Benign Neoplasms of the Ovary, Fallopian Tube, and Parovarian Region
Authors
INTRODUCTION
Benign pelvic tumors are the result of aberrant development, physiological variation, or growth disorders.. Therefore, knowledge of the development, growth, and physiology of the pelvic tissues forms an important background for understanding benign tumors. The coelomic cavity develops within a mass of undifferentiated mesodermal cells, and those cells that line this cavity differentiate into epithlelial cells. Current concepts suggest that this process occurs because common precursor cells recognize their relative position in the tissues and develop into the appropriate type of tissue for their site.1 If the same precursor cells are sited elsewhere, they differentiate in another way. The implication then is that the epithelium of the coelomic cavity is derived from the same common precursors that give rise to the connective tissue wall of the cavity.
During the fifth week of embryonic life, the müllerian ducts develop bilaterally on the lateral surface of the urogenital ridges as multiple evaginations of the coelomic cavity. These multiple evaginations fuse into a single lumen. The multiplicity of origins of this lumen produce the fimbria. The müllerian duct subsequently extends into the pelvis. There, the two ducts from each side fuse with each other and become the genital canal-the fallopian tubes, the uterus, and the upper vagina. Thus the fallopian tubes, the uterus, and the upper vagina are simply extensions of the coelom and share a common epithelium and mesenchymal wall with the larger cavity.2
During the fourth, fifth, and sixth weeks of embryonic life, the germ cells migrate into the medial wall of the urogenital ridge and induce gonadal development.3 Achieving a position immediately beneath the surface of the epithelium, the germ cells divide mitotically and induce mitotic division of the epithelial cells. The result is a mass of proliferating epithelium and germ cells on the medial wall of the urogenital ridge. As development proceeds, this cortical mass is gradually divided up into smaller and smaller nests of epithelium and germ cells by gonadal stroma.4 Eventually, a single germ cell with only a single cell layer of epithelium surrounding it is the largest collection of cells remaining. This process begins in the hilum of the gonad and moves peripherally. Germ cells not properly encapsulated in this manner die. This presumably is the usual result if two germ cells are encapsulated in the same follicle, a consistent but unusual occurrence.5
If germ cells migrate to other subepithelial coelomic sites, aberrant gonads develop there. Consequently, it may be seen that gonadal epithelium is not different from peritoneal epithelium, and the whole may be viewed as a field. Further, it becomes rational to view the female genital canal and the coelomic cavity as a continuous lumen lined by a continuous epithelium and supported by a continuous mesenchymal wall. At various sites along this continuous lumen, specific tissues develop (e.g., the endometrium or the ovary).
BENIGN TUMORS THAT RESULT FROM PHYSIOLOGICAL VARIATION
When development proceeds normally, the germ cells that migrate into the medial wall of the urogenital ridge during the second month of embryonic life induce a proliferation of surface epithelium that encapsulates the proliferating germ cells. As described earlier, this mass of proliferating surface epithelium and proliferating germ cells is eventually divided by the original mesenchyme of the gonad into small compartments containing a single germ cell totally invested by a single cell layer of epithelium, the pregranulosa. This structure is a primordial follicle. When successfully encapsulated in a primordial follicle, the germ cell ceases mitotic proliferation. It enters prophase of meiosis I and stops at that point until subsequent development of the follicle. The first clinically important example of follicular development takes place in the peripartum. Cysts that develop from the follicular apparatus are known as functional cysts.
A newborn girl has just been removed from an environment in which she was exposed to high levels of maternal hormones, but these are rapidly being eliminated from her circulation. Associated with either these high levels, their rapid disappearance, or other unknown endogenous factors, some newborns develop large functional cysts. Rarely these possess a histologic appearance suggesting ovulation. Ultrasonography has made it apparent that ovarian cysts in neonates are more frequent than previously thought.6 They may be quite large and associated with apparent pain. Torsion may occur with apparent pain. Bleeding into these cysts has been sufficient to decrease infants' hematocrit. Despite occasional severe clinical symptoms, most of the surgery used as a response to these lesions is unnecessary, and unwarranted gonadectomy is common. Because most such tumors will resolve, expectancy is to be preferred. Malignancy in a neonate is not unheard of but is incredibly rare.
