Postpartum Hemorrhage
Authors
INTRODUCTION
Although postpartum hemorrhage (PPH) has declined in frequency as a cause of maternal death, it remains a significant cause of maternal morbidity and mortality. Postpartum hemorrhagic complications (including ruptured uterus) account for 6–10.5% of nonabortive maternal deaths in the United States.1, 2 In developing countries, maternal death rates from hemorrhage are even higher. Because PPH is one of the more common and, usually, easily treated complications of delivery, the obstetrician may be lulled into underestimating the volume and impact of PPH until a catastrophic situation has developed. PPH is best managed by a high level of awareness of the causes of hemorrhage and a systematic approach to management when this problem develops.
DEFINITION
Postpartum hemorrhage is widely defined as a blood loss of more than 500 mL after delivery of the placenta. This definition is problematic, because studies of mean blood loss for vaginal delivery have varied findings, often as a result of differences in measurement techniques or patient populations. Careful investigations reported an average blood loss of approximately 500 mL in the first 24 hours after vaginal delivery.3,4 Pritchard and colleagues3 noted that 7% of women lost more than 1000 mL of blood after vaginal delivery. Blood loss in a patient with a small circulating blood volume is more significant than a similar blood loss in a patient with a larger blood volume. An alternative definition of PPH is blood loss of greater than 15% of the total estimated blood volume.5 In any case, to avoid the situation of unexpected hypotensive shock resulting from major hemorrhage, the obstetrician should always remain cognizant of the approximate total blood loss and the rate of ongoing bleeding.
CAUSES
Uterine Atony
The most common cause of PPH is uterine atony. Patients at increased risk for uterine atony include those with high parity, overdistended uterus (e.g., multiple gestation, polyhydramnios), prolonged or rapid labor, use of oxytocin for induction or augmentation, and use of magnesium sulfate. Uterine tone can usually be assessed by abdominal palpation after delivery; even when uterine tone seems normal, initial treatment for PPH is frequently directed toward uterine atony. However, when initial management efforts fail, the obstetrician should not waste time treating presumed uterine atony before evaluating other potential causes of hemorrhage.
Laceration
Lacerations of the perineum, vagina, cervix, or uterus may result in visible or concealed hemorrhage. Careful examination of the birth canal, including both inspection and palpation, is necessary to eliminate laceration as a source of PPH.
The obstetrician should also be aware of the potential of uterine rupture to cause massive hemorrhage. Although uterine rupture occurs most often with a prior uterine scar, it can occur spontaneously. High parity, oxytocin use, and obstetric procedures (e.g., forceps, breech delivery) are risk factors for uterine rupture. The increasing frequency of vaginal birth after cesarean section makes it important to consider uterine rupture in all cases of hemorrhage in this population.
Retained Placenta
Retained placenta causes uterine atony by preventing uterine contraction, which compresses the myometrial spiral arteries. Retained products may cause delayed PPH by interfering with involution of the placental site. At the time of delivery, the maternal surface of the placenta should be carefully inspected to ensure that no fragments are missing. The fetal surface is then examined, with particular attention to the margins to look for severed blood vessels that may have led to a succenturiate placental lobe. Routine uterine exploration to rule out retained products is uncomfortable for the patient and may increase the risk of postpartum infection; however, if doubt exists about the potential for retained products, uterine exploration is appropriate.
Placenta Accreta
Placenta accreta occurs when placental villi attach directly to or invade the myometrium, preventing normal placental separation. Placenta accreta is commonly associated with placenta previa (64% of placenta accreta) or a history of prior cesarean section, dilatation and curettage, or abortion. An anterior placenta previa in a patient with prior cesarean section should make the obstetrician particularly wary of an accreta.6 Accreta should be considered whenever a retained placenta occurs or when manual removal of the placenta is particularly difficult. Although most cases of accreta require hysterectomy, some patients can be managed with curettage, suture of the bleeding site, or hypogastric artery ligation. Modern management of this complication is credited with reducing the maternal mortality rate in this condition from 37% to 3%.7
Coagulopathy
Immediate hemostasis after delivery occurs by uterine compression of myometrial spiral arteries. However, most coagulopathies (e.g., idiopathic thrombocytopenic purpura, von Willebrand's disease) may also cause PPH. Disseminated intravascular coagulation from abruptio or severe preeclampsia may also result in PPH. Coagulopathies have the potential to cause PPH up to several days after delivery.8 Although the obstetrician is usually aware of these problems before delivery, there is a potential for a coagulopathy to make its initial presentation as PPH.
