Serious Postpartum Infections
Authors
INTRODUCTION
Endometritis is the most common infection that occurs post partum. The incidence of postpartum endometritis has been reduced by the widespread use of prophylactic antibiotics. The incidence following cesarean section has dropped from 50% to 90% without antibiotic prophylaxis to 15% to 20% with prophylaxis.1 However, infection still occurs in 10% to 20% of patients who are given prophylaxis, as well as in the large number of women who do not receive prophylaxis, so it is apparent that infection still occurs in this era of prophylaxis. In addition, the effect of antibiotic prophylaxis on serious infection seems to be minimal, in part because prophylaxis apparently has less impact on the development of serious infection than on post-operative fever and in part because a large number of women who develop serious infection have insufficient risk factors to warrant prophylaxis. Many of the most serious infections are due to unusual organisms that seem to cause infection by chance more often than by the presence of predictable risk factors. At the same time, changes in obstetrical practices continue to increase patient risk for infection. Cesarean sections account for an ever-increasing proportion of deliveries; post-partum endometritis is tenfold to 20-fold more common among women who delivered by cesarean section than women who delivered vaginally.2 In addition, an increasing number of women have invasive procedures performed before or during labor. There is a trend, particularly among gestations with marked prematurity, for longer delays between rupture of membranes and delivery. Women with severe medical disease that potentially increases infectious rates, such as diabetics, renal transplant patients, and those on long-term steroid therapy, are now encouraged to become pregnant when formerly they were either unable to become pregnant or were advised against pregnancy.
On the other hand, serious complications resulting from infection have become unusual in this era. Maternal death from infection is no longer one of the traditional top three leading causes of maternal mortality.3 Postpartum endometritis is usually adequately treated by any of a large number of effective and safe antibiotics. In fact, reliance on the effectiveness of antibiotics has been so great that the vast majority of febrile women are treated without a thorough physical examination or without a culture. However, not all febrile women have postpartum endometritis and not all infected patients respond adequately to antibiotic therapy. There is a tendency for today's practicing physician to place almost blind reliance on the effectiveness of antibiotics. In this chapter it will become evident that, although they are rare, serious post-partum infections still occur. A serious infection must be rapidly and accurately diagnosed. It will also become evident that many serious potentially life-threatening postpartum infections do not respond solely to antibiotic therapy, and surgical intervention usually becomes necessary.
It is beneficial to have an understanding of the pathophysiology of postpartum infection. The vagina contains a large number of organisms (from 104 to over 109 organisms per milliliter of vaginal fluid) in proximity to the normally sterile upper genital tract.4 While the majority of these vaginal organisms are of low virulence, smaller concentrations of high-virulence organisms also reside in the vagina. A small number of women will even carry large quantities of virulent organisms. Organisms from the vagina are frequently introduced into the uterus during the labor and delivery process. Amniotic fluid colonization and postpartum infection rates are directly related to the duration of labor and rupture of the membranes. The uterine cavity has two characteristics that contribute to the possibility of infection: a large surface area on which infection can occur and, following placental delivery, large open venous channels under the placenta that are directly accessible to bacteria within the uterus. The uterus also serves as an anatomic barrier that is effective in preventing infection; however, breaks in this anatomic barrier enable infections to become establ4ished. Infections are much more common when an anatomic barrier is breached by cesarean section, urinary catheter placement, intravenous line placement, and anesthetic blocks than when these procedures are not performed. Organisms introduced into normally sterile areas as a result of the breach frequently cause infection of susceptible areas.
It is helpful to divide postpartum infections into those occurring in the early postpartum period (<48 hours) and those occurring in the late post-partum period (3 days-6 weeks).5 Patients can develop infections, occasionally serious infections, within the first 24 hours post partum, and early infections require the same prompt and effective treatment as those occurring later. Febrile post-partum women manifest bacteremia as commonly in the first 24 hours as in the next several days.6 Over 90% of women with a fever 38.5°C or higher in the first 24 hours post partum require treatment,7 and the old concept that infection does not occur in the first 24 hours post partum must be discarded. In fact, life-threatening infection may occur within hours of delivery. On the other hand, low-grade fever with temperatures up to 38°C within the first 24 hours of delivery, particularly intermittent fever, usually does not represent infection or require antibiotic therapy.7
EARLY INFECTION
Early infections most frequently result from subclinical infection or from excessive bacterial colonization of amniotic fluid and the endometrical deciduae prior to delivery, both of which are frequently caused by prolonged labor or ruptured membranes. Usually organisms that produce early infections are able to divide rapidly or produce toxin. Organisms, toxins, or both easily gain access to the vascular system of postpartum patients because of the large exposed venous channels in the uterus or because of interruption of natural barriers.
A thorough assessment of the patient by a complete history and physical examination is essential when symptoms or signs of severe infection develop. Symptoms of severe pain that appear out of proportion to that usually expected may occur with severe infection. Sudden pain with diaphoresis can indicate a sudden event. The more common symptoms and signs associated with severe infection are listed in Table 1. Unusual anxiety or disorientation may indicate impending circulatory shock. Prostration indicates a serious condition. Unusual temperature elevations, leukocytosis, leukopenia, hemoconcentration, low hematocrits, or poor urinary output should prompt close scrutiny for severe infection. Additional unusual manifestations indicate serious infection in the septic patient: septic shock, adult respiratory distress syndrome, disseminated intravascular coagulation, pulmonary emboli, hemolysis, sudden anasarca, or cardiac failure. Increasing areas of cellulitis with adequate antibiotic therapy or necrosis of tissue also indicates a severe infection. As stressed later, surgical removal of infected areas is required when these additional signs occur. Intensive care monitoring should be undertaken so that vital signs, blood gases, and urine function can be closely documented (Table 2). Ultrasound or imaging tests may be indicated. Patients with symptoms and signs of serious infection should have consultation with an infectious disease specialist or be transferred to a tertiary center once the clinical condition is stable.