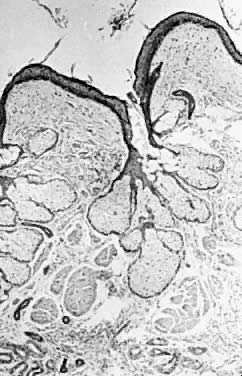
In a gonadotropin-independent process that continues from prepuberty until menopause, primordial follicles develop into primary follicles that are about 150/μm in diameter. In the presence of follicle-stimulating hormone (FSH), these will subsequently develop into cystic antral preovulatory follicles that may average 20 mm in diameter.7, 8 There is individual variation in this process, and 25-mm-diameter cysts may occur, particularly if they are clomiphene induced. These structures consequently rupture (i.e., ovulate). They then seal over and re-expand, in some cases acutely, as a result of intrafollicular hemorrhage. The result is a corpus luteum, which also varies in size but may have a diameter of several centimeters. This physiological process, for unknown reasons, is frequently associated with the development of either preovulatory or postovulatory cysts that are enlarged and persistent. Cysts lacking any histologic evidence that they have participated in an ovulation are termed follicular cysts (Fig. 1). If significant luteinization is present, they are called theca lutein cysts. If they have been the site of an ovulation, then they are named corpus luteum cysts. The term luteinization refers to an increased amount of cytoplasm in cells producing steroid hormones. It is derived from the fact that tissue consisting of such cells accumulates cholesterol and then is yellow grossly. Thus luteinization is termed microscopically but referent to a gross observation.
|
Functional cysts may be asymptomatic or may be associated with pain. They may result in torsion, and they frequently cause anxiety for both the obstetrician and the patient. Even normal ovulation may be responsible for significant episodes of clinical pain, and one of the frequent sources of laparotomy for what is eventually found to be a “cold” appendix is normal ovulation. It is traditional to wait a month or so, with or without hormonal suppression of the ovary, before operating on these cysts, because most will resolve in a single cycle. Those that persist will also resolve in time, however, and it is usually a statistically misplaced concern about malignancy in young women that prompts surgery.9 Unilocular cysts in women of any age are highly unlikely to be malignant.10 Oral contraceptives have been used to suppress functional cysts and recurrences of the same phenomenon in the next cycle. This approach is often useful. Multiphasic oral contraceptives have ironically been blamed for the development of functional ovarian cysts, but the quantitative importance of any such association has been questioned.11
In the course of the artificial induction of ovulation with gonadotropins or other drugs, ovaries may be hyperstimulated, usually bilaterally. This is more likely to occur in the face of polycystic ovarian disease, which can itself be the source of enlarged ovaries. This condition, of unknown etiology, has been attributed to defects in almost all tissues. An abnormality in the 5 α-reductase levels in the skin and liver has been suggested.12 The condition is associated with increased serum androgens, an elevated luteinizing hormone (LH) level, and enlarged, polycystic ovaries with thecal hyperplasia and much luteinization. With or without polycystic: ovaries, if the ovaries are hyperstimulated, very large theca lutein cysts may be generated in sufficient numbers to cause both ovaries to swell to fill the peritoneal cavity. Symptoms are a feeling of fullness and pain. Ascites may develop. These large cystic ovaries occasionally swell so rapidly that they rupture, and life-threatening hemorrhage occurs. Pregnancy rarely results in the same phenomenon, particularly if multiple pregnancy or erythroblastosis is present. However, hyperstimulated ovaries with multiple theca lutein cysts have also been encountered with normal singleton pregnancies. The histology associated with hyperstimulated ovaries has been termed hyperreactio luteinalis.
Theca lutein cysts are common in the presence of trophoblastic disease, especially in the case of true hydatidiform moles. Here, as in the other conditions mentioned earlier, it has been presumed that the ovarian stimulation is due to elevated levels of human chorionic gonadotropin (hCG). However, it has been shown that the clinical behavior of theca lutein cysts does not correlate one to one with hCG titers.3 Theca lutein cysts may grow in the presence of falling β-hCG titers, and they may persist for prolonged periods after β-hCG is no longer present. Therefore, individual variation. or other factors must play a role. The histology of all these lesions is one of multiple cystic follicles with luteinization of both the follicles and, variably, of the surrounding hyperplastic stroma.
More discrete single, multiple, or bilateral nodules of luteinized stroma in pregnancy are referred to as pregnancy luteomas.14 These lesions may be associated with androgen production and virilization of the mother. They will masculinize a female fetus.15 They regress postpartum but may recur in subsequent pregnancies.