Uterine Inversion
Uterine inversion has been reported in the past to be extremely rare (1:20,000 deliveries) and to have an associated high mortality rate.9 Recent studies suggest that the rate of uterine inversion is approximately 1:2000.10, 11 The high mortality rate of uterine inversion in the past may have resulted from the practice of not performing routine vaginal and cervical examination immediately postpartum.12 The major complication of uterine inversion is PPH. Shock is directly related to the volume of blood lost.10, 11
Uterine inversion can occur spontaneously but usually is associated with uterine fundal pressure and cord traction to deliver the placenta. Primiparity, uterine hypotonia, and fundal placental implantation are associated with an increased risk of uterine inversion. A 10-year review of uterine inversions at the University of Michigan, from 1970 to 1980, revealed 10 uterine inversions, with an incidence of 1:1770 deliveries (Table 1). The degree of shock was related to hemorrhage. In all of the cases the uterus was replaced manually. Immediate recognition of uterine inversion and prompt replacement appear to be the keys to preventing death and complications.
Table 1. Cases of uterine inversion, University of Michigan, 1970–1980
Patient | Age | Para | Estimated blood loss (mL) | Shock | Postpartum complications | Postpartum stay (days) |
1 | 23 | 0 | 2500 | ++ | Fever | 4 |
2 | 24 | 1 | 2000 | None | 5 | |
3 | 26 | 1 | 2000 | ++ | None | 4 |
4 | 19 | 0 | 1900 | Fever | 3 | |
5 | 30 | 0 | 1500 | + | None | 4 |
6 | 29 | 1 | 1250 | + | None | 4 |
7 | 28 | 2 | 1000 | + | None | 3 |
8 | 26 | 0 | 750 | + | None | 4 |
9 | 18 | 0 | 500 | None | 3 | |
10 | 22 | 0 | 500 | None | 3 |
Late Postpartum Hemorrhage
Late (or secondary) PPH is defined as hemorrhage occurring more than 24 hours after delivery. Causes include subinvolution of the placental site, retained products, lacerations, and coagulopathy.13, 14 Although infection is occasionally noted with delayed PPH, it is uncommon. The management of delayed PPH follows the same sequential process as that of early PPH.
MANAGEMENT
Management of PPH begins before excessive blood loss has occurred by carefully observing for the rate of bleeding immediately after delivery. It is common practice in the United States to await spontaneous expulsion of the placenta (expectant management of third stage). In contrast, active management of the third stage involves early cord clamping, prophylactic administration of uterotonic agents before placental delivery, and controlled cord traction. A recent review in the Cochrane Library15 identified four controlled trials, performed in the United Kingdom, Ireland, and Abu Dhabi, of active versus expectant management. The meta-analysis found a somewhat shorter third stage of labor (mean difference −9.8 minutes, 95% confidence interval −10.0 to −9.5), an average of 79 mL less mean blood loss, and a lower risk of postpartum hemorrhage exceeding 500 mL and exceeding 1000 mL. Active management was associated with increased maternal side effects, including nausea and hypertension. Vigorous cord traction may be associated with uterine inversion, but this was not evaluated in the studies. Controlled trials of active versus expectant management in the context of U.S. practice are still needed.
A schematic approach to PPH is presented in Table 2. Immediately after placental delivery, bimanual massage of the uterus promotes uterine contraction and hemostasis. If uterine bleeding does not promptly diminish, the obstetrician should proceed in serial fashion to consider possible causes of bleeding and institute therapeutic interventions. If a maneuver is unsuccessful in stopping hemorrhage, an alternative should be attempted. When less invasive measures are not initially successful, it is usually fruitless to repeat them while the patient continues to bleed.
