Anal Incontinence
Authors
INTRODUCTION
Anal incontinence, the involuntary loss of flatus or stool per anus, afflicts far more women than is often appreciated.1, 2, 3 Social embarrassment, fear about the cause, or even a misconception that incontinence is part of the normal aging process may prevent a patient from revealing these symptoms to her gynecologist.4, 5, 6 The severity of the condition often mandates continuous protection from soiling. These symptoms may persist for years before a patient vocalizes complaints and obtains relief.
Although variable, the most common cause of anal incontinence is injury to the anal sphincter during parturition.3 There are two factors most commonly involved in anal incontinence. One is colonic contraction or motility, and the other is the sphincter injury or sphincter defect. Anal incontinence is classified in three different categories: fecal seepage – conscious leakage of fecal matter after a normal bowel action and during or after excercise; passive incontinence – when the patient is not aware of fecal leakage; and urge incontinence – when the patient is unable to actively defer a bowel movement. Sphincter injury or sphincter defect falls into the fecal seepage category. Inadequate repair or poor healing of obstetric perineal injuries may present as anal incontinence within days to weeks of delivery. In fact, some authors have reported an incidence of anal incontinence after third- or fourth-degree laceration as high as 40–60%.5, 7 In these European studies, between 45% and 68% of women had mediolateral episiotomy, a technique associated with third- or fourth-degree laceration in approximately 25% of women.8 In a separate group of 35 women who did not undergo mediolateral episiotomies but experienced obstetric sphincter injury, the incidence of incontinence of flatus was 17% compared to 3% in the intact sphincter group.9 Incontinence of liquid or solid stool was not significantly different in the study versus control groups.
Other causes include perineal trauma from straddle injuries or aberrant healing from surgical procedures such as perineoplasty, hemorrhoidectomy, fissurectomy, or radical oncologic procedures. Rectal prolapse is a very common cause in older women. Anal incontinence may even occur as the result of a primary neoplastic process. Less frequently, fecal incontinence may result from congenital anomalies, neurologic disorders affecting the perineum and anal sphincter, or demyelinating diseases that alter multiorgan systems. Fecal incontinence is not common as either an acute or chronic side effect of radiotherapy.10, 11
This chapter addresses the repair of intrinsic anatomic muscular dysfunction. Systemic, malignant, or neurologic disease processes lie outside the scope of the surgical procedures discussed here.
ANATOMY
The anatomy and physiology of the anal canal have been well studied. Current literature expands upon Shafik's original work on the triple loop theory.12, 13, 14, 15, 16 The anal canal measures 2.5–4 cm in length and extends from the mucocutaneous junction caudally through the dentate line, above which columnar colonic mucosa is found. Here the anal mucosa is composed of cuboidal cells formed into eight to 14 folds called the rectal columns of Morgagni, which overlie an internal hemorrhoidal plexus. The arterial blood supply originates from the superior hemorrhoidal artery (the terminal branch of the inferior mesenteric artery) and from the middle and inferior hemorrhoidal arteries, which arise from the anterior division of the internal iliac artery. The venous drainage returns to the portal system by way of the superior hemorrhoidal and inferior mesenteric veins. The middle and inferior hemorrhoidal veins return to the internal iliac system. The boundaries of the anal canal are the coccyx posteriorly, with interposed adipose and muscular tissues; and the ischiorectal fossa, with Alcock's canal laterally.
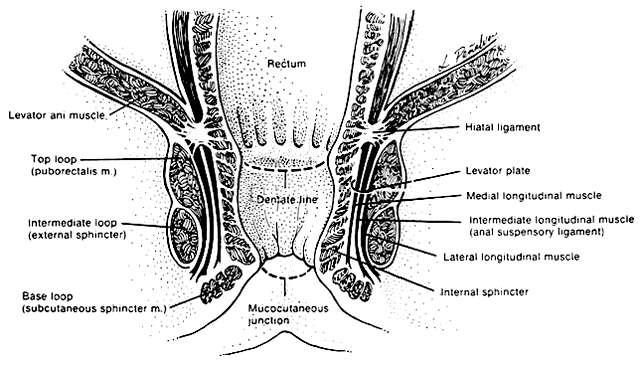
The anal canal is surrounded by two major muscle groups, the involuntary internal and the voluntary external sphincter systems (Fig. 1). These two systems provide a fine balance between continence and controlled intermittent evacuations of fecal material. The capacity to distinguish between flatus and variable stool consistency is achieved through a complicated integration of pelvic musculature.
|
The involuntary internal anal sphincter is composed of autonomically innervated circular smooth muscle fibers that lie within the wall of the anal canal. These fibers remain in a steady state of contracture, yielding tone to the anal canal and maintaining it in a collapsed position when nonfunctioning. As the rectal ampulla fills and distends, the sensory stimulus for evacuation begins the process of defecation. The contribution of the internal sphincter to anal continence has been studied with magnetic resonance imaging.6 In this study, the internal sphincter was found to contribute 54% of the anterior thickness of the sphincter in nulliparous women. Application of this knowledge has important implications in the repair of third- and fourth-degree perineal lacerations and contrasts with the traditional understanding portrayed in the medical literature.