BENIGN TUMORS DUE TO ABERRANT DEVELOPMENT
Aberrant development of the fallopian tubes is frequent. If one or more of the multiple evaginations that form the tube fail to coalesce into a single lumen, then tubal diverticuli or paratubal cysts develop. If small, these are the classic hydatid cysts of Morgagni. They may also be large enough to represent more significant clinical masses. Most probably enlarge on the basis of fluid accumulation rather than neoplastic growth. This is particularly the case in pregnancy. In one series, hydatid cysts were the most common adnexal tumors encountered in pregnancy.16
Although other paratubal and parovarian cysts have for many years been attributed to mesonephric duct remnants, the only two studies that have ever examined this issue both have concluded that most paratubal and parovarian cysts are in fact mullerian. 17, 18 They are usually lined by tubal-type epithelium and are found in the mesosalpinx, where tubal anomalies would be expected. Many possibly arise as inclusion cysts as the residua of endometriosis or inflammation. Once the peritoneal epithelium has been internalized in the cyst, its site has been changed, and the mesothelium becomes a tubai-type epithelium. Whether paratubal, parovarian, or truly ovarian, most simple benign epithelial cysts are probably not truly autonomous neoplasms but grow in response to hormone stimulation or fluid accumulation. Again, the incidence of such cysts in a series of adnexal masses in pregnancy is suggestive.16 Equally suggestive is the relatively frequent appearance of parovarian or ovarian cysts of an epithelial nature during the course of estrogen treatment in normal pubertal girls in an effort to control their growth. 19
When on rare occasion epithelial, paratubal, or parovarian cysts are malignant, their histology is that of typical ovarian cancer. Two different studies have examined the frequency with which this occurs, and both found that approximately 2% of parovarian cysts were malignant.18, 20 Unfortunately, it is impossible to make this distinction through a laparoscope. Malignancies are not confined to postmenopausal women. However, if a cyst is unilocular by ultrasonographic examination and if it lacks papillary projections, then malignancy is considerably less likely.10 It also remains a matter of debate whether or not aspiration of a malignant cyst alters prognosis.
When development proceeds normally, germ cells are encapsulated in a single cell layer of pregranulosa, but various aberrant encapsulations may occur. One of these is the inappropriate encapsulation of two germ cells within a single layer of pregranulosa.5 It has been hypothesized that this causes the stimulation of one oocyte by the other in a manner that results in parthenogenesis. Parthenogenesis is believed to be the source of benign cystic teratomas on more secure genetic grounds.21 The encapsulation of the oocyte, properly or improperly, and its long period of maintenance in the ovarian cortex is consistent with the occurrence of benign cystic teratomas in relatively young women and their almost total absence in males. Experimental evidence that may further bear on the female-male difference in the incidence of these neoplasms is derived from experimental work in mice.22 If an oocyte is fertilized, by micromanipulation, with an additional female pronucleus, then the result is the development of nearly normal amounts of embryonic tissue components but underdeveloped extraembryonic tissues such as the chorion. The reverse experiment, in which an oocyte contains two male pronuclei, is associated with the development of extensive extraembryonic structures and deficient embryonic ones.
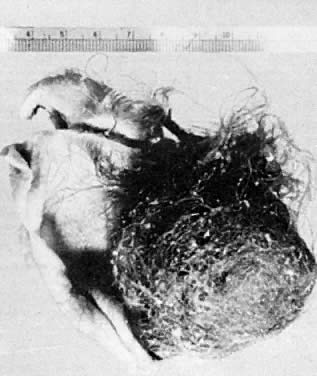
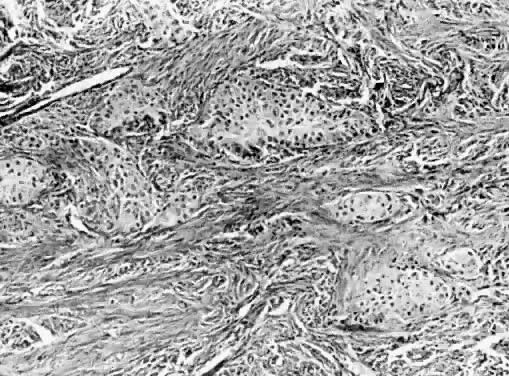
Benign cystic teratomas are quite common. They represent 70% of benign ovarian neoplasms in women up to age 40.9 Between age 40 and 50, they represent 40%; between age 50 and 60, they represent 20%. They comprise 15% of benign ovarian tumors in the seventh decade of life. They commonly consist primarily of ectoderm and mesoderm. The stratified squamous epithelium of the skin, its appendages, and neural tissue are the most common ectodermal derivatives (Fig. 2). The mesoderm is routinely represented by dermal connective tissue, but cartilage, bone, and muscle are also seen. The endoderm is represented by respiratory epithelium or gastrointestinal tract epithelium. However, endodermal derivatives are less commonly seen than the other types of tissue. This scarcity of endodermal derivatives is curious and of particular interest, because mesodermal induction in vertebrate embryos requires endoderm.23 Thyroid, another endodermal tissue, is present with some frequency. When it represents a significant component of the tumor. the term struma ovarii is used. Strumas occur in slightly older women than is usual for benign cystic teratomas. If the tissue functions autonomously, then clinical thyroid disease may occur. The carcinoid syndrome may also have its origin in typical carcinoids arising in benign cystic teratomas.
|
The cystic component of a dermoid is routinely lined by ectodermal skin, and its lumen is filled with sebum and hair (Fig. 3). This material may erode through the epidermal lining and elicit an inflammatory reaction in the connective tissue of the tumor or surrounding ovary. Histologically, this reaction is characterized by a dramatic giant cell response. The process results in the destruction of the wall of the cyst with progressive dissection by the cyst contents. Dissection may eventually extend deep into the pelvic soft tissues or to the peritoneal surfaces. This is the mechanism that results in spontaneous rupture of benign cystic teratomas. Rarely, the cystic spaces in these neoplasms contain cerebrospinal fluid rather than sebum and are lined by neural tissue with choroid plexus and not skin.