Table 2. Management scheme for postpartum hemorrhage
Diagnostic maneuver | To determine etiology | Therapeutic maneuver |
Palpate uterus | Uterine atony | Uterine massage |
Establish intravenous access (if not established) | ||
Oxytocin | ||
Methylergonovine | ||
Prostaglandin (Carboprost or alternative) | ||
Catheterize bladder | ||
Obtain blood for transfusion (if not already available) | ||
Prevent/treat shock | ||
Examine perineum, vagina, cervix | Lacerations | Repair lacerations |
Manually explore uterus | Retained products | Manual re moval |
Dilatation and curettigs | ||
Uterine inversion | Replace uterus | |
Surgical replacement | ||
Uterine rupture | Laparotomy for repair or hysterectomy | |
Coagulation studies | Coagulopathy | Specific factor replacement |
If above measures are unsuccessful, presume uterine atony, uterine rupture, or intra-abdominal laceration | ||
Repeat prostaglandin | ||
| MAST suit (if available) | |
| Uterine artery embolization | |
Uterine tamponade (e.g., SOS Bakri™ balloon tamponade) | ||
| or | |
| Laparotomy | |
B-Lynch compression suture | ||
Hypogastric artery ligation | ||
Hysterectomy |
Uterotonic Agents
OXYTOCICS
Prophylactic use of oxytocics postpartum reduces the risk of postpartum hemorrhage by approximately 40%.16 In Britain and Europe, oxytocics are often administered at the time of the delivery of the anterior shoulder or immediately after delivery of the infant. In most US hospitals, it is common practice to begin an infusion of oxytocin only after the delivery of the placenta; 10–20 units oxytocin in 1000 mL crystalloid is administered at 100 to 200 mL per hour through a previously established intravenous site. If an intravenous line was not started earlier, it should be placed immediately on the suspicion of increased postpartum bleeding. Direct intravenous bolus injection of as little as 5 units of oxytocin has been associated with hypotension; therefore, a continuous drip is preferable.17
ERGOT
Methylergonovine or ergonovine maleate 0.2 mg may be administered intramuscularly. There is no clear evidence suggesting superior efficacy of ergot derivatives over oxytocin, although ergot preparations appear to be associated with a higher incidence of hypertension and should not be used in hypertensive patients. Ergot derivatives should not be administered intravenously because of the potential for severe vasospasm and hypertensive crisis.
PROSTAGLANDINS
Prostaglandins F and E promote strong uterine contractions that are effective in the treatment of uterine atony unresponsive to either oxytocin or methylergonovine stimulation. Reported series in which prostaglandin was used selectively for uterine atony showed a success rate of approximately 85%.18, 19 Prostaglandins may be less effective in the presence of chorioamnionitis or after a cesarean section.
Multiple forms of prostaglandin are available. The principal parenteral prostaglandin formulation is 15-methyl prostaglandin F2α, also known as carboprost (Hemabate, Pharmacia & Upjohn). Hayashi and coworkers18 outlined a safe and effective protocol using an intramuscular dose of 0.25 mg carboprost every 15 minutes for up to five injections over 1.5 hours. Carboprost may also be injected directly into the myometrium and may have a faster onset of action by this route. If carboprost is not immediately available, other forms of prostaglandin are effective in controlling uterine atony. Prostaglandin E2 20-mg vaginal suppositories (Prostin E, Pharmacia & Upjohn) may be administered vaginally or rectally.20 Recently, rectal administration of 1000 μg (five 200-μg tablets) of misoprostol (Cytotec, Searle) was described for treatment of severe postpartum hemorrhage.21
Prophylactic use of prostaglandins, both injectable carboprost and oral misoprostol, as an alternative to oxytocin or methylergonovine has been investigated.22 Prostaglandin administration during the third stage of labor appears to be effective in preventing postpartum hemorrhage, but data are insufficient to evaluate relative cost and safety. Similar concerns exist as voiced above about this approach versus current common practice in the United States.
When using prostaglandins, the obstetrician must be cognizant of the systemic effects of these agents and any underlying medical condition of the patient. Side effects of diarrhea, hypertension, vomiting, fever, flushing, and tachycardia are common.19 Patients with significant cardiac or pulmonary disease will be at high risk should systemic side effects develop; thus, prostaglandins should be used with extreme caution in these patients.
It may be beneficial to catheterize the bladder in cases of uterine atony. Catheterization also reduces the risk of bladder trauma during other maneuvers.