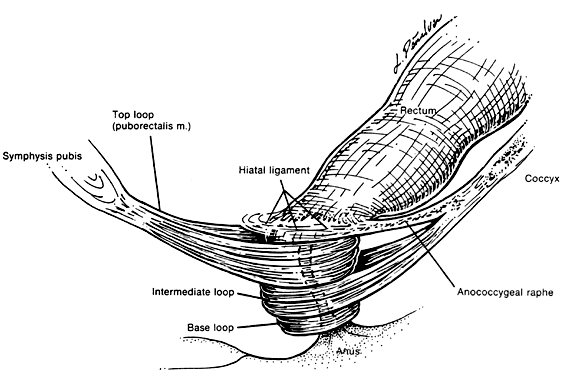
The voluntary external anal sphincter (Fig. 2), composed of multiple interrelated skeletal muscle loops (separated by their fascial coverings), lies in close approximation to the levator ani and the muscles of the urogenital diaphragm. This sphincter system contains three muscular loops that surround the anal canal. The top loop is the deepest and fuses with the puborectalis muscle of the levator ani group. It attaches to the pubic symphysis and encircles the colon posteriorly at the anorectal verge. Innervation is provided by the inferior hemorrhoidal branch of the pudendal nerve. This superior loop functions to angulate the anal canal forward in order to maintain continence. The intermediate loop portion of the external sphincter is found anteriorly around the midportion of the anal canal as a fleshy muscle. These fibers terminate posteriorly in an interdigitating fashion and attach to the tip of the coccyx in a fibrous tendon. On voluntary stimulation by the perineal branch of S4, contraction of this intermediate sphincter retracts the anal canal posteriorly to align with the rectum. The third and most superficial muscle of this complex sphincter system is the base loop. The smallest of the three loops, it is composed of skeletal muscle fibers that completely encircle the anal canal distally and attach to the mucocutaneous junction. It is innervated by the inferior hemorrhoidal nerve. These three muscle groups function in concert to provide the capacity for both continence and defecation (Fig. 3).
|
Just above the top loop and puborectalis muscles is the pubococcygeus muscle of the levator ani group. Its circular skeletal muscle fibers extend from the pubic bone to the coccyx, where they fuse as decussating tendons called the anococcygeal raphe. The fleshy, muscular portion of the pubococcygeus encircles the hiatal space, through which pass the pelvic viscera. The pubococcygeus muscle adheres to the colonic wall by dense fascial fibers called the hiatal ligament. Muscular traction on the hiatal ligament effects posterior circumferential distention and elevation of the anal canal.
Between the internal sphincter and the three circular muscle loops of the external sphincter lie the longitudinal fibers of the levator plate. These muscle fibers anchor the U-shaped external sphincter loops to the anal wall and perianal skin. Longitudinal bundles of muscle fibers descend from the pubococcygeus muscle between the internal sphincter and the three loops of the external sphincter system. The most medial smooth-muscle group, the medial longitudinal muscle, attaches the levator plate to the anal canal wall, actually fusing with the wall. The intermediate longitudinal muscle group, also called the suspensory ligament, inserts as the central tendon that attaches above the external anal sphincter base loop and the perianal skin. The lateral longitudinal muscle bundle is a condensation from the top loop of the external anal sphincter, which attaches to the medial surface of the intermediate loop, inserting just above the base loop. These three longitudinal muscle bundles provide continuity and attachments between the internal and external sphincter systems.
Interspersed among these muscle fibers are six perirectal spaces. Four intersphincteric spaces lie among the three longitudinal muscle layers and separate their dense fascial coverings. Between the skin and the base loop is the subcutaneous space, which is continuous with the perirectal spaces. The central space is low around the anal canal and communicates with each of the surrounding spaces. It separates the base loop from the intermediate loop and is an insertion site for the longitudinal muscle tendon bundles. These potential spaces play an important role in the development and repair of perirectal abscesses and rectocutaneous fistulae.
Physiology of the sphincter systems
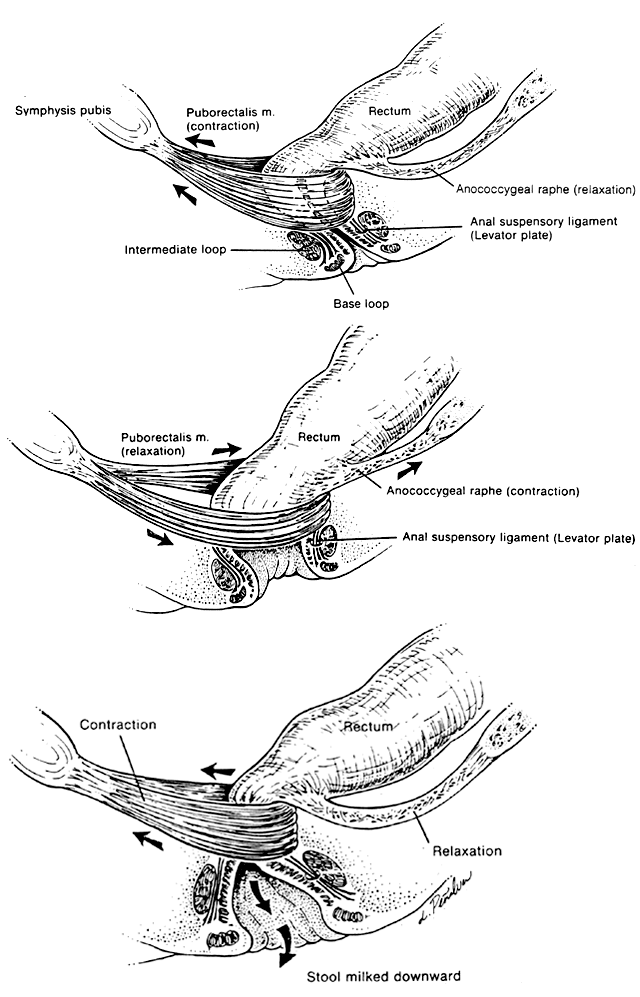
Much investigation has centered on the mechanism of defecation.17, 18, 19, 20 Electromyography, manometric pressure testing, and radiography have been used to explore the function of these muscle systems. When the rectal ampulla fills and distends, the signal for defecation is triggered (see Fig. 3). Defecation is initiated by Valsalva's maneuver, which increases intra-abdominal pressure. In a reflex manner, the internal anal sphincter must relax to accommodate the bolus. Simultaneous voluntary relaxation of the top loop of the external anal sphincter allows the anal canal to relax posteriorly. Voluntary contraction of the levator plate and the longitudinal muscle bundles acts to widen the anal inlet by means of traction on the hiatal ligament. This action simultaneously shortens the canal length, flares the base loop of the external sphincter outward, and opens the anal orifice. The passage of fecal material distally is completed by intermittent vermicular contractions of the intermediate and base loops, which compress the flow outward. Once emptying is complete, the external loops, anococcygeal raphe, and levator plate all relax, returning to their normal positions, while the puborectalis muscle and the top loop contract, repositioning the canal forward. The process is completed with reflex contraction of the internal sphincter system to recollapse the anal canal, thereby maintaining continence.