Clinically, benign cystic teratomas present in young women, either as asymptomatic masses or in association with pain or pressure. Torsion is occasionally responsible for acute pain. For unknown reasons, benign cystic teratomas tend to be found anteriorly in the pelvis. This is a useful clinic al sign. Bilaterality occurs in about 25% if subsequent recurrence in the opposite gonad is included in the definition. At initial diagnosis, they are bilateral about 10% to 15% of the time.
The radiologic identification of calcification or teeth is also clinically useful, and dental development has been observed.24 Therapy consists of the surgical excision of the tumor and the closure of the residual ovary. Expanding from a single cell of origin and possessing a pushing border, dermoids invariably thin the surrounding ovary but do not destroy it. The exception is the dissection caused by sebum and hair that has eroded the wall of the cyst. The terrible chemical peritonitis that results when this material escapes into the peritoneal cavity can result in death, but this outcome is the result of spontaneous rupture and widespread peritoneal involvement. The small amount of controllable leakage that occurs during the course of a careful attempt to resect a cyst does not constitute this kind of a threat. Fear of it is not a reason to sacrifice an ovary that is otherwise desirable.
If dermoid cysts are unnoticed and persist into the postmenopausal years, secondary malignancy may develop within them.25 Although many types of secondary cancer may be encountered, the most common is a squamous cell cancer of the “skin” lining the cyst. These are nearly always detected late and have a dismal prognosis.
BENIGN TUMORS RESULTING FROM GROWTH DISORDERS
Whether all the benign cysts or adenomas of the adnexal regions are true growth disorders is unclear. Other factors may contribute to their development. Inclusion cysts derived from ovulation, old inflammation, endometriosis, or unknown factors result in an epithelium that is in an anomalous site. It has become internalized rather than being external, and as a result it displays a bizarre differentiation. Again. many “cysts” in the adnexae probably enlarge without neoplasia, but on the basis of continued secretion.
Current concepts suggest that all cells start off with identical genetic components but differentiate into various tissues depending on which part of their genome is activated and that in most complex organisms the signals that tell them how to do this are derived from their position within the embryo. Consequently, their final form is a combination of their genes plus their environment interacting in an intimate way. It is also believed that malignant growth is the result of a series of genetic accidents that alter this pattern in such a way that cells continue to proliferate rather than to differentiate. It follows that oncogenes may result in benign tumors. In some tumor systems, benign tumors are composed of cells with genetic damage similar to that found in carcinoma. This idea has been most extensively developed in colon carcinomas, in which villous adenomas have been shown to possess ras oncogenes.26
In any case, differentiation in benign tumors is modified. The tissue site apparently exerts less control over differentiation than is normal, because although benign tumors may display differentiation that is normal for their site, they may display alternative forms of differentiation as well. Furthermore, there may be significant variation in the type of differentiation displayed by the cells within a given tumor. It is as if their site within the tumor varies or different cells interpret site-specific messages differently. In any case, it is unusual for epithelial tumors in the female pelvis to be homogeneous in cell type even when benign. However, it is convenient to describe benign tumors as if they were composed of a single cell based on their predominant pattern.
Among benign ovarian tumors, epithelial tumors account for 30% of the total up to age 40 years, 50% between ages 40 and 49, 70% between ages 50 and 70, and 90% thereafter.9 As determined by ultrasonography, unilocular cysts without papillary vegetations on their walls may be assumed to be benign.10 In 296 unilocular cysts seen on ultrasonography, only one was malignant. The patient was 60 years old and had papillary vegetations on her cyst wall.