TABLE 1. Common Manifestations That Indicate Severe Postpartum Infection
Physical Examination | Laboratory Examination |
Anxiety | Marked leukocytosis ( |
Disorientation | Marked leukopenia (<1,000) |
Prostration | Hemoconcentration (hematocrit > 45%) |
Severe tenderness | Low hematocrit (<20%) |
Unusual temperature elevation ( | Low urinary output (<20 ml/hr) |
Cardiac failure |
|
TABLE 2. Unusual Signs That Virtually Always Indicate a Serious Infection Which Usually Requires Surgical Removal
Septic Shock | Hemolysis |
Adult respiratory distress syndrome | Increasing area of cellulitis |
| Necrosis of tissue |
Disseminated intravascular coagulation |
|
ENDOMETRITIS (UTERINE-PERITONEAL INFECTIONS)
Early postpartum endometritis usually results from colonization or infection of the amniotic fluid prior to delivery.8 Often amniotic fluid infection will not be recognized during labor, particularly if fever has not developed. Risk factors important for amniotic fluid infection are also important for early postpartum endometritis and include those events likely to contaminate amniotic fluid (i.e., prolonged labor, prolonged rupture of the membranes, multiple cervical examinations, and lower socioeconomic status).9 Patients in lower socioeconomic groups probably have increased rates of virulent vaginal organisms that produce upper tract infection.10 Prolonged labor and rupture of membranes contribute to amniotic fluid colonization from organisms colonizing the lower genital tract. Colonizing organisms usually do not invade the endometrium or produce infection if patients deliver vaginally, because the contaminated amniotic fluid drains into the vagina in these cases. However, during cesarean section, bacteria in the amniotic fluid no longer remain within the uterus but have the potential to contaminate the peritoneal cavity and incisions of the uterus and abdominal wound. In fact, postpartum endometritis (uterine-peritoneal infections) is tenfold to 20-fold more common in patients delivered by cesarean section than among patients who deliver vaginally.2
Early postpartum endometritis is usually diagnosed on the basis of a temperature of 38.5°C or higher in the first 24 hours or 38°C or higher for 4 consecutive hours beyond the first 24 hours from delivery. Uterine tenderness is expected because most patients will have both a uterine and an abdominal wound from the cesarean section. Since wide variations occur in the degree of uterine tenderness, the finding of uterine tenderness is not a precise guide to establish whether or not uterine infection is present. Some organisms, particularly streptococci, may produce little or no uterine tenderness. Careful physical examination will usually reveal signs of peritonitis with an ileus and rebound tenderness in both upper and lower quadrants of the abdomen. It is important during the physical examination to exclude other sources of fever, particularly from wound, intravenous line, or lung infection.
Many physicians do not routinely obtain endometrial cultures because the organisms that cause early postpartum endometritis have been well described and, until recently, have been relatively similar between hospitals. Certainly unreliable culture results occur when cervicovaginal cultures are used or transcervical endometrial cultures are obtained by pushing an unprotected swab through the cervix.11 Instead, protected swabs should be used to obtain endometrial culture, because they reduce (although they do not eliminate) cervicovaginal contamination. Endometrial or amniotic fluid cultures taken at the time of delivery accurately reflect the endometrial flora for 24 hours post partum and can be substituted for a transcervical endometrial culture during this period. However, endometrial cultures should be obtained for the remaining patients to detect an unusual or particularly virulent organism. Organisms that are commonly isolated from patients with postpartum endometritis have been described elsewhere in these volumes and are not discussed in detail here. Cultures typically recover a wide variety of facultative bacteria, including group B streptococci, other facultative streptococci, Gardnerella vaginalis, and Escherichia coli, and a wide variety of anaerobic bacteria, including Bacteroides and Peptostreptococcus species.6,12 Blood cultures recover similar organisms from approximately 15% to 25% of febrile patients.6 Bacteremia per se does not predict the severity or the course of infection, although the isolation of certain virulent organisms can be predictive of severe infection. Despite the high frequency of positive blood cultures, young, otherwise healthy patients rarely develop septic shock.