Maintenance of a minimal baseline anal pressure is necessary for fecal continence. The internal sphincter contributes approximately 50% of the force necessary to keep the anus closed when empty, and the external sphincter 25–30%. Variable filling of the internal hemorrhoidal plexuses provides the remainder of the pressure required to seal the anus by conforming around the sphincters.21 As the anus is distended, the contribution of the internal sphincter declines progressively, while stretching of passive canal wall elements other than the sphincters limits capacity and provides the force to maintain continence. The importance of the involuntary internal sphincter and the role of autonomic dysfunction in fecal incontinence can therefore be understood. Manometric evaluation reveals a distinctive pressure profile of the anal canal resulting from the combined effects of the different muscles that constitute the anal sphincters.22 In the upper anal canal, radial pressure measurements indicate a lower pressure anteriorly, corresponding to the posterolateral location of the puborectalis muscle. In the distal anus, the pressure is less posteriorly because of the orientation of the intermediate loop.
MEASUREMENT OF ANORECTAL PRESSURE
Simple manometric pressure measurements in some incontinent patients do not always distinguish true deficiencies. More sophisticated techniques involving measurement of anorectal pressure gradients or three-dimensional manometry (vector manometry) may prove more accurate, but are not widely available.23, 24
Biofeedback techniques using visual manometry have been successful in the treatment of some patients with fecal incontinence.25 Of motivated patients, 50–80% experience improvement in symptoms. An increase in both resting and squeezing pressures can be demonstrated.26 The efficacy persists for at least 5 years in successfully treated patients.27
Of women with idiopathic fecal incontinence, 75–80% have evidence of nerve injury to the pelvic floor musculature.28 Such patients have slowing of nerve conduction, which is measured as pudendal nerve terminal motor latency (PNTML) on electromyographic tests.29 Approximately 60% of patients with sphincter injury have concomitant pudendal neuropathy.28 The contributing role of vaginal delivery is underscored by the finding that 42% of women demonstrate slowed conduction in the pudendal nerve (prolonged PNTML) immediately after delivery, of whom 60% recover by 2 months.30
Fiber density, an electromyographic index of the number of muscle fibers innervated by a single nerve axon, is increased in anal incontinence. This effect is believed to result from injury, causing denervation with subsequent reinnervation. Increases in PNTML and fiber density are present in incontinent patients and are associated with aging, multiparity (if delivered vaginally), increased birth weight, and a long duration of the second stage of labor.30 Increases are not found after episiotomy or first- and second-degree perineal lacerations, but do occur after third- or fourth-degree obstetric perineal tears. Patients with anal incontinence have decreased sphincter length, lower maximum resting pressure, and decreased maximum voluntary contraction pressure. Such patients also have decreased sensitivity to electrical stimulation and temperature sensation in the anus.31, 32
In normal, continent patients, sensations of temperature and pressure from stool or flatus in the anal canal are better appreciated and evoke an increase in sphincter tone.33 Differences in sensation are postulated to contribute to the difficulty that incontinent patients typically experience in differentiating flatus from solid stool. Pudendal neuropathy is most accurately assessed by measurement of PNTML; however, this test is not widely available and may be inaccurate unless performed by an experienced clinician. Manometry is not thought to be useful in the determination of pudendal nerve malfunction.34 One preliminary prospective, controlled trial demonstrates that a component of routine manometry, the rectoanal excitatory reflex, may prove useful in this diagnosis.35 The recognition of pudendal nerve injury and the distinction of this disorder from sphincter injury is vital.
Defecography (evacuation proctography), a video radiographic technique to evaluate defecation, can demonstrate rectocele, enterocele, rectal prolapse or rectal intussusception. It is an excellent test to evaluate the causes of passive incontinence in older women as a result of all the problems mentioned above.36, 37
Anal endosonography has been applied to the evaluation of fecal incontinence. The normal anatomy of the external and internal sphincters as imaged by ultrasound has been described.38 Comparison of anal ultrasonography with anal manometry in normal volunteers demonstrates no correlation of sphincter muscle thickness with squeeze pressure.39
However, defects in the external and/or internal anal sphincters demonstrated ultrasonographically correlate with manometric findings.40, 41 In addition, electromyographic mapping of sphincter abnormalities confirms anal endosonographic results.41, 42, 43 A prospective study of 127 women compared anal manometry and anal ultrasonography before and after vaginal delivery.44 Sphincter defects can be demonstrated by anal ultrasound in 25% of incontinent women believed to have intact sphincters on the basis of a digital examination. This discrepancy may be related, in part, to difficulty in distinguishing scar tissue during the examination. Ultrasound characteristics of scar tissue differ from those of normal muscle.40, 45 Incontinent patients with normal manometry may also have sphincter abnormalities seen on ultrasound examination.45 Finally, a small number of patients with idiopathic incontinence resulting from pudendal neuropathy and confirmed by abnormal PNTML will have coexistent sphincter defects.41, 43, 45 Thus, anal endosonography can provide information that may not be obtained by digital examination or traditional testing, and this information is valuable for those making treatment decisions.
PHYSICAL EXAMINATION AND TESTING
All gynecologic patients and certainly parous women should be questioned for a history of anal incontinence. The inability to maintain flatus or solid and liquid stool should be investigated thoroughly. The history should elicit duration, frequency, and severity of symptoms; an inciting event; and the necessity for sanitary protection. External anal sphincter and puborectalis weakness, as measured by maximum squeezing pressure on manometry, is associated with fecal and urinary urge incontinence, fecal incontinence en route to the toilet, and urinary stress incontinence.46
Many patients experience loss of flatus or diarrhea, but not solid stool. Patients must be questioned for symptoms of rectocele, such as perineal pressure or protrusion of the rectum into the vaginal introitus. Patients should also be questioned about rectal prolapse. In addition, inquiry regarding a prior history of inflammatory bowel disease, vulvar or vaginal infections, venereal diseases, neurologic deficits, or nontraditional sexual practices (e.g., anal intercourse, foreign body manipulation, rape, or sexual abuse) should direct a practitioner toward a diagnostic evaluation before attempted surgical repair.