Repair Lacerations
Depending on the conduct of the delivery and palpation of the uterine tone after placental delivery, treatment of uterine atony may precede inspection for lacerations. If uterotonic agents are unsuccessful in reducing bleeding, a careful visual and manual examination of the perineum, vagina, and cervix is carried out. Brisk bleeding may occur from an episiotomy in the absence of other lacerations or extensions. Vaginal sidewall lacerations may bleed profusely and should be repaired carefully. Repair of vaginal lacerations, as with episiotomy, must extend above the apex of the laceration because vessels can retract and cause a late hematoma. Small cervical lacerations need not be repaired unless they are bleeding. When necessary, repair of cervical lacerations should also begin with a deep suture above the apex of the laceration to reduce bleeding and to provide adequate control of the cervix for the repair.
Manual Removal of Placenta and Uterine Exploration
Signs of placental separation (lengthening of the umbilical cord, a show of blood, and a change of shape of the uterine fundus) are well known to obstetricians. Increased bleeding without evidence of placental separation suggests the need to remove the placenta manually. Anesthesia or analgesia may be necessary to allow adequate exploration of the uterus. Aseptic surgical technique is important to reduce the risk of infection. Placental delivery is performed using the following technique:
- With the nondominant hand grasping the fundus through the abdominal wall, use the other hand explore the uterine cavity to find the edge of the placenta.
- Break through the membranes and enter the decidual plane.
- Sweep the hand over the placental surface to separate it from the uterine attachment.
- Grasp the entire placenta and withdraw it.
- Carefully remove remaining membranes using a ring forceps.
Manual exploration after placental delivery is performed to find and remove retained placental remnants (which may be present even if the placenta appears intact on visual examination) or clots and to rule out uterine inversion or rupture. We have found that manual evacuation of clot from the uterine cavity frequently allows uterine contraction and promptly reduces the bleeding. The discovery of a uterine rupture leads to either immediate laparotomy for repair or hysterectomy, as discussed below.
When performing uterine exploration, some obstetricians wrap a gauze around their glove to remove placental fragments by abrasive action. If placental fragments cannot be removed manually, curettage with a large curette should be performed. Postpartum curettage must be performed with extreme caution because of the risk of perforating the soft postpartum uterus.
Prevent or Treat Shock
If initial attempts at control of hemorrhage by uterotonics and inspection and repair of lacerations are unsuccessful, the obstetrician should ascertain that adequate blood replacement is available. Anesthesia personnel should be available in the event that surgical treatment is necessary. Vital signs must be monitored regularly. The first sign of impending shock is tachycardia; it is only when this compensatory mechanism cannot maintain adequate perfusion that the blood pressure will drop.
Coagulation Studies
If uterine bleeding persists, coagulation studies should be performed. Coagulopathy may cause PPH or may result from profuse bleeding and volume replacement. Coagulation disorders are treated by specific factor replacement (e.g., platelet transfusion, cryoprecipitate).
Uterine Packing
In recent years uterine packing has fallen into disfavor. Packing of the uterus can be considered an art; it is difficult to develop expertise in this technique without some practice. Unless one is trained and experienced in this technique, it is not recommended.
The use of a large Foley catheter has been reported to control PPH.23, 24 Recently a commercial device, the SOS Bakri Balloon™ catheter became available (Cook® OB/Gyn).25 This device has been useful in a number of patients, but one should keep in mind the potential of masking continued intrauterine bleeding.
MAST Suit
The military antishock trousers (MAST) suit was perfected during the Vietnam experience and recently has received attention for its use in gynecologic and obstetric hemorrhage.26, 27 The MAST suit exerts its effect by returning more peripheral vascular blood supply to the central circulation owing to a direct pressure effect on the vessels within the suit, as well as controlling uterine hemorrhage by direct pressure on the uterus. The suit does appear to increase blood pressure dramatically when it is applied and to control hemorrhage by decreasing the rate of bleeding. The use of the MAST suit may control bleeding to the extent that surgery is not necessary.27 Use of the MAST suit depends on availability and familiarity with its use. The reports on its use are encouraging, and as obstetric units obtain more experience with this suit it may become not only readily available but also an important addition to the armamentarium of the obstetrician.
Arterial Embolization
As interventional radiology techniques become more widely available, arterial embolization is a reasonable choice for control of continued hemorrhage when lacerations have been excluded or repaired and uterotonics are not effective. This approach has also been effective in situations of continued hemorrhage after hypogastric artery ligation or hysterectomy.28
Surgery
When uterotonic agents have failed, a decision must be made either to proceed with arterial embolization or perform laparotomy. The MAST suit, described above, may be helpful in this situation to control hypotension while the patient is being transported and prepared.