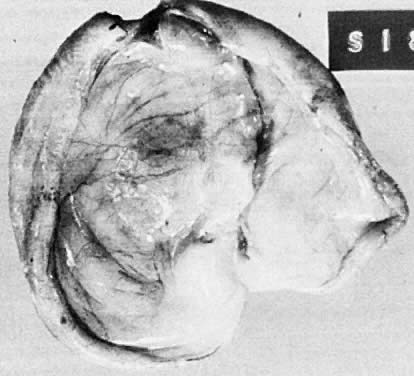
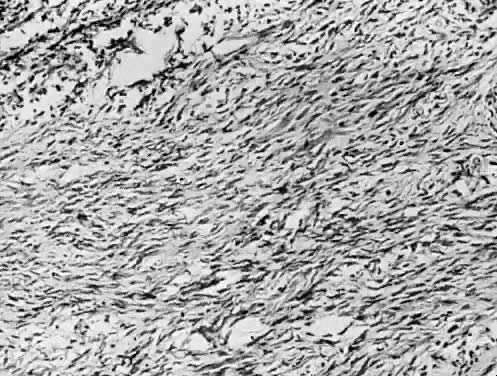
Benign serous tumors are usually unilocular cysts (Fig. 4). They may possess a few papillary vegetations. The presence of a significant stromal component is defined as serous cystadenofibroma. Serous tumors tend to be surface phenomena in the pelvis. There is also an incidence of bilaterality that may amount to 20%, and here, as elsewhere, bilaterality must be interpreted to mean a recurrence in a conserved gonad after successful operative removal of the other. When bilaterality exists at the time of initial diagnosis, it increases the risk that the patient has a malignant rather than a benign tumor.27 In contrast to this finding, when ovarian tumors recur in a conserved gonad, their grade is usually unchanged. That is, if a benign epithelial tumor is removed from one ovary and another tumor occurs in the other gonad a few years later, it is nearly always also benign.
Although benign serous tumors are not the most common type that produces hormones they rarely do so. They are the most common tumors to undergo torsion in postmenopausal women, which is a clinically useful piece of information because malignancy is not associated with torsion.28 When epithelial inclusions are found in pelvic lymph nodes or elsewhere in the pelvis, they are most often serous in cell type.
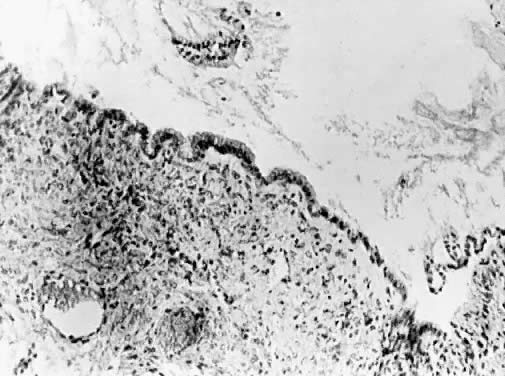
Cysts more deeply placed within the ovary or beneath the peritoneum frequently are mucinous (Fig. 5). Ultrastructural studies have demonstrated that both endocervical and gastrointestinal types of epithelium are present in these lesions, and for that reason many believe that at least a portion of mucinous tumors are teratomatous in origin.29 They are more often multilocular than serous lesions, and only rarely do they have enough fibrotic stroma to be diagnosed as adenofibromas. Squamous metaplasia may be encountered, apparently converting a mucinous cyst to one linked by squamous epithelium. Mucinous tumors often possess a prominent stromal component. It is likely to retain its thecal character and may be luteinized. For this reason, mucinous cysts may present with symptoms of increased estrogen production. Rarely they produce androgen. If peritoneal inclusion cysts develop in the retroperitoneal space, their aberrant site tends to promote mucinous differentiation. These retroperitoheal mucinous cysts are more frequently encountered in women, but they also occur in men.30
|
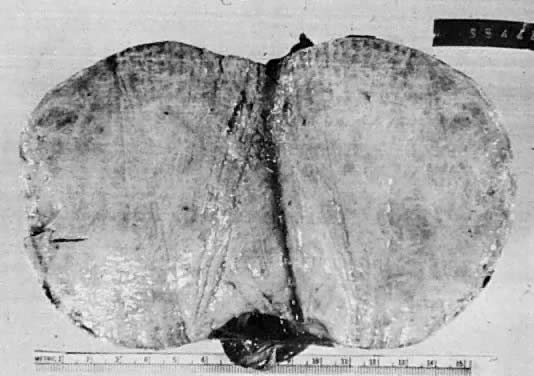
Mucinous cysts or cystadenomas may become huge and fill the peritoneal cavity. They are the largest neoplasms in the human body, and the clinical picture of a woman who is basically healthy but has a huge distended abdomen with an ovarian mass is almost diagnostic. Despite gross distention of the abdomen, they are usually free, and simple removal is all that is required to effect a cure. Before mucinous tumors are judged to be benign, however, they should be thoroughly sectioned, because it is characteristic of them to possess isolated foci that are malignant.31
Endometriosis is discussed elsewhere in these volumes, but endometriomas also tend to be intraovarian. Benign endometrioid-type adenofibromas may occur in the ovary or elsewhere in the pelvic soft tissues, principally in the broad ligament. Clinically, they may be confused with myomas until examined histologically. Brenner tumors occur in borderline and malignant forms, but the vast majority are benign (Fig. 6). They consist of squamous-type epithelial nests in a fibrous or thecomatous stroma, and although there are exceptions, they tend to be on the ovarian surface. Accounting for only a small percent of all ovarian tumors, they are bilateral about 5% of the time. Most are small and are discovered incidentally. The stroma is often hormone producing, and Brenner tumors, for this reason, have been associated with clinical signs and symptoms of hyperestrogenism, including hyperplasia and endometrial cancer. Alternatively, the stroma may be fibrotic and calcified.
|
The appearance of squamous metaplasia in mucinous tumors has led some to believe that Brenner tumors routinely arise in this manner. Indeed mucinous and Brenner tumors are often mixed. Additional speculation about the origin of Brenner tumors has been occasioned by the fact that the epithelium in the nest is often transitional in type rather than squamous. Many such neoplasms are, for this reason, termed urothelial. In any case, the epithelial nests are clearly continuous with the ovarian surface.32 This is another example of an altered site giving rise to an altered form of differentiation.