The physical examination of these patients follows the sequence of a standard gynecologic visit. The perineum is inspected with attention to muscular weakness, asymmetry, or inability to contract the muscles of the urogenital diaphragm or the anal sphincter. Injury to the external sphincter is evidenced by absence of the radial creases anterior to the anus.47 With the patient instructed to tighten her anal sphincter, inspection of the perineum should reveal both anal constriction and an inward motion of the anal orifice.47 The detection of horse-shoe defect anteriorly is very classic for anterior sphincter injury.
Sensory stimuli should be tested, and an intact bulbocavernosus reflex should be evoked bilaterally by stroking the labia majora and observing tightening of the anal orifice. Perception of sharp pain stimulation over the perineum may be altered. Palpation of the labia majora and the perineal body may reveal laxity or a mass effect. Most commonly, a thinned rectovaginal septum or perineal body indicates functional loss. In a patient with separation of the sphincter, neural dysfunction may be demonstrated by the absence of perianal skin dimpling with anal constriction.47 This sign is important because simple repair of the sphincter will not correct the incontinence.47 Manometric measurements of maximum squeezing pressure correlate with digital assessment of sphincter strength.48
On digital examination, squeezing of the urogenital diaphragmatic musculature, with tightening of the bulbocavernosus and the levator ani muscles, gives an indication of neuromotor stability. To determine the integrity of the septum and the external anal sphincter loops, rectovaginal palpation is performed. The patient is instructed to tighten her anal sphincter around the examining finger; assessment of the levator ani muscles is performed by palpation of the muscular sling located posterior and cephalad to the external sphincter.47 The anal canal is elevated toward the symphysis pubis when the patient contracts these muscles.
Finally, the length and thickness of the rectovaginal septum at the vaginal introitus versus the mid and upper third of the vagina should be assessed. The desire to retain sexual capacity should be discussed before attempting surgical correction. Following this thorough history and physical examination, additional testing may be necessary to confirm or clarify the clinical impression. Manometry, electromyographic studies, electrophysiologic studies, and anal ultrasonography may all be useful. To achieve accurate results, experience in test performance and interpretation is necessary. Also, these studies are not readily available at all institutions. When the history and physical examination are suggestive, close attention should be directed to the possibility of a rectovaginal fistula, and dye or barium studies should be performed. For the woman with demonstrable sphincter disruption and no evidence of pudendal neuropathy on examination, surgical repair of the sphincter without extensive evaluation is appropriate. Idiopathic anal incontinence attributed to pudendal neuropathy is diagnosed when a sphincter defect is not apparent on examination. In this instance, pudendal neuropathy may be confirmed by prolonged PNTML on electrophysiologic studies. Pure neuropathic anal incontinence is beyond the scope of surgery. Patients with pudendal neuropathy may also have sphincter abnormalities that are not appreciated on clinical examination, but may be discerned on ultrasound. These women with sphincter defects and concomitant neuropathy will have lower success rates with simple sphincter repair unless postanal repair is included.28
More extensive testing may be especially helpful in patients who have had prior anal surgery, who have not improved with therapeutic interventions, or in whom the clinical picture is unclear.
PREOPERATIVE AND POSTOPERATIVE CARE
Before entering into a surgical procedure, the patient must be screened for complicating problems, medications, allergies, and prior surgeries. The basic laboratory evaluation includes a complete blood count and urinalysis, with chest radiography, electrocardiography, or other testing as indicated. Colonoscopy with or without biopsy is a must when it is indicated. The preoperative bowel preparation should empty all solid fecal material by clear liquids for 24 hours in conjunction with an isosmotic bowel preparation such as Golytely or Miralax the afternoon before surgery. Finally, on the evening before surgery a vaginal cleansing douche should guarantee a clean operative field. Preoperative intravenous prophylaxis for both aerobic and anaerobic bacteria is the only antibiotic therapy required. Only one dose of preoperative antibiotic is necessary and should be given half an hour prior to incision.
The preoperative counseling should outline the operative preparations and the general procedure in detail. A summary of the postoperative course, including catheters, intravenous fluids, diet, analgesics, ambulation, and anticipated discharge, will prepare the patient for hospitalization. Finally, a thorough review of all complications, including hemorrhage requiring transfusion, infection, injury to the anus or rectum, failure of repair, or stenosis of the vaginal introitus should be discussed with the patient, including the possibility of dyspareunia or introital and perineal body pain.
Postoperatively, urinary catheterization should continue overnight or until the patient becomes fully ambulatory. The patient should remain on clear liquids until the first postoperative day, and then progress to a low-residue diet. Preferably, no solid fecal evacuation should challenge the repair until late in the postoperative period. Postoperative sitz baths and perineal heat lamps aid in healing. Spontaneous passage of flatus should not be resisted, nor should the sphincter musculature be voluntarily contracted for 2 weeks. At that time, slow regular exercising of the external sphincter by Kegel exercises or perineal squeezing may begin. These are increased to ten voluntary contractions four times daily. Gradual restoration of anal control may be attempted. Daily stool softeners and cathartics will ease the passage of fecal bulk and minimize resistance against the sphincter suture line. Normal anal continence should be appreciated by the 6-week postoperative visit.
SURGICAL PROCEDURES
In obstetrics and gynecology practice, the majority of patients require surgical repair after a postpartum presentation in which the rectovaginal septum has been weakened, or the intermediate or base loops of the external sphincter have been severed. Less commonly, surgical manipulations, such as posterior colporrhaphy, rectovaginal fistulectomy, or incision and drainage of a perirectal abscess, may precede the symptoms. Generally, repair requires dissection of the rectovaginal septum with definition of the rectal wall, external anal sphincter, levator musculature, and vaginal mucosa. After excision of cicatrix, a multilayered repair of healthy, mobile, well-vascularized tissue may be performed with minimal tension, using additional support from the muscles of the urogenital diaphragm. The following repair techniques are the more common approaches available to gynecologists in treating anal incontinence secondary to obstetric or gynecologic trauma.