When the abdomen is entered at the time of PPH, the patient is often in a compromised state, and the obstetrician must proceed with a well-conceived plan. The abdomen should be entered rapidly and the uterus compressed within both hands and elevated on traction. Upward traction on the uterus, as well as the bimanual pressure, decreases blood loss and allows for some stabilization of the patient. Should the patient be unstable or the blood loss rapid, aortic compression may be beneficial.
UTERINE ARTERY LIGATION
Uterine artery ligation is well described by O'Leary and O'Leary.29 This technique uses a large Mayo needle with a 1 chromic suture. The needle is passed into and through the myometrium from anterior to posterior 2–3 cm medial to the uterine vessels and brought through the avascular area of the broad ligament lateral to the artery and the vein. This appears to be effective by reducing the pulse pressure to the uterus, because approximately 90% of the blood flow to the uterus is from the uterine artery. Uterine artery ligation was originally described at the time of cesarean section; the procedure failed in only 9 of 90 patients. It is technically less difficult than hypogastric artery ligation, and the risk of venous bleeding in the retroperitoneal space is avoided.
UTERINE COMPRESSION PROCEDURES
B-Lynch described a uterine compression suture technique to avoid the need for hysterectomy.30 The B-Lynch suture mechanically compresses the uterus to treat hemorrhage resulting from intractable atony. Baskett report a series of 28 cases treated with this technique over a 7-year period.31 Hemorrhage was not controlled in five patients (18% failure rate). Seven patients went on to have successful subsequent pregnancies.31 Complications of this technique, including ischemic necrosis of the uterus and erosion of the sutures have been reported.32, 33, 34 Although these appear to be isolated case reports it is reasonable to reserve this technique for situations where all other alternatives to hysterectomy have been exhausted.
HYPOGASTRIC ARTERY LIGATION The reported success rate of hypogastric artery ligation varies from 40% to 80%.35, 36 It works by reducing pulse pressure and not by absolute control of blood flow; it decreases ipsilateral blood flow by approximately 50% and decreases pulse pressure by approximately 85%.37 Although articles have been written on the efficacy and ease with which hypogastric artery ligation can be performed, this operation can be somewhat difficult and potentially dangerous for an operator without some experience in the retroperitoneal space. This can be especially true under the circumstances in which it is usually performed. Often the patient is continuing to lose blood, is hypotensive, and may be in profound shock. This obviously heightens the anxiety of the operator, and should the operator be inattentive to detail because of the pressures of the situation or be unfamiliar with the technique, a more serious and potentially catastrophic event can occur, with damage to the internal or external iliac vein. For that reason, if the operator is not familiar with this operation and assistance is not readily available from someone accomplished in the operation, proceeding directly to hysterectomy may be the more prudent course of action.
HYSTERECTOMY
Hysterectomy can be a life-saving operation, and temporizing measures can lead to further deterioration of the patient's hemodynamic status. The choice of total versus subtotal (supracervical) hysterectomy is dictated by the patient's status at the time of operation, as well as by the difficulty of the procedure. The cervix should be removed if it is technically feasible and the patient is stable; however, one must remember that the indication for the operation is massive hemorrhage. In a patient with a normal Papanicolaou smear and no evidence for cervical disease, a continued effort to remove the cervix is not necessary if it risks excessive blood loss or if the patient is unstable.
When performing a hysterectomy for PPH, the obstetrician must be cognizant of the tremendous blood supply and the dilatation that occurs in the ovarian and uterine vessels. Careful attention must be paid to hemostasis, and all pedicles should be clearly identified and secured. Pedicles should be smaller than normally would be used, and vascular pedicles should be doubly clamped and doubly ligated. An excessive amount of skeletonization in the broad ligament should be avoided. Incorporating both leaves of the broad ligament into the pedicles should be attempted to prevent oozing from denuded peritoneal surfaces. Attention should also be paid to the amount of back bleeding that will occur as a result of the increased collateral blood supply.