A wide variety of antibiotics have been used to successfully treat postpartum endometritis. The antibiotic should be active against the most common facultative and anaerobic bacteria.13 Over 90% of patients with postpartum endometritis readily respond to antibiotic therapy. Several possibilities must be checked when patients do not respond to antibiotic therapy (Table 3). The administration of too low an antibiotic dose is the most common cause of treatment failure. Pregnant and postpartum women require a 40% increase in antibiotic dose over that required when they are no longer pregnant.14 The 40% increase in blood volume, extracellular volume, and glomerular filtration rate that occurs during pregnancy is maintained in the immediate postpartum period, and antibiotic concentrations must be high enough to achieve bacterial inhibition in the postpartum patient. Thus, most antibiotics, particularly those excreted through the kidneys, need to be administered at a high dosage. For example, the increased doses that should be given post partum calculate to between 8 and 12 g/day of a β-lactam (penicillin or cephalosporin) antibiotic. Other causes of antibiotic treatment failure include infection from resistant organisms (unusual), wound infection, abscess formation, and the development of septic thrombophlebitis.13
TABLE 3. Possibilities to Check When Patients Fail to Respond to Antibiotic Therapy
Subtherapeutic antibiotic dose | Wound infection |
Resistant organisms | Septic thrombophlebitis |
Abscess formation |
|
URINARY TRACT INFECTION
Approximately 5% of the patients who develop immediate and postpartum infections have a sterile endometrial cavity but 105 organisms or more per milliliter in the urine culture.6 Most of these patients will have had asymptomatic antepartum bacteriuria, which becomes symptomatic following bladder catheterization or the trauma of delivery. Costovertebral angle tenderness and other clinical signs of acute pyelonephritis are not usually present. Therefore, on the basis of clinical signs, these patients are difficult to differentiate from those with early postpartum endometritis. Urine sediments usually contain large numbers of white blood cells, and urine cultures are positive for uropathogens. Urinary tract infection usually responds readily to common antibiotics used to treat endometritis. However. an unusual serious antepartum complication of pyelonephritis has been reported with severe respiratory distress and disseminated intravascular coagulopathy requiring prolonged assisted ventilation.15 Physicians must be aware that similar severe manifestations of pyelonephritis could occur in the early postpartum period.
DISTURBED ABSCESSES
lntra-abdominal abscesses are unusual during pregnancy. Appendicitis and, rarely, abscess from salpingitis16 constitute the most likely sources of an intra-abdominal abscess. Relatively asymptomatic abscesses occur because infection within the abscesses remains separated from the vasculature and from adjacent organs by the abscess wall. During pregnancy, the uterus usually constitutes part of the abscess wall. At delivery, the uterine portion of the abscess wall is disturbed by the collapse of the uterus, and pus leaks into the peritoneal cavity. Infection can then spread, causing frank peritonitis, bacteremia, or both. A ruptured abscess may be difficult to recognize, particularly if the leak is slow and there are no sudden peritoneal findings. Prompt recognition of a ruptured abscess from the history and physical findings can prevent uncontrolled and potentially fatal sepsis. The patient may have had vague lower abdominal pain or even an episode resembling appendicitis during pregnancy. Unsuspected rupture of an abscess occurs among vaginally delivered patients because the abscess would have been discovered during cesarean section. Possible abscess rupture is most easy to recognize in vaginally delivered patients without prolonged labor or rupture of membranes who otherwise would not be expected to suddenly develop septic shock or signs of sepsis. Recognition becomes more difficult when patients have recognized risk factors for infection. Immediate abdominal exploration under antibiotic coverage is necessary to control sepsis from a disturbed ruptured abscess (Table 4).
TABLE 4. Most Common Cause of Serious Infection Occurring Early in the Postpartum Period (<48 Hours of Delivery)
Postpartum endometritis
Urinary tract infection
Disturbed abscess
Rapidly developing soft tissue infection
Necrotizing fasciitis
Intravenous fluid contamination
Intravenous catheter infection
RAPIDLY DEVELOPING SOFT TISSUE INFECTION
Rapidly developing soft tissue infections are potentially lethal infections of the subcutaneous tissue, muscle, or myometrium, most commonly caused by clostridia or group A streptococci. Patients with these rapidly developing infections usually have a history and physical examination similar to patients with other causes of postpartum infection. These infections commonly masquerade as postpartum endometritis, and patients may have the usual risk factors for infection, including prolonged labor or rupture of membranes, although many others will have no particular risk factors. However, two clinical features suggest a more serious infection: the severity of clinical manifestations and indications of septic shock. Patients with these infections invariably exhibit marked systemic signs and symptoms, including severe pain out of proportion to that usually expected, prostration, and, at times, disorientation initially or later in the course of infection. Temperature may be high (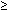 40°C), leukocytosis may be marked (>30,000), and hemoconcentration may occur as large shifts of fluid leak from the vascular compartment infected area. These patients frequently develop the features of particularly severe infection, including adult respiratory distress syndrome, disseminated intravascular coagulopathy, or renal shutdown. Signs of septic shock often eventually develop.
40°C), leukocytosis may be marked (>30,000), and hemoconcentration may occur as large shifts of fluid leak from the vascular compartment infected area. These patients frequently develop the features of particularly severe infection, including adult respiratory distress syndrome, disseminated intravascular coagulopathy, or renal shutdown. Signs of septic shock often eventually develop.