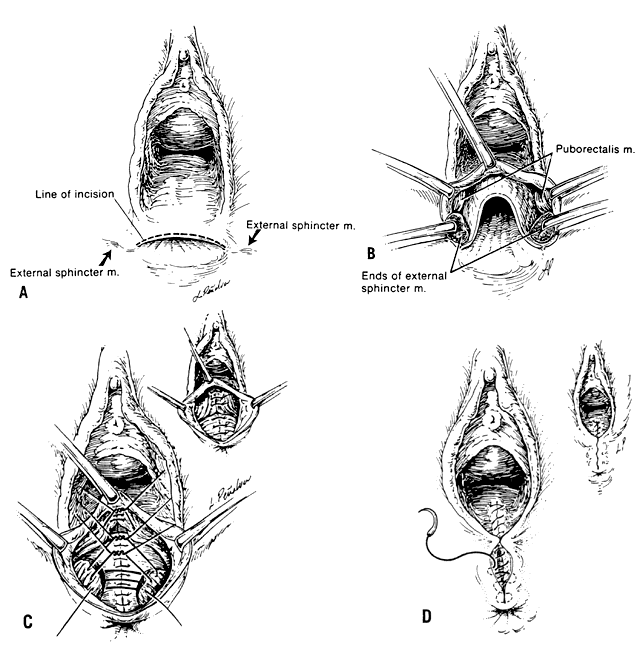
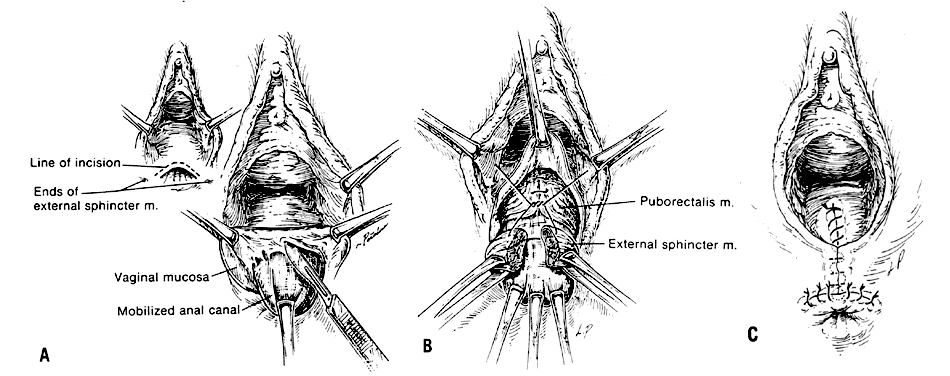
The layered repair is a common procedure that closes an anovaginal defect by individually dissecting and then reapproximating each tissue plane. A transverse incision (Fig. 4 A) is extended from both edges of the retracted external sphincter and carried along the inferior margin of the rectovaginal septum. Using a scalpel and Metzenbaum's scissors, the vaginal mucosa is separated from the rectal mucosa in the midline, and this plane is extended laterally. The external sphincter muscle fibers are identified and dissected free of cicatrix (Fig. 4 B). After reapproximation of the rectal mucosa with an interrupted 4-0 delayed-absorbable submucosal suture, the base and intermediate-loop layers of the external sphincter are united by interrupted 0 delayed-absorbable sutures. Plication of the superior puborectalis muscle loop with an interrupted 0 delayed-absorbable suture adds additional support to the repair (Fig. 4 C). Care must be taken to avoid compromising the vaginal lumen at this step. Plication of the transverse perineal muscles of the urogenital diaphragm reinforces the perineal body as needed. This is followed by closure of the repair with interrupted 2-0 delayed-absorbable subcutaneous sutures and a continuous 2-0 chromic mucosal stitch (Fig. 4 D).
The Warren flap is a relatively simple dissection of the thinned rectovaginal septum to provide additional length and depth while minimizing the risk of infectious sequelae.49 An inverted-U incision is extended laterally beyond the margins of the retracted external sphincter muscle. The rectovaginal septum is dissected, separating off the vaginal mucosa and creating a flap (Fig. 5 A). The tissue planes are defined laterally, cicatrix is removed, and the edges of the external sphincter are identified. The rectovaginal septum is thickened by plication of the puborectalis musculature with interrupted 0 delayed-absorbable sutures. This step is followed by reapproximation of the base loop with interrupted 0 delayed-absorbable figure-of-eight sutures (Fig. 5 B). The subcutaneous tissues are approximated with interrupted 2-0 delayed-absorbable sutures after reinforcement of the perineal body by interrupted delayed-absorbable simple stitches through the transverse perineal muscles. The vaginal mucosa is then closed with a continuous 2-0 chromic stitch (Fig. 5 C). Excessive flap tissue is removed either during surgery or via resorption during healing.