In the fallopian tubes, the most common benign tumor is the adenomatoid tumor. Microscopic to 3cm in size, this whitish nodule is incidental and clinically insignificant but histologically impressive. Multiple cleftlike spaces lined by flattened mesothelial, columnar, or cuboidal cells are so complex in appearance that in the past they have led to an erroneous diagnosis of malignancy. The cells are clearly differentiated ones, however. Ultrastructural studies demonstrate that the adenomatoid tumor is more accurately interpreted as a benign mesothelioma.33 Although usually found in the tube, an adenomatoid tumor may also be encountered in art ovary or elsewhere in the pelvic soft tissues.
The sex cord stromal tumors (i.e., granulosa cell tumors and Sertoli-Leydig cell tumors) occur in both benign and malignant forms. It has been a challenge to predict accurately in all cases which is which. In general, those examples possessing the, most differentiated cell types, the smallest gross size, and the most active hormone production have tended to be benign. Most granulosa cell tumors are estrogenizing, and most Sertoli-Leydig cell tumors are virilizing, but there are exceptions in both cases. Particularly with granulosa cell tumors has the diagnostic confusion presented a clinical problem. A normal, unluteinized granulosa cell is undifferentiated and lacks cytoplasm. A population of such cells also displays mitoses, but the mitoses and the interphase nuclei of the normal granulosa cell are morphologically typical. This, however, may be the only significant distinction between these cells and highly malignant small cells in a high-grade neoplasm. If the small cells in a high-grade neoplasm are arranged in strands or display a few follicular spaces, many pathologists identify them as granulosa cells. From the pathologic viewpoint, this is not necessarily incorrect, but it does mean that the term granulosa cell tumor is relatively useless to most clinicians, who must pursue this diagnosis to get some information about grade.
Among Sertoli-Leydig cell tumors there is also considerable variation in differentiation, some of which has been appropriately honored with separate designations. An ovarian sex cord tumor with annular tubules is highly associated with the Peutz-Jeghers syndrome when bilateral.34 It has also been associated with well-differentiated cervical adenocarcinoma.35
STROMA
Benign stromal tumors of the ovary are not common. After the age of 50 years they account for only 15% of the total of benign tumors, but because they may be responsible for bizarre clinical syndromes, they have much fascination. If they are not hormonally active, then the histology has little clinical relevance. Myomas are the least common, with combinations of fibrous and thecal tissue being the most frequent. Regardless of their histology, they present as asymptomatic pelvic masses, with pain produced by pressure or by torsion (Fig. 7). Particularly in postmenopausal women they may be calcified, with a radiologic picture that resembles that of calcified uterine myomas. In younger women bilateral fibromas with or without calcification are frequently associated with basal cell nevus syndrome.36 Benign ovarian stromal tumors are often found at surgery performed for myomas.
Most benign ovarian tumors can be associated, rarely, with a clinical picture typical of Meigs syndrome, but fibromas are the most common histologic type and the one originally reported.37 In 1937, Meigs described a case of ovarian fibroma with ascites and hydrothorax.38 Both the ascites and hydrothorax cleared when the tumor was removed, although this clinical picture has never been definitively explained. A plausible explanation is that the surface of the tumor mass is responsible for increased ingress of fluid into the peritoneal cavity. If a large amount of fluid enters rapidly enough, then it will exceed the capacity of the subdiaphragmatic lymphatics to remove it. If., as a result, flow through the right thoracic duct is large, then fluid that normally drains through this channel from the right thorax may be relatively obstructed and thus may accumulate.
There is a continuous spectrum between those neoplasms that are purely fibrous and those that are purely thecal, with mixtures being the most common (Fig. 8). There is not a one-to-one correlation between cell morphology and function, and in general, if a stromal neoplasm stains positively for intracellular lipid or is hormonally active at the clinical level, it is termed a thecoma. Thecomas may be large and may be responsible for significant estrogen production. For this reason, their clinical presentation is that of hyperestrogenism. Bleeding and endometrial hyperplasia may be the problem that brings a patient to a physician, and endometrial cancer has been associated with the comas.
|
Some thecomas reveal individual cell luteinization within the otherwise fibroblastic stroma. These are more likely to be androgen producers, although there is no one-to-one correlation, and peripheral conversion also complicates the clinical picture. Rarely, dramatic luteinization of a the coma is associated with progesterone production.