A relatively high (approximately 20%) bacteremic rate occurs with postpartum endometritis.6 However, few otherwise healthy postpartum women with uncomplicated postpartum endometritis or uncomplicated postpartum infection develop septic shock. Even in young patients without underlying disease, the mortality rate remains between 20% and 30% when septic shock occurs.17 Patients in septic shock require immediate resuscitation with fluids, respiratory support, circulatory support, and antibiotics. Intensive care monitoring becomes mandatory. The differential diagnosis includes amniotic fluid embolism, pulmonary embolism, cardiogenic shock (from drugs, cardiac disease, or aortic dissection), and diabetic ketoacidosis. Although unusual, rupture of an abscess with release of gram-negative anaerobic and aerobic bacteria must also be considered if septic shock occurs. The causative organism and the infectious source must be identified with certainty using physical examination, diagnostic tests, and appropriately obtained cultures. Poor prognostic signs include leukopenia, severe disseminated intravascular coagulopathy, high levels of lactate, and adult respiratory distress syndrome. Successful outcome usually depends on surgical removal of the source of infection. Virtually all postpartum septic shock develops from a source of infection that is amenable to surgical drainage or removal. Therefore it becomes the duty of the surgeon-obstetrician to absolutely exclude the possibility of surgically treatable infection when signs of severe infection occur. The surgeon must not relinquish this responsibility when the patient with shock is transferred to an intensive care specialist. Fluid resuscitation often restores the blood pressure to normal, giving a false sense of security. Thus, unless it can be established that the infection originates from the lung, kidney, or heart or from another site outside the pelvis, wound exploration, an examination under anesthesia, curettage, or exploratory laparotomy may be necessary for diagnosis and treatment. Surgery should be performed as soon as possible after restoration of adequate circulation. Surgery that becomes necessary despite a deteriorating circulatory function is hazardous but occasionally lifesaving if a defined focus of infection exists. Endotracheal intubation with minimal balanced general anesthesia is preferable to regional anesthesia.
Some causes of rapidly developing infection or shock are more common than others. Among these, myonecrosis caused by either Clostridium perfringens or other clostridia, including C. sordellii, is high on the list of possibilities, particularly if an unusual amount of tissue trauma has resulted from a difficult forceps delivery or during cesarean section. Other evidence of clostridial infection includes the classic signs of severe pain, hemolysis, renal shutdown, and gas formation. Other toxin-producing organisms, such as group A streptococci, or other organisms, including E. coli, can produce rapidly developing cellulitis without myonecrosis. Bacterial toxins usually produce necrosis in the area of cellulitis, and the combination of tissue necrosis and the systemic release of toxins causes unusually severe manifestations. Necrosis can occur in subcutaneous tissue, striated muscle, or myometrial tissue. Patients with necrosis exhibit progressive local infection and the alarming systemic signs previously noted. The source of infection may be particularly difficult to locate for physical or imaging examination if group A streptococci or clostridia produce deep striated muscle or uterine muscle necrosis. Necrotizing fasciitis from either an abdominal or an episiotomy wound is another cause of rapidly developing infection and shock that will be covered in more detail below.
Immediate antibiotic therapy must cover gram-negative facultative and anaerobic bacteria and clostridia, in particular. Three antibiotics are initially indicated for patients with septic shock or the severe manifestations of infection described above. Either an aminoglycoside or a first-generation cephalosporin is chosen to inhibit facultative aerobic bacteria. Clindamycin, imipenem-cilastatin, or metronidazole is given to inhibit anaerobic bacteria, and either penicillin or ampicillin is used for its effect on clostridia and for its synergistic action with aminoglycosides against enterococci. However, it cannot be overemphasized that antibiotic therapy alone in these serious infections is almost never sufficient to control sepsis, and surgical exploration is usually required (Table 5). Failure to recognize myonecrosis or necrotic cellulitis often proves fatal for patients with these serious infections. Antibiotics alone are without effect in necrotic tissue.
TABLE 5. Therapy for Rapidly Developing Soft Tissue Infections and Necrotizing Fasciitis
Three antibiotics
- Choice of one to inhibit gram-negative and gram-positive facultative aerobic bacteria
Gentamicin
Tobramycin
Amikacin - Choice of one to inhibit anaerobic bacteria
Clindamycin
Imipenem-cilastatin
Metronidazole - Choice of one to inhibit enterococci and clostridia
Penicillin
Ampicillin
Surgical removal of infected tissue
Obvious cellulitis of the subcutaneous tissue needs to be explored with the patient under general anesthesia, as described for necrotizing fasciitis. Tissue culture and Gram stain are required to identify the responsible organisms. Frozen tissue section may be required to identify necrosis.18 Necrotic tissue needs to be removed to the point of viability. Debridement serves to remove both necrotic tissue and the source of bacterial toxins. The involved area may be small in cases of early necrotizing cellulitis or extensive when infection has gone uncontrolled. Clostridial myonecrosis can involve muscles in the uterus or elsewhere in the pelvic diaphragm or sacral area.
Exploratory laparotomy can be lifesaving for patients with unchecked bacteremia and uncontrolled intra-abdominal sepsis. Laparotomy should be particularly considered when no apparent source of infection is evident. Obviously infected or necrotic structures need to be removed, whereas normal organs and tissue should be conserved. Removal or drainage of an abscess can be undertaken without removal of the uterus or fallopian tubes, if these organs are not extensively infected. The extensively infected uterus is usually pale, yellow, or obviously necrotic, often with extensive thrombosis of ovarian and adnexal vessels. When these findings are present and patients have increasingly severe and life-threatening disseminated coagulopathy or respiratory distress, hysterectomy can be lifesaving. The uterus usually contains areas of frank necrosis or micro-abscesses in the myometrium. Life-threatening coagulopathy or respiratory distress from sepsis slowly resolves with removal of infected tissue.
On the other hand, there are rare circumstances in which ascites and edematous bowel, uterus, and adnexae are present without obviously infected tissue. Considerable clinical judgment is required. We have had these rare cases that have progressive life-threatening coagulopathy and adult respiratory distress at maximal levels of oxygen and positive end-expiratory pressure in which the uterus contained only normal necrotic deciduae. Resolution of these life-threatening signs began immediately although slowly following hysterectomy. It is likely that in these rare circumstances the sepsis that began the coagulopathy and respiratory distress was successfully treated with antibiotics but the normal necrosis of deciduae continued to release enough thromboplastin or other factors to maintain the vicious cycle of coagulopathy and respiratory distress.