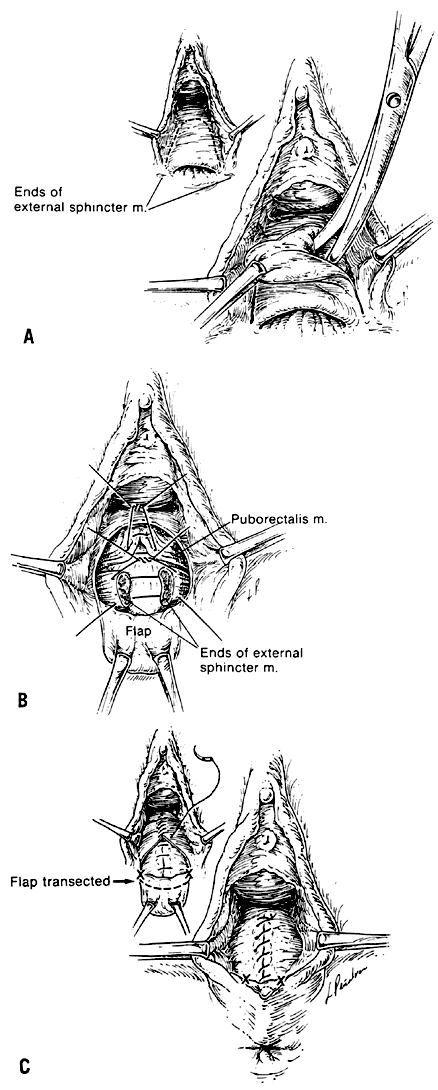
The Noble-Mengert repair (or anal pull-through repair) provides increased rectovaginal septal length while avoiding contamination of the incision site.50, 51 A transverse incision is made along the edge of the rectovaginal septum. The vagina is separated from the anterior rectal mucosa to mobilize the anal canal (Fig. 6 A). With the anal wall advanced, the dissection is continued laterally, identifying and freeing the ends of the external sphincter muscle. The ends of the external sphincter muscle are united with interrupted 0 delayed-absorbable sutures. The puborectalis muscle can also be approximated with interrupted 0 delayed-absorbable sutures (Fig. 6 B). Interrupted 2-0 delayed-absorbable stitches plicate the pararectal fascia and subvaginal tissues to thicken the septum. Finally, the vaginal mucosa is closed with a continuous 2-0 chromic suture and the anal mucosa sutured to the perianal skin with interrupted 2-0 chromic stitches (Fig. 6 C).
Currently surgical treatments for anal incontinence are mostly performed by "colon rectal surgeons". The most common surgical treatment is an "overlapping sphincteroplasty" for anal incontinence secondary to sphincter injury.52, 53 The anal mucosa and vagina are dissected off the sphincter muscle and the scar tissue in the midline is saved. The scar tissue in the midline is then transected sharply with a knife. Sphincter muscles are then overlapped on each other as a double breasting technique. The levator muscle could also be sutured together to lenghthened the anal canal. This part of the procedure is known as "levatoroplasty". Complications of this procedure are difficulty in voiding, hemorrhage, abscess, and fecal impaction. Success in repair may be as high as 80–90%.1, 54 Postanal pelvic floor repair was advocated in 1975 by "Sir Alan Parks" from London England.55 He believed that one of the major contributing factors for maintaining continence is the valve effect caused by the double right-angle which normally exists between the anal canal, the angle being created by puborectalis muscle. So he advocated posterior repair of puborectalis muscle to recreate the anorectal angle. Many of the studies reporting alternative procedures discuss the use of a diverting loop colostomy before attempting the repair of anal incontinence. Generally, the North American surgical literature reflects a trend away from this procedure.56 Most recent series suggest a thorough preparatory bowel cleansing with postoperative dietary manipulation to minimize perioperative fecal soiling.
Artificial anal sphincter
Indications for artificial anal sphincter are failed multiple repairs, end stage anal incontinence, neurogenic incontinence, and congenital incontinence. Gracilis muscle transposition, gluteus muscle transposition, dynamic graciliplasty, and artificial anal sphincter are the few methods to replace the damaged sphincter.
Several techniques for muscle transposition exist.57, 58 The pelvic floor musculature including the puborectalis muscle is dissected and then transected. Using an overlapping suture technique, the muscles are wrapped around the anal canal to provide a spiraling sphincter.59 This same technique may be used with the gluteus maximus or gracilis muscle, exercising caution to preserve the vascular supply and innervation.59, 60, 61, 62 Generally, these series report success rates of 70–80%. Some authors have described reconstruction of the puborectalis sling63 using anterior abdominal wall fascia or a Dacron (Dow Corning Medical Products, Midland MI) or Marlex (Meadox, Oakland, NJ) graft.64, 65, 66 The thrust of these techniques is to reposition the angle of the anal canal anteriorly and reinforce the effect of the puborectalis muscle. Dynamic graciloplasty is encirclement of the anal canal with a transposed gracilis muscle, which is then stimulated electrically with an implantable pulse generator that lasts 7–8 years.67
In the anal encircling procedure a Dacron-impregnated Sialastic sheet is used as a prosthesis to encircle the anus. This procedure is helpful for patients with pudental injury. Complications are infection, stricture, fecal impaction, persistent incontinence, and pain.
Artificial anal sphincter implantation (ACTION (AMS) DEVICE)68 is an artificial implantable device that has been successfully used for urinary incontinence. In 1987, Christiansen (Minessota) and Lorentzen (Copenhagen) were the first to report the insertion of this device for anal incontinence. The device consists of three Sialastic components – an inflatable cuff, a pressure-regulating balloon, and a control pump – which can be activated and deactivated. The cuff is wrapped around the anorectum, the pump is placed in the labia for women or scrotum for men. The balloon is placed in the the space of Retzius. The patient is taught to squeeze the fluid in the balloon to activate the pump and to relax the cuff, and patient will then have a bowel movement. The fluid automatically returns to balloon after a predetermined time and the cuff is activated again. Multiple complications can occur following this procedure. Nonetheless, the artificial anal sphincter offers the best alternative for restoring continence, especially for those who have no other option except a stoma.