Ovarian lipid cell tumors were historically referred to as adrenal rest tumors because they are composed of cells that have the morphology of cells that make up the adrenal cortex. Alone or in association with surrounding ovarian stroma, they may produce large amounts of dehydroepiandrosterone sulfate (DHEAS) as well as testosterone, further stimulating adrenal tissue.39 However, the best evidence is that they are ovarian stromal in origin. First of all, they are found entirely within the ovary, and secondly, they may arise in the background of preexisting ovarian stromal luteinization.40 ,41 True adrenal rests do occur in the mesosalpinx or along the ovarian vessels, but these insignificant structures are extraovarian. Their origin is debated.42 Some lipid cell tumors are malignant, but these are usually large, greater than 8 cm, and lack differentiation.43 Lipid cell tumors may be diagnosed with (6 β-131I) iodomethylnorcholesterol (NP-59) scans.
In the ovarian hilum of young girls, one occasionally encounters a benign androgen-producing tumor composed of tubules and interstitial cells. Such arrhenoblastomas are quite rare. On the other hand, hilar cell hyperplasia is routine in postmenopausal women unsupported with hormones, because these cells apparently respond to elevated gonadotropins. Perhaps it is for this reason that hilar cell tumors are characteristically found in older women. They are small hilar masses, occasionally bilateral, that may produce large quantities of testosterone.44 Histologically, they consist of sheets of hilar cells and may possess crystalloids of Reinke.
Ovarian cancer is a terrible disease, and its possibility justly evokes major clinical responses. However, it is well to remember that when an ovarian tumor is present, the chance that it represents malignancy is not high except in older patients.9 For tumors with low malignant potential, the rate is on the order of 5% across age-groups. For higher-grade lesions, it is about 5% to 10% up to age 30, 15% between ages 30 and 40, 35% between ages 40 and 50, 45% between ages 50 and 70, and perhaps lower after that.
REFERENCES
Edelman GM: Topobiology. New York, Basic Books, 1988 |
|
Parmley TH: Embryology of the female genital canal. In Kurman RJ (ed): Blaustein's Pathology of the Female Genital Tract, 3rd ed. New York. Springer-Verlag, 1987 |
|
Witschi E: Migration of the germ cells of human embryos from the yolk sac to the primitive gonadal folds. Contrib Embryol 32: 67, 1948 |
|
Konishi I, Fujii S, Okamura H et al: Development of interstitial cells and ovigerous cords in the human fetal ovary: An ultrastructural study. J Anal 148: 121, 1986 |
|
Sherrer CW, Getson B, Woodruff JD: The incidence and significance of polynuclear follicles. Am J Obstet Gynecol 128: 6, 1977 |
|
Nussbaum AR, Sanders RC, Hartman DS et al: Neonatal ovarian cysts: Sonographic-pathologic correlation. Radiology 168: 817, 1988 |
|
Schwartz NB: Novel peptides in ovarian follicular fluid: Implications for contraceptive development. Research Frontiers in Fertility Regulation 2: 1, 1982 |
|
Fossum GT, Vermesh M, Kletzky OA: Biochemical and biophysical indices of follicular development in spontaneous and stimulated ovulatory cycles. Obstet Gynecol 75: 1, 1990 |
|
Koonings PP, Campbell K, Mishell DR et al: Relative frequency of primary ovarian neoplasms: A 10 year review. Obstet Gynecol 74: 921, 1989 |
|
Granberg S, Wikland M, Jansson I: Macroscopic characterization of ovarian tumors and the relation to the histologic diagnosis: Criteria to be used for ultrasound evaluation. Gynecol Oncol 35: 139, 1989 |
|
Grimes DA, Hughes JM: Use of multiphasic oral contraceptives and hospitalizations of women with functional ovarian cysts in the United States. Obstet Gynecol 73: 1037, 1989 |
|
Stewart PM, Shackleton CHL, Beastall GH et al: 5 X-reductase activity in polycystic ovary syndrome. Lancet 335: 431, 1990 |
|
Montz FJ, Schlaerth JB, Morrow CP: The natural history of theca lutein cysts. Obstet Gynecol 72: 247, 1988 |
|
Sternberg WH, Barclay DL: Luteoma of pregnancy. Am J Obstet Gynecol 95: 165, 1966 |