NECROTIZING FASCIITIS
Necrotizing fasciitis is a rapidly evolving acute infection of the superficial fascia (subcutaneous tissue). The superficial fascia contains two layers of subcutaneous tissue that become infected: a superficial fascia of Camper and a deeper Scarpa's fascia. Necrotizing fasciitis usually begins as a wound infection that spreads unchecked in part because of the lack of natural tissue barriers. In an abdominal wound, infection spreads superiorly, laterally, and inferiorly in the abdominal wall, and in an episiotomy wound, infection spreads onto the abdominal wall, laterally to the inner thighs, or posteriorly to the buttocks. More important, the synergistic action of at least two organisms causes tissue necrosis. A large variety of organisms can combine to produce a synergistic infection, including nonhemolytic streptococci and staphylococci, mixed aerobic and anaerobic bacteria (including clostridia), and group B hemolytic streptococci and anaerobes. The combined effect of the organisms produces extensive subcutaneous tissue necrosis and in many cases toxins that cause further severe systemic effects. Necrosis prevents antibiotics from reaching infected tissue, and organisms continue to proliferate and release toxin in the necrotic areas. The deep fascia and muscle layers are not involved unless the infection is totally out of control or clostridia are present. In addition, skin is not primarily infected. Therefore, the extent of necrosis often cannot be appreciated from the appearance of the skin until late in the disease when bullae or frank necrosis of the skin appears.
Although infections have been associated with diabetes mellitus,19 most postpartum patients with necrotizing fasciitis have no preexisting medical disease. Usually the antepartum course and delivery are uncomplicated. Extensive episiotomy tears are not associated with necrotizing fasciitis. Mortality rates range from 20% to 75%; the mortality rate is directly related to the promptness of diagnosis and surgical debridement.20 Fatalities occur most frequently 4 days after onset.
Typical clinical features include severe local pain and progressive erythema and edema of the area surrounding the wound despite appropriate antibiotic therapy. Infection can occur within hours of making the surgical wound. This progression can be documented over several hours in exceedingly rapid infections, particularly among diabetics. More often, infection progresses over a period of days. Erythema and woody edema occur beyond the boundary normally expected for a simple wound infection, and progression is a particularly characteristic feature.21 In addition, any patient with cellulitis who develops septic shock should be considered to have necrotizing fasciitis. Patients invariably have marked systemic features, including a high temperature (which occasionally can be normal), marked prostration, anemia (which may be masked by hemoconcentration as a vast amount of fluid collects within the extra-cellular compartment), shock, marked leukocytosis (often 20,000–75,000), and disseminated intravascular coagulopathy. Hypocalcemia is common, caused by the action of bacterial lipases, which degrade fat into fatty acids that in turn combine with calcium to produce soap.
Any patient suspected of having necrotizing fasciitis must have the diagnosis immediately confirmed by wound exploration under general anesthesia. Wounds should not be opened at bedside without adequate anesthesia because characteristic findings of infection, such as pus, are not usually present and limited exploration frequently leads to the conclusion that only minimal infection is present. On opening the wound, a thin, watery, usually nonodorous “dishwater”-appearing exudate is present but pus is not found. The tissue does not have an obviously necrotic appearance, but rather appears edematous. Necrosis is confirmed by easily dissecting tissue free with a blunt instrument. Frozen tissue diagnosis of suspected necrotic areas may be useful when the clinician is unfamiliar with the disease or when clinical findings are atypical.18 Tissue culture and immediate Gram stain must be done to exclude the presence of large gram-positive rods suggesting clostridia. The presence of large rods should prompt a search for deeper infection, including myonecrosis.
Therapy for this infection is obvious when the correct diagnosis is established. This uncommon disease is usually unfamiliar to the obstetrician. Many general surgeons have had experience with this infection, but consulting inexperienced physicians, even infectious disease specialists, may result in misleading recommendations. Therapy includes immediate debridement of all necrotic tissue. Necrotic tissue must be removed to the point where bleeding occurs. At times overlying viable skin can be saved and used as a skin graft, since skin is not primarily infected. Even with proper debridement, only 50% of patients survive.20 However, survival is nearly zero without surgical debridement. Survival is also low when patients have developed septic shock or clostridial infection. Wide-spectrum antibiotic therapy needs to be instituted, but it must be emphasized that most people who die of this infection received optimal antibiotic therapy from the time diagnosis is made. Multiple debridements are often required, and the patient should have further tissue removed under anesthesia as frequently as needed, often with daily debridements until the infection stabilizes.
INTRAVENOUS INFECTION
Fluid Contamination
Intravenous fluid contamination is an uncommon but lethal infection in which unusual organisms (Erwinia sp, Enterobacter cloacae, Pseudomonas stutzeri, Mima sp, or Herellea sp) colonize intravenous fluids and disseminate intravenously when the fluid is administered.22 Organisms in high concentrations or organisms that produce toxins are especially lethal. It is estimated that a large number of these infections occur annually, but most episodes go unrecognized because patients who receive intravenous fluids often have debilitating diseases with high mortality rates. The obstetrician can recognize this infection more readily when a rapid onset of sepsis developed in an otherwise healthy patient. Intravenous fluid contamination causes a rapid onset of sepsis or a persistent gram-negative sepsis with a hectic fever, leukocytosis, hypotension, disseminated intravascular coagulation, respiratory distress, and often renal shutdown. The intravenous bottle is usually not cloudy, and diagnosis requires a high degree of suspicion and the recovery of the same organism from both the patient's blood and the intravenous fluid. Therapy includes the immediate change of the intravenous line and bottle; the administration of antibiotics, including aminoglycosides to cover gram-negative facultative bacteria; and the usual resuscitative therapy for shock and serious sepsis. In many cases the offending source will not be identified, particularly if manifestations occur late or are present in a patient who had another apparent source of infection.