Intravenous antibiotic therapy should be instituted at the time of hysterectomy. Broad-spectrum coverage with ampicillin, gentamicin, and clindamycin is reasonable, or a second- or third-generation cephalosporin may be used. The morbidity related to hysterectomy for obstetric hemorrhage is considerable; however, it must be borne in mind that this is a life-saving operation. Potential complications include transfusion (96%), febrile morbidity (50%), wound infection (12%), coagulopathy (6%), ureteral injury (4%), cardiac arrest (4%), septic pelvic thrombophlebitis (3%), and maternal death (1%).38
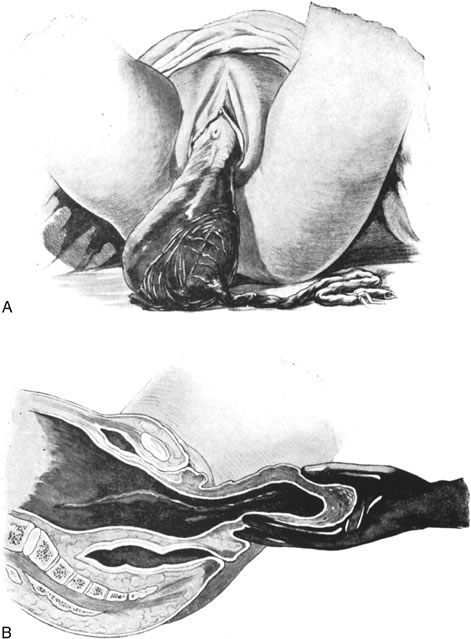
Replacement of a Uterine Inversion
Uterine inversion may present as the uterine fundus protruding through the cervix and vaginal introitus (complete inversion) or as only a depression of the uterine fundus into the endometrial cavity (partial inversion). The uterus can usually be replaced by steady pressure against the fundus or by gradually replacing the uterus from the edges with pressure from the fingertips (Fig. 1). Replacement of the uterus may be facilitated by tocolytic drugs, (β-sympathomimetics, or magnesium sulfate).39, 40, 41 Use of tocolytic agents for uterine relaxation may obviate the need to induce deep halothane anesthesia.
Once the uterus is replaced, it should be held firmly with bimanual compression until uterine tone develops. An oxytocin infusion or intramuscular administration of ergonovine may be helpful; carboprost may also be beneficial. The obstetrician should be aware of the potential for spontaneous reinversion of the uterus. In this situation, immediate replacement and adjuvant use of uterotonics are indicated.42, 43
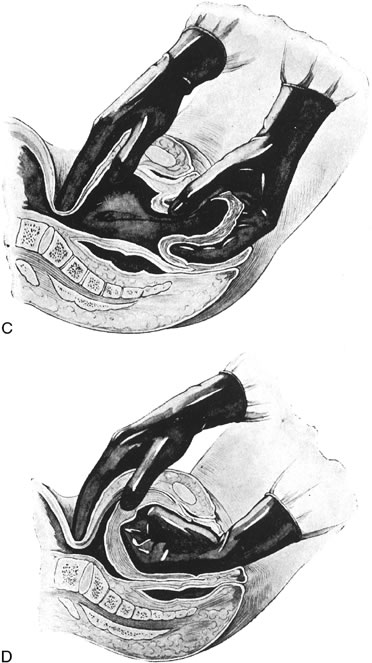
In rare instances, vaginal replacement of the inverted uterus is not possible. In this case laparotomy should be performed to reposition the uterus. The uterus is grasped within the contraction ring with Allis forceps, and gentle traction is applied. As the uterus is gradually restored to normal position, the forceps are advanced on the fundus.44 In some cases an incision may be made through the contraction ring on the posterior side of the uterus to reduce constriction and a combined abdominal-vaginal repositioning may be performed.45 Although other surgical approaches, such as Spinelli's vaginal procedure, have been described, the rarity of this condition precludes an evaluation of the merit of these approaches.