|
Hensleigh PA, Woodruff JD: Differential maternal-fetal response to androgenizing luteoma or hyperreactio luteinalis. Obstet Gynecol Surv 33: 262, 1978 |
|
Sunoo CS, Terada KY, Kamemoto LE et al: Adnexal masses in pregnancy: Occurrence by ethnic group. Obstet Gynecol 75: 38, 1990 |
|
Gardner GH, Greene RL, Peckham BM: Tumors of the broad ligament. Am J Obstet Gynecol 73: 536, 1957 |
|
Genadry R, Parmley TH, Woodruff LJD: The origin and clinical behavior of the parovarian cysts. Am J Obstet Gynecol 129: 873. 1977 |
|
Wettenhall HMB, Cahill C, Roche AF: Tall girls: A survey of fifteen years of management and treatment. J Pediatr 86: 602, 1975 |
|
Stein AL, Koonings PP, Schlaerth JB et al: Malignant parovarian tumors. Obstet Gynecol 75: 1029, 1990 |
|
Linder D, McCow BK, Hecht F: Parthenogenetic origins of benign ovarian tumors. N Engl J Med 292: 63, 1975 |
|
Surani MAH: Evidences and consequences of difference between maternal and paternal genomes during embryogenesis in the mouse. In Rossant J, Pedersen RA (eds): Experimental Approaches in Mammalian Embryonic Development, p 401. New York, Cambridge University Press, 1986 |
|
Slack JMW: Peptide regulatory factors in embryonic development. Lancet 1: 1312, 1989 |
|
Nogales F: Germ cell tumors of the ovary. In Fox H (ed): Haines and Taylor Obstetrical and Gynecological Pathology, p 637. New York, Churchill Livingstone, 1987 |
|
Genadry R, Parmley TH, Woodruff JD: Secondary malignancy in benign cystic teratomas. Gynecol Oncol 8: 246, 1979 |
|
Forrester K, Almoguera C, Han K et al: Detection of high incidence of K-ras oncogenes during human colon tumorigenesis. Nature 327: 298, 1987 |
|
Koonings PP, Grimes DA, Campbell K et al: Bilateral ovarian neoplasms and the risk of malignancy. Am J Obstet Gynecol 162: 167, 1990 |
|
Koonings PP, Grimes DA: Adnexal torsion in postmenopausal women. Obstet Gynecol 73: 11, 1989 |
|
Fenoglia CM, Ferenczy A, Richart RM: Mucinous tumors of the ovary. Ultrastructural studies of the mucinous cystadenomas with histogenetic considerations. Cancer 36: 1709, 1975 |
|
Pennell TC, Gusdon JP Jr: Retroperitoneal mucinous cystadenoma. Am J Obstet Gynecol 160: 1229, 1989 |
|
Hart WR, Norris HJ: Borderline and malignant mucinous tumors of the ovary. Cancer 31: 1031, 1973 |
|
Arey LB: The origin and form of the Brenner tumor. Am J Obstet Gynecol 81: 743, 1961 |
|
MacKay B, Bennington JL, Skoglund RW: The adenomatoid tumor: Fine structural evidence for a mesothelial origin. Cancer 27: 109, 1971 |
|
Young RH, Scully RE: Ovarian Sertoli-Leydig cell tumors: A clinical pathological analysis of 207 cases. Am J Surg Pathol 9: 543, 1985 |
|
Young RH, Welch WR, Dickersin GR et al: Ovarian sex cord tumor with annular tubules: Review of 74 cases including 27 with Peutz-Jeghers syndrome and 4 with adenoma malignum of the cervix. Cancer 50: 1384, 1982 |
|
Glendenning WE, Herdt JE, Black TB: Ovarian fibromas and mesenteric cysts: Their association with hereditary basal cell cancer of the skin. Am J Obstet Gynecol 87: 1008, 1963 |
|
Nicoll JJ, Cox PJ: Leiomyoma of the ovary with ascites and hydrothorax. Am J Obstet Gynecol 161: 177, 1989 |
|
Meigs JV: Fibroma of ovary with ascites and hydrothotax. Am J Obstet Gynecol 67: 962, 1954 |
|
Shenker Y, Malozowski SN, Ayers J et al: Steroid secretion by a virilizing lipoid cell ovarian tumor: Origins of dehydroepiandrosterone sulfate. Obstet Gynecol 74: 502, 1989 |
|
Taylor HB, Norris HJ: Lipid cell tumors of the ovary. Cancer 20: 1953, 1967 |
|
Davidson BJ, Waisman J, Judd HL: Long-standing virilism in a woman with hyperplasia and neoplasia of ovarian lipidic cells. Obstet Gynecol 58: 753, 1981 |
|
Adashi E, Rosenshein NB, Parmley TH et al: Histogenesis of the broad ligament adrenal rest. Int J Gynecol Obstet 18: 102, 1980 |
|
Roth LM: Sex cord-stromal and lipid cell tumors of the ovary. In Fox H (ed): Haines and Taylor Obstetrical and Gynecological Pathology, 3rd ed, p 623. New York, Churchill Livingstone, 1987 |
|
Baramki T, Leddy A, Woodruff JD: Bilateral hilar cell tumors of the ovary. Obstet Gynecol 62: 128, 1983 |