Catheter Infection
Intravenous catheter infections are associated with plastic catheters that are in place for more than 48 hours. These infections are reduced when professionally trained intravenous teams place and care for the catheters. Further preventive methods include the use of steel needles and upper extremities, careful washing of hands before line placement, a secure catheter anchoring, and coverage of the site with sterile dressing. Blood should not be drawn through the intravenous lines. However, changing the catheter site at least every 48 hours provides the most protection against these infections.
Clinical features of this infection include the presence of a plastic catheter (usually in place for more than 48 hours) and fever. with tenderness and redness along the vein or pus at the site when the catheter is removed. A palpable vein cord may also be present. Persistent septicemia or hypotension or multiple pulmonary emboli should also raise suspicion of this infection. Organisms involved include Staphylococcus aureus (in approximately 50% of cases) and Klebsiella, Enterobacter, and Serratia species. Therapy includes immediate intravenous catheter removal and culture of the catheter. Rolling the catheter on a blood plate provides a simple method for semi-quantitative culture.23 The presence of more than 15 colonies on the blood plate correlates with infection, whereas fewer colonies indicate colonization of the catheter by skin flora. Appropriate intravenous antibiotic therapy to inhibit Staphylococcus aureus is required through a new intravenous site. Surgical removal of the vein should be considered when intraluminal pus is discovered, particularly if pulmonary embolization continues despite appropriate antibiotic therapy.
LATE INFECTION
In contrast to the early postpartum infections previously discussed, most late postpartum infections do not have an associated high mortality rate. Many late infections develop slowly and insidiously. Both the pathogenesis and the microbiology of late postpartum infections differ from those of early postpartum infections. Late infections are often more difficult to diagnose and include a larger range of possibilities than early postpartum infections. Clinical features are often less obvious. For example, some patients will lack fever or leukocytosis. Despite the often insidious nature of late infections, they are still capable of causing serious morbidity (Table 6).
TABLE 6. Most Common Cause of Serious Infections Occurring Late in the Postpartum Period (3 Days to 6 Weeks)
Continuation of postpartum | Septic thrombophlebitis |
endometritis | Pudendal and paracervical |
Abscess | block infection |
Abdominal wound infection | Breast infection |
Toxic shock syndrome | Pseudomembranous colitis |
infection | Pneumonia |
Episiotomy infection |
|
New ascending uterine |
|
infection |
|
CONTINUATION OF EARLY POSTPARTUM ENDOMETRITIS AND SOFT TISSUE INFECTION
Physical examination of the patient is the key to diagnosing the continuation of the earlier infections. The patient should be checked carefully for a wound infection and for other sources of fever outside the pelvis. A thorough physical examination should include a bimanual pelvic examination. If a pelvic examination reveals only minimal tenderness and no mass; the remaining physical examination reveals no other source of infection, but only a fever; and the elevated pulse rate, leukocytosis, abdominal tenderness, and ileus have resolved, resolution of fever can be expected within 24 hours and additional or new antibiotic therapy is not indicated. The patient should be observed carefully. However, if a fever together with any of the other features remain or reappear, the following checklist should be used:
- Check for an appropriate antibiotic dose or level.
- Check the antibiotic sensitivities for resistant organisms.
- Thoroughly reexamine for wound infection, including, at times, aspirating the wound with a needle.
- Search for an abscess, extragenital infection, or septic pelvic thrombophlebitis.13
Two antibiotics should be administered as a rule when patients have a prolonged or serious infection.24 Broad-spectrum antibiotic coverage with either clindamycin or an aminoglycoside will inhibit the majority of aerobic and anaerobic bacteria. Metronidazole and a cephalosporin or other antibiotic combinations are other possible choices. Imipenem-cilastatin, given alone, can also be used.
Rarely, patients with early postpartum endometritis will remain seriously ill with a prolonged course or will develop signs of uncontrolled sepsis such as persistent bacteremia or increasingly severe disseminated intravascular coagulopathy or adult respiratory distress syndrome. The same careful clinical judgment needed to manage persistent early sepsis is also required for persistent late sepsis. Patients with these continued manifestations of infection must be carefully scrutinized for either a surgically treatable infection or inappropriate antibiotic therapy. As is true of unchecked early infection, unchecked late infection with these serious manifestations usually represents a collection of pus or necrotic infected tissue that requires surgical removal. If there is no other obvious source of the infection, such as a wound or extragenital site, consideration should be given for a laparotomy.
ABSCESS FORMATION
Well-recognized clinical features of abscess formation include spiking temperatures despite appropriate antibiotic coverage, persistent tachycardia, persistent leukocytosis, and a demonstrable mass. The mass may be difficult to feel on pelvic examination in the early stages of abscess formation, but well-established abscesses may be demonstrated by either pelvic examination or ultrasound, computed tomography (CT), or magnetic image (MI) scanning. Abscesses usually contain both aerobic and anaerobic bacteria, including one or more of the Bacteroides species, usually B. bivius, B. disiens, or B. fragilis.