Newer options for endstage incontinence, when all other options have failed
Sacral nerve stimulation (SNS) is a new and safe procedure. Sacral nerve endings are stimulated by an implanted electrode which causes contraction of sphincter muscles. This is a promising option, however, its safety and efficacy are still being investigated.68
Injectable bulking agent, autologous fat, Teflon, etc. have been injected in the submucosa of the anus. Injectable PTQ(TM) implants are solid, textured silicone particles suspended in a carrier hydrogel. This material has been injected into defects in the sphincter or around a degenerated spinchter to augment muscle bulk. Results are usually short lasting.69
The Secca procedure consists of delivery of temperature-controlled radiofrequency energy to the muscles of the anal canal. It is believed that heat from the energy source causes collagen contraction, healing, and remodeling, leading to shorter and tighter fibers.70
The Malone antegrade continence enema involves a continent stoma being formed by implanting the tip of the appendix into the cecum by tunneling to create a one-way valve. The base of the appendix is brought out to the anterior abdominal wall. The patient can then introduce antegrade enemas via the appendicostomy to clear the colon and rectum.71
Anal plugs are disposable devices that control continence by blocking the passage of stool as a result of self-expansion when they are soaked in fecal content.72
When all other forms of treatment have failed, or are unsuitable, a colostomy remains a safe and viable option to treat fecal incontinence.55
SUMMARY
Fecal continence is maintained through a delicate yet complex interaction between several neuromuscular systems. These may become impaired in postobstetric or gynecologic patients. Gynecologists should be attuned to the symptoms of this defect, as well as to approriate evaluation and intraoperative management. When appropriate the patient should be referred to a colon rectal surgeon. With minimal intervention, the correction of anal incontinence can significantly improve these patients' quality of life.
REFERENCES
Ctereteko GC, Fazio VW, Jagelman DG et al: Anal sphincter repair: A report of 60 cases and review of the literature. Aust NZ J Surg 58: 703, 1988 |
|
Stricker JW, Schoetz DJ, Coller JA et al: Surgical correction of anal incontinence. Dis Colon Rectum 31: 533, 1988 |
|
Schoetz DJ: Operative therapy for anal incontinence. Surg Clin North Am 65: 35, 1985 |
|
Sorensen SM, Bondesen H, Istre O et al: Perineal rupture following vaginal delivery: Long-term consequences. Acta Obstet Gynecol Scand 67: 315, 1988 |
|
Haadem K, Ohrlander S, Lingman G: Long-term ailments due to anal sphincter rupture caused by delivery--a hidden problem. Eur J Obstet Gynecol Reprod Biol 27: 27, 1988 |
|
Aronson MP, Lee RA, Berquist TH: Anatomy of anal sphincters and related structures in continent women studied with magnetic resonance imaging. Obstet Gynecol 76: 84, 1990 |
|
Mellerup-Sorensen S, Bondesen H, Istre O, Vilman P: Perineal rupture following vaginal delivery: Long-term consequences. Acta Obstet Gynecol Scand 67: 315, 1988 |
|
Gass MS, Dunn C, Stys SJ: Effect of episiotomy on the frequency of vaginal outlet lacerations. J Reprod Med 31: 240, 1986 |
|
Crawford LA, Quint EH, Pearl MH et al: Incontinence following rupture of the anal sphincter during delivery. Obstet Gynecol 82: 527, 1993 |
|
Birnbaum EH, Dreznik Z, Myerson RJ et al: Early effect of external beam radiation therapy on the anal sphincter: A study using the anal manometry and transrectal ultrasound. Dis Colon Rectum 35: 757, 1992 |
|
Birnbaum EH, Myerson RJ, Fry RD et al: Chronic effects of pelvic therapy on anorectal function. Dis Colon Rectum 37: 909, 1994 |
|
Shafik A: A new concept of the anatomy of the anal sphincter mechanism and the physiology of defecation: The external anal sphincter: A triple loop system. Invest Urol 12: 412, 1975 |
|
Shafik A: Anatomy of the levator ani muscle with special reference to puborectalis. Invest Urol 13: 175, 1975 |
|
Shafik A: The longitudinal anal muscle: Anatomy and role in anal sphincter mechanism. Invest Urol 13: 271, 1976 |
|
Shafik A: Anatomy of the perianal spaces. Invest Urol 13: 424, 1976 |
|
Shafik A: A concept of the anatomy of the anal sphincter mechanism and the physiology of defecation. Dis Colon Rectum 30: 970, 1987 |
|
Parks AG: Anorectal incontinence. Proc R Soc Med 68: 21, 1975 |
|
Milligan ETC, Morgan CN: Surgical anatomy of the anal canal. Lancet 2: 1150, 1934 |
|
Gaston EA: The physiology of fecal continence. Surg Gynecol Obstet 87: 280, 1985 |
|
Goligher JC, Leacock AG, Brossy J: The surgical anatomy of the anal canal. Br J Surg 43: 51, 1955 |
|
Lestar B, Penninck F, Kerremans R: The composition of anal basal pressure: An in vivo and in vitro study in man. Int J Colorect Dis 4: 118, 1989 |
|
Santini L, Pezzulo L, Guadagno P et al: Radial manometric study of the anal canal: Preliminary results. Ital J Surg Sci 4: 347, 1988 |
|
Rasmussen OO, Sorensen M, Tetzschner T et al: Anorectal pressure gradient in patients with anal incontinence. Dis Colon Rectum 35: 8, 1992 |
|
Braun JC, Treutner KH, Dreuw B et al: Vector manometry for differential diagnosis of fecal incontinence. Dis Colon Rectum 37: 989, 1994 |
|
Evans J: Anorectal manometry. Med J Aust 147: 263, 1987 |
|
Keck JO, Staniunas RJ, Coller JA et al: Biofeedback training is useful in fecal incontinence but disappointing in constipation. Dis Colon Rectum 37: 1271, 1994 |
|
Enck P, Daublin G, Lubke HJ et al: Long-term efficacy of biofeedback training for fecal incontinence. Dis Colon Rectum 37: 997, 1994 |