REFERENCES
Kaunitz AM, Hughes JM, Grimes DA et al: Causes of maternal mortality in the United States. Obstet Gynecol 65: 605, 1985 |
|
Chang J, Elam-Evans LD, Berg CJ et al: Pregnancy-related mortality surveillance--United States, 1991--1999. MMWR Surveill Summ 2003 Feb 21;52(2):1-8 |
|
Pritchard JA, Baldwin RM, Dickey JC, Wiggins KM: Blood volume changes in pregnancy and the puerperium II. Red blood cell loss and changes in apparent blood volume during and following vaginal delivery, cesarean section, and cesarean section plus total hysterectomy. Am J Obstet Gynecol 84: 1271, 1962 |
|
Newton M: Postpartum hemorrhage. Am J Obstet Gynecol 94: 711, 1966 |
|
Nelson GH: Consideration of blood loss at delivery as percentage of estimated blood volume. Am J Obstet Gynecol 138: 1117, 1980 |
|
Clark SL, Koonings PP, Phelan JP: Placenta previa/accreta and prior cesarean section. Obstet Gynecol 66: 89, 1985 |
|
Read JA, Cotton DB, Miller FC: Placenta accreta: Changing clinical aspects and outcome. Obstet Gynecol 56: 31, 1980 |
|
Strickland DM, Galey WT, Hauth JC: Hypofibrinogenemia as a cause of delayed postpartum hemorrhage. Am J Obstet Gynecol 143: 230, 1982 |
|
Bunke JW, Hofmeister FJ: Uterine inversion: Obstetrical entity or oddity? Am J Obstet Gynecol 91: 934, 1965 |
|
Watson P, Besch N, Bowes WA Jr: Management of acute and subacute puerperal inversion of the uterus. Obstet Gynecol 55: 12, 1980 |
|
Platt LD, Druzin ML: Acute puerperal inversion of the uterus. Am J Obstet Gynecol 141: 187, 1981 |
|
Henderson H, Alles RW: Puerperal inversion of the uterus. Am J Obstet Gynecol 56: 134, 1948 |
|
Thorsteinsson VT, Kempers RD: Delayed postpartum bleeding. Am J Obstet Gynecol 107: 565, 1970 |
|
Rome RM: Secondary postpartum haemorrhage. Br J Obstet Gynaecol 82: 289, 1975 |
|
Prendiville WJ, Elbourne D, McDonald S: Active versus expectant management in the third stage of labour (Cochrane Review). In: The Cochrane Library, Issue 2, 2001. Oxford: Update Software |
|
Prendiville W, Elbourne D, Chalmers I: The effects of routine oxytocic administration in the management of the third stage of labour: An overview of the evidence from controlled trials. Br J Obstet Gynaecol 95: 3, 1988 |
|
Hendricks CH, Brenner WE: Cardiovascular effects of oxytocic drugs used post partum. Am J Obstet Gynecol 108: 751, 1970 |
|
Hayashi RH, Castillo MS, Noah ML: Management of severe postpartum hemorrhage with a prostaglandin F-2-alpha analogue. Obstet Gynecol 63: 806, 1984 |
|
Oleen MA, Mariano JP: Controlling refractory atonic postpartum hemorrhage with Hemabate sterile solution. Am J Obstet Gynecol 162: 205, 1990 |
|
Hertz RH, Sokol RJ, Dierker LJ: Treatment of postpartum uterine atony with prostaglandin E2 vaginal suppositories. Obstet Gynecol 56: 129, 1980 |
|
O'Brien P, El-Refaey H, Gordon A et al: Rectally administered misoprostol for the treatment of postpartum hemorrhage unresponsive to oxytocin and ergometrine: A descriptive study. Obstet Gynecol 92: 212, 1998 |
|
Gülmezoglu AM: Prostaglandins for prevention of postpartum haemorrhage (Cochrane Review). In: The Cochrane Library, Issue 2, 2001. Oxford: Update Software |
|
Bowen LW, Beeson JH: Use of a large Foley catheter balloon to control postpartum hemorrhage resulting from a low placental implantation. A report of two cases. J Reprod Med 30: 623, 1985 |
|
Marcovici I, Scoccia B. Postpartum hemorrhage and intrauterine balloon tamponade. A report of three cases. J Reprod Med 44:122, 1999 |
|
Bakri YN, Amri A, Abdul Jabbar F: Tamponade-balloon for obstetrical bleeding. Int J Gynaecol Obstet 2001 Aug;74(2):139-42 |
|
Peligra R, Sandberg EC: Control of intractable abdominal bleeding by external counterpressure. JAMA 241: 708, 1979 |
|
Pearse CS, Magrina JF, Finley BE: Use of MAST suit in obstetrics and gynecology. Obstet Gynecol Survey 39: 416, 1984 |
|
Pelage JP, Le Dref O, Jacob D et al: Selective arterial embolization of the uterine arteries in the management of intractable post-partum hemorrhage. Acta Obstet Gynecol Scand 78: 698, 1999 |