The therapy for abscesses includes administration of antibiotics and the possibility of surgical drainage if abscesses are large (greater than 6 cm). Several methods can be chosen for surgical drainage, depending on the clinical situation and physician experience. Traditionally, abscesses have either been drained vaginally or drained or surgically removed at laparotomy. In the past, hysterectomy or bilateral salpingo-oophorectomy was also frequently performed. Hysterectomy usually is not indicated when the abscess is well established and infection of the uterine myometrium appears unlikely. In recent studies, over 90% of intra-abdominal abscesses have been successfully treated with percutaneous catheter drainage.25 Experience with obstetric and gynecologic infection indicates that similar rates of success also occur with percutaneous drainage of pelvic abscesses.26 In many cases, catheters can be placed at the time of minilaparotomy or under ultrasound guidance if the abscess is pointing close to the abdominal surface and bowel is not present between the abscess and the anterior abdominal wall. Open laparoscopy can also be used to avoid bowel and the large postpartum uterus and to place percutaneous catheters. The abscess is directly visualized through the laparoscope, bowel is dissected from the abscess surface, and the abscess is confirmed by the aspiration of pus, using a percutaneously placed 18-gauge spinal needle. A drainage catheter placed over a sharp trocar can be inserted through the skin into the abscess under direct vision. Pus is aspirated through the catheter, and sterile water is used to gently irrigate the abscess cavity. It is important that irrigation fluid not be forced into the abscess cavity, but rather irrigation fluid should be allowed to flow by gravity to prevent bacteria in the abscess wall from disseminating into the vascular system and causing septic shock. When the abscess drainage clears, 2 g of a broad-spectrum cephalosporin can be placed in the abscess through the catheter and the catheter can be clamped for 2 hours. The catheter is then attached to a closed drainage system bag and allowed to drain to gravity. The catheter is pulled when drainage has ceased, usually 24 to 48 hours later. A 90% success rate has been achieved in reports of a small number of patients.26 Percutaneous drainage procedures allow abscess drainage without prolonged hospitalization or postoperative recovery because of a laparotomy. Contraindications include the absence of a clear-cut source of the abscess and the possibility of an intestinal source. Close follow-up is required to ensure continued resolution of the abscess. Systemic antibiotics should be continued until the infection clinically responds.
If the abscess is not surgically treated, it is best to continue intravenous antibiotics until the abscess has been reduced to 50% or less of its original size. Patients with this degree of resolution who have no fever, leukocytosis, or large amount of pelvic tenderness may be placed on oral antibiotics for an additional 10 to 14 days. Oral doses of clindamycin, metronidazole, or Augmentin (amoxicillin and clavulanate potassium) should be used because they are active against anaerobic bacteria, develop appropriate tissue levels, and penetrate into abscesses. The patient should be closely followed to ensure total resolution.
ABDOMINAL WOUND INFECTIONS
Approximately 5% (range 2%–10%) of women undergoing cesarean section develop an abdominal wound infection.1,27 Factors that increase the rate of wound infection include amniotic fluid infection, a long duration of labor or rupture of membranes.27 urgency of operation, obesity, diabetes, preoperative shaving, and the use of wound drains and electrocautery.28,29 Some factors can be eliminated, including excessively high electrocautery amperage and the use of drains, particularly Penrose drains brought out through the abdominal excision. If required, drainage should be established with a closed catheter drain brought out of the wound through a separate skin incision. An additional measure preventing wound infection includes the use of delayed primary closure whenever patients manifest well-established infection as determined by either grossly purulent exudate or foul-smelling amniotic fluid at cesarean section. Wound infection rates in these conditions approach 25% with immediate primary closure.28 The peritoneum and fascia can be closed, but the subcutaneous tissue and wound should be left open. Delayed primary closure can be undertaken 2 to 4 days post partum.
Wound infection usually causes erythema and tenderness of the wound and a fever. Pus can usually be demonstrated with an 18-gauge needle aspiration or with opening of the wound. If pus is not present, streptococcal cellulitis or necrotizing fasciitis needs to be considered. Wound cultures should be obtained either of tissue removed from the deep portion of the wound or of a swab taken of pus deep in the wound. Gram stain of the specimen should be reviewed to identify streptococci or clostridia. Therapy includes the continued use of wide-spectrum antibiotics until the cellulitis clears, as indicated by resolution of adjacent erythema, edema, and tenderness. Wound infections should be opened and sharply debrided of all necrotic debris under anesthesia. A wet gauze is packed into the wound, and the wound is then gently irrigated three times daily. Vigorous brushing, scraping, or other trauma to the wound will only prevent further wound healing.30 It may be necessary to remove new or necrotic debris surgically with additional debridement. Following control of the wound infection, the wound can be allowed to close by secondary intent, although large, open clean wounds can be secondarily closed.