|
Roig JV, Villoslada C, Lledo S et al: Prevalence of pudendal neuropathy in fecal incontinence: Results of a prospective study. Dis Colon Rectum 38: 952, 1995 |
|
Swash M: Anorectal incontinence: Electrophysiologic tests. Br J Surg 72 (suppl): S14, 1985 |
|
Snooks SJ, Swash M, Henry MM et al: Risk factors in childbirth causing damage to the pelvic floor innervation. Br J Surg 72 (suppl): S15, 1985 |
|
Miller R, Bartolo DCC, Roe A et al: Anal sensation and the continence mechanism. Dis Colon Rectum 31: 433, 1988 |
|
Miller R, Lewis GT, Bartolo DCC et al: Sensory discrimination and dynamic activity in the anorectum: Evidence using a new ambulatory technique. Br J Surg 75: 1003, 1988 |
|
Akervall S, Fasth S, Nordgren S et al: Manovolumetry: A new method for investigation of anorectal function. Gut 29: 614, 1988 |
|
Vaccaro CA, Cheong DMO, Wexner SD et al: Pudendal neuropathy in evacuatory disorders. Dis Colon Rectum 38: 166, 1995 |
|
Sangwan YP, Coller JA, Barrett RC et al: Distal rectoanal excitatory reflex: A reliable index of pudendal neuropathy? Dis Colon Rectum 38: 916, 1995 |
|
Kelvin FM, Maglinte DDT, Benson JT: Evacuation photography (defecography): An aid to the investigation of pelvic floor disorders. Obstet Gynecol 83: 307, 1994 |
|
Nielsen MB, Buron B, Christiansen J et al: Defecographic findings in patients with anal incontinence and constipation and their relation to rectal emptying. Dis Colon Rectum 36: 806, 1993 |
|
Law PJ, Bartram CI: Anal endosonography: Technique and normal anatomy. Gastrointest Radiol 14: 349, 1989 |
|
Gantke B, Schafter A, Enck P et al: Sonographic, manometric and myographic evaluation of the anal sphincters' morphology and function. Dis Colon Rectum 36: 1037, 1993 |
|
Falk PM, Blatchford GJ, Cali RL et al: Transanal ultrasound and manometry in the evaluation of fecal incontinence. Dis Colon Rectum 37: 468, 1994 |
|
Felt-Bersma RJF, Cuesta MA, Koorevaar M et al: Anal endosonography: Relationship with anal manometry and neurophysiologic tests. Dis Colon Rectum 35: 944, 1992 |
|
Law PJ, Kamm MA, Bartram CI: A comparison between electromyography and anal endosonography in mapping external anal sphincter defects. Dis Colon Rectum 33: 370, 1990 |
|
Law PJ, Kamm MA, Bartram CI: Anal endosonography in the investigation of fecal incontinence. Br J Surg 78: 312, 1991 |
|
Sultan AH, Kamm MA, Hudson CN et al: Anal sphincter disruption during vaginal delivery. N Engl J Med 329: 1905, 1993 |
|
Cuesta MA, Meijer S, Derksen EJ et al: Anal sphincter imaging in fecal incontinence using endosonography. Dis Colon Rectum 35: 59, 1992 |
|
Hill J, Corson RJ, Brandon H et al: History and examination in the assessment of patients with idiopathic fecal incontinence. Dis Colon Rectum 37: 473, 1994 |
|
Toglia MR, Delancy JOL: Anal incontinence and the obstetrician-gynecologist. Obstet Gynecol 84: 731, 1994 |
|
Felt-Bersma RJ, Klinkenberg-Knol EC, Meuwissen SG: Investigation of anorectal function. Br J Surg 75: 53, 1988 |
|
Warren JC: A new method of operation for the relief of rupture of the perineum through the sphincter and rectum. Trans Am Gynecol Soc 7: 322, 1982 |
|
Noble GH: A new technique for complete laceration of the perineum designed for the purpose of eliminating infection from the rectum. Trans Am Gynecol Soc 27: 357, 1982 |
|
Mengert WF, Fish SA: Anterior rectal wall advancement. Obstet Gynecol 5: 262, 1955 |
|
Christiansen J, Lorentzen M: Implantation of artificial sphincter for anal incontinence: Report of five cases. Dis Colon Rectum 32: 432, 1989 |
|
Motson RW: Sphincter injuries: Indications for and results of sphincter repair. Br J Surg 72 (suppl): 19, 1985 |
|
Fang DT, Nivatvongs S, Vermeulen FD et al: Overlapping sphincteroplasty for acquired anal incontinence. Dis Colon Rectum 27: 720, 1984 |
|
Kottmeier PK, Velcek FT, Koltz DH et al: Results of levatorplasty for anal incontinence. J Pediatr Surg 21: 647, 1986 |
|
Ferguson EF Jr: Puborectalis sphincteroplasty for anal incontinence. South Med J 77: 423, 1984 |
|
Christiansen J, Pedersen K: Traumatic anal incontinence: Results of surgical repair. Dis Colon Rectum 30: 189, 1987 |
|
Leguit P Jr, Van Baal JG, Brummelkamp WH et al: Gracilis muscle transposition in the treatment of fecal incontinence: Long-term follow-up and evaluation of anal pressure recordings. Dis Colon Rectum 28: 1, 1985 |
|
Onishi K, Maruyama Y, Shiba T: A wrap-around procedure using the gluteus maximus muscle for the functional reconstruction of the sphincter in a case of anal incontinence. Acta Chir Plast 31: 56, 1989 |
|
Gemsenjager E: How I do it: Transverse pelvic floor division for the posterior approach to the rectum and anus. Int J Colorect Dis 4: 67, 1989 |
|
O'Rourke DA, Egerton MF: A puborectal sling in the management of anal incontinence and rectal prolapse. Aust NZ J Surg 55: 493, 1985 |
|
Horn HR, Schoetz DJ Jr, Coller JA et al: Sphincter repair with a Silastic sling for anal incontinence and rectal procidentia. Dis Colon Rectum 28: 868, 1985 |
|
Labow SB, Hoexter B, Mosenson MD et al: Modification of Silastic sling repair for rectal procidentia and anal incontinence. Dis Colon Rectum 28: 684, 1985 |
|
Larach SW, Vazqiez B: Modified Thiersch procedure with Silastic mesh implant: A simple solution for fecal incontinence and severe prolapse. South Med J 79: 307, 1986 |
|
Satava RM, King GE: An artificial anal sphincter: Phase 2: Implantable sphincter with a perineal colostomy. J Surg Res 46: 207, 1989 |
|
Christiansen J, Lorentzen M: Implantation of artificial sphincter for anal incontinence. Lancet 2 (8553): 244, 1987 |