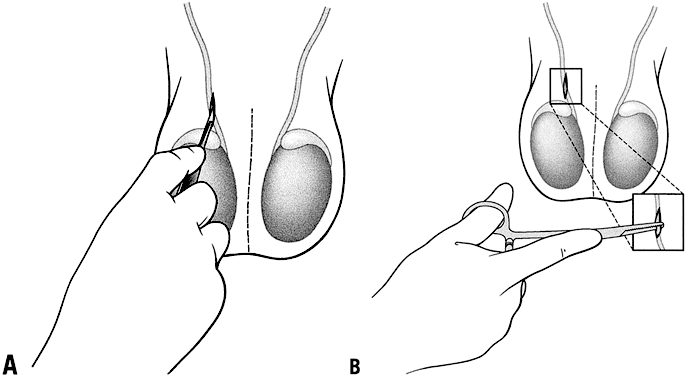
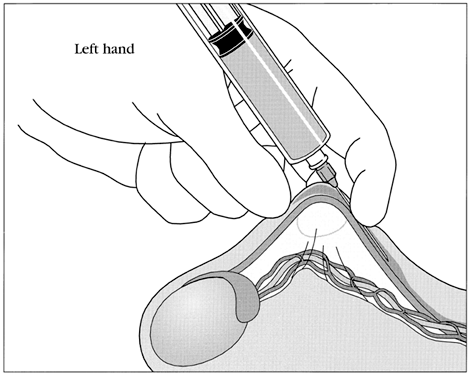
The vas deferens may be divided into five portions: the sheathless epididymal vas, the scrotal vas, the inguinal vas, the retroperitoneal or pelvic vas, and the ampulla (Fig. 2). The portion of the vas of clinical interest in relation to vasectomy is the scrotal vas in the midscrotal area. Here the vas is located within the spermatic cord, which is made up of fascia, arteries, veins, and nerves and which suspends the testis in the scrotum (Fig. 3). The firm, thick structure of the vas can be easily palpated and differentiated from other structures in the spermatic cord. Also in the spermatic cord is the testicular artery, which supplies blood to the testis and epididymis, and the testicular veins, which form the pampiniform plexus that returns blood from the testis and epididymis.
|
|
The vas deferens is composed of three layers of smooth muscle: the outer and inner longitudinal layers and the middle circular layer. It is capable of powerful peristaltic motion. There is a thick sheet of connective tissue exterior to the muscle layer. The lumen of the vas, like that of the epididymis, is lined with pseudostratified epithelium and contains longitudinal folds lined with microvilli.
The blood supply of the vas deferens is from the artery of the vas deferens (deferential artery), a branch of the superior vesical artery that is also important in collateral circulation for the testicle. This artery is easily separated from the vas during vasectomy; however, it may be a source of hemorrhage during vasectomy if it is not separated or ligated.
The nerves of the testes (the superior spermatic nerves) arise from the renal plexus and intermesenteric nerves and travel in association with the testicular arteries, whereas the inferior spermatic nerves arise from the hypogastric plexus and course around and along the vas deferens to innervate the epididymides. At the junction of the epididymis and vas deferens, the amount of adrenergic innervation increases, with innervation of the vas consisting of short, postganglionic neurons.4 Adrenergic fibers, which are found in all three smooth muscle layers, are most likely the motor supply of the vas muscle. The sheath of the vas in the scrotal portion contains pain nerves. Careful infiltration of the sheath with local anesthetic agents is effective in reducing pain during the procedure.
Blood vessels and nerves involved in erection and ejaculation, including the internal pudendal artery, dorsal and cavernous veins, pudendal nerves (sensory nerves to the penis), and nerves from the pelvic plexus arising from sacral nerves S2-S4 (nerves involved in erection), are located well away from the procedure site and are therefore unaffected by vasectomy.