TOXIC SHOCK SYNDROME
Clinical features of toxic shock syndrome have been well described. They include hypotension (<90 mm Hg systolic or 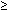 15 mm Hg orthostatic drop), fever (>38°C), diffuse macular rash, multi-system involvement, and desquamation of the skin, usually the palms and soles,1 to 2 weeks after the onset of infection.31 Toxin-producing Staphylococcus aureus and, rarely, Staphylococcus epidermidis cause this disease. Toxic shock syndrome has recently been recognized in post-partum patients.32 Typical clinical features of this syndrome in a postpartum patient should alert the clinician to the diagnosis. An immediate search needs to be instituted for the source of S. aureus toxin. The site of infection may be difficult to detect in the postpartum patient. Toxic shock syndrome from S. aureus can occur from wound, endometrial, or even breast infection. Although signs of typical wound infection may be present, toxic shock syndrome has also occurred from wounds with minimal erythema or even from initially normal-appearing wounds that develop manifestations of infection several days after the onset of toxic shock syndrome.33 Thus, diagnosis depends on finding typical clinical features of the syndrome in a patient with S. aureus infection. Therapy for toxic shock syndrome includes immediate removal of the source of the toxin. Wound infections should be debrided, and a search should be made for endometrial, breast, or other infections. Supportive therapy is required, and adequate volume replacement is crucial; the patient must be closely monitored for renal failure or adult respiratory distress syndrome. Nafcillin, 1 g every 4 hours, is recommended to prevent recurrent infection, but the value of antibiotics in the acute phase of the syndrome may be minimal.
15 mm Hg orthostatic drop), fever (>38°C), diffuse macular rash, multi-system involvement, and desquamation of the skin, usually the palms and soles,1 to 2 weeks after the onset of infection.31 Toxin-producing Staphylococcus aureus and, rarely, Staphylococcus epidermidis cause this disease. Toxic shock syndrome has recently been recognized in post-partum patients.32 Typical clinical features of this syndrome in a postpartum patient should alert the clinician to the diagnosis. An immediate search needs to be instituted for the source of S. aureus toxin. The site of infection may be difficult to detect in the postpartum patient. Toxic shock syndrome from S. aureus can occur from wound, endometrial, or even breast infection. Although signs of typical wound infection may be present, toxic shock syndrome has also occurred from wounds with minimal erythema or even from initially normal-appearing wounds that develop manifestations of infection several days after the onset of toxic shock syndrome.33 Thus, diagnosis depends on finding typical clinical features of the syndrome in a patient with S. aureus infection. Therapy for toxic shock syndrome includes immediate removal of the source of the toxin. Wound infections should be debrided, and a search should be made for endometrial, breast, or other infections. Supportive therapy is required, and adequate volume replacement is crucial; the patient must be closely monitored for renal failure or adult respiratory distress syndrome. Nafcillin, 1 g every 4 hours, is recommended to prevent recurrent infection, but the value of antibiotics in the acute phase of the syndrome may be minimal.
EPISIOTOMY INFECTION
Most episiotomy infections represent simple wound infections that can be treated in a manner similar to an abdominal wound infection. However, extensive cellulitis from toxin-producing organisms and necrotizing fasciitis can also occur in episiotomy wounds. Patients with serious systemic manifestations, septic shock, or signs of wound infection beyond the immediate episiotomy area must be scrutinized for these possibilities, particularly necrotizing fasciitis. Necrotizing fasciitis of the episiotomy wound has the same lethal consequences as necrotizing fasciitis in other areas of the body, and several deaths have been reported.21
Serious episiotomy infection may be difficult to appreciate. Virtually all episiotomies in primiparous women cause pain, often severe pain. Edema, bruising, and hematomas following a long second stage of labor are also frequent causes of discoloration of the area. Most episiotomy infections readily respond to simple drainage and antibiotic therapy. However, serious episiotomy infection must be considered when systemic toxicity or signs beyond the immediate episiotomy exist.21 Surgical therapy of clostridial myonecrosis and necrotizing fasciitis is the same as when these infections are present in other areas.
NEW ASCENDING UTERINE INFECTION
In contrast to early postpartum uterine infections, which are usually related to cesarean section, late-occurring uterine infections usually develop in patients who delivered vaginally.5 The onset of infection most often occurs 2 weeks (range of 3 days-6 weeks) post partum. It is most likely that the pathogenesis of late uterine infection involves the ascent of bacteria along mucosal surfaces analogous to that occurring in nonpregnant patients with pelvic inflammatory disease. Patients usually have mild clinical symptoms with minimal pain. They are usually afebrile, and they often experience minimal abdominal and uterine tenderness.
Chlamydia trachomatis has been isolated from the cervix and endometrium in half of the patients.34 The remaining patients have either aerobic or anaerobic bacteria or genital mycoplasmas. It is necessary to obtain cervical cultures for C. trachomatis and other cultures as indicated. Therapy should be initiated with antibiotics that inhibit C. trachomatis (erythromycin or, for non-breast-feeding women, tetracyclines). Other broad-spectrum antibiotics may need to be given in conjunction with these antibiotics, particularly if patients are seriously ill or require hospital admission. Despite minimal abdominal pain and uterine tenderness, the diagnosis is important to establish because of possible long-term sequelae. A recent association has been made between chlamydiae and infertility.35 About half of the patients who are infertile because of tubal obstruction have never had a recognized episode of pelvic inflammatory disease.35 From one third to two thirds of patients who are infertile because of obstructed fallopian tubes have been previously pregnant.35 Generally, 5% to 10% of pregnant women carry C. trachomatis in the cervix. Although the late postpartum infection rates following a normal vaginal delivery are unknown, salpingitis occurs in up to 20% of women with C. trachomatis following induced abortion.36 If similar infection rates were to occur post partum in women with C. trachomatis, it is possible that a considerable amount of infertility results from these late ascending postpartum endometritis-salpingitis infections. Therefore, despite the often mild clinical features, it seems important that these infections be recognized and