|
O'Leary JL, O'Leary JA: Uterine artery ligation for control of postcesarean section hemorrhage. Obstet Gynecol 43: 849, 1974 |
|
B-Lynch C, Coker A, Lawal AH et al: The B-Lynch surgical technique for the control of massive postpartum haemorrhage. An alternative to hysterectomy? Br J Obstet Gynaecol 104: 372, 1997 |
|
Baskett TF: Uterine compression sutures for postpartum hemorrhage: efficacy, morbidity, andsubsequent pregnancy. Obstet Gynecol 2007 Jul;110(1):68-71 |
|
Joshi VM, Shrivastava M: Partial ischemic necrosis of the uterus following a uterine brace compressionsuture. BJOG 2004 Mar;111(3):279-80 |
|
Treloar EJ, Anderson RS, Andrews HS et al: Uterine necrosis following B-Lynch suture for primary postpartum haemorrhage. BJOG 2006 Apr;113(4):486-8 |
|
Grotegut CA, Larsen FW, Jones MR et al: Erosion of a B-Lynch suture through the uterine wall: a case report. J Reprod Med 2004 Oct;49(10):849-52 |
|
Clark SL, Phelan JP, Yeh S et al: Hypogastric artery ligation for obstetric hemorrhage. Obstet Gynecol 66: 353, 1985 |
|
Montgomery L, Belfort M, Allon M, Moise K Jr: Hypogastric artery ligation is an effective and safe alternative to hysterectomy in patients with severe postpartum hemorrhage [abstract]. Am J Obstet Gynecol 172: 291, 1995 |
|
Burchell RC: Physiology of internal iliac artery ligation. J Obstet Gynaecol Br Commonw 75: 642, 1968 |
|
Clark SL, Yeh S, Phelan JP et al: Emergency hysterectomy for obstetric hemorrhage. Obstet Gynecol 64: 376, 1984 |
|
Grossman RA: Magnesium sulfate for uterine inversion. J Reprod Med 26: 261, 1981 |
|
Catanzarite VA, Moffitt KD, Baker ML et al: New approaches to the management of acute puerperal uterine inversion. Obstet Gynecol 68: 7S, 1986 |
|
Thiery M, Delbeke L: Acute puerperal uterine inversion: two-step management with a beta-mimetic and a prostaglandin. Am J Obstet Gynecol 153: 891, 1985 |
|
O'Connor MC: Recurrent postpartum uterine inversion. Br J Obstet Gynaecol 84: 789, 1977 |
|
Heyl PS, Stubblefield PG, Phillippe M: Recurrent inversion of the puerperal uterus managed with 15(s)-15-methyl prostaglandin F-2-alpha and uterine packing. Obstet Gynecol 63: 263, 1984 |
|
Huntington JL, Irving FC, Kellogg FS: Abdominal reposition in acute inversion of the puerperal uterus. Am J Obstet Gynecol 15: 34, 1928 |
|
Haultain FWN: The treatment of chronic uterine inversion by abdominal hysterotomy, with a successful case. Br Med J 2: 974, 1901 |


COMMENTARY
By: Louis Keith
Affiliation: Emeritus Professor of Obstetrics & Gynecology, Feinberg School of Medicine, Northwestern University, Chicago, Illinois, USA
Date Submitted: 27-10-2008 15:00:27
Comment:
Although this chapter is dated for revision in 2009, it should not be overlooked because the information it contains is valuable. In particular, clinicians should realize that the active management of labor has now been shown to dramatically reduce the incidence of PPH in all circumstances, i.e., in both the developed and developing worlds. In addition, there have now been at least 1800 reported cases of the use of the B-Lynch suture (C. B-Lynch, personal communication, 2008) in which there have been only 17 failures. When the failures were analyzed, they were determined to be due to late application or to poor or incorrect technique. One of the issues that the chapter fails to emphasize is the fact that bolus administration of oxytocin is often followed by hypotension, and that this is particularly vexing in the case of cesarean delivery.1 Further reading is available on the internet at www.sapienspublishing.com in the Textbook on Postpartum Hemorrhage, edited by B-Lynch, Keith, Karoshi and LaLonde. This latter volume is the only up-to-date (2006) textbook available and represents the opinions of authorities throughout the world.
1. Pinder AJ, Dresner M, Calow C et al: Hemodynamic changes causes by oxytocin during cesarean section under spinal anesthesia. Int J Obstet Anes 2002: 11, 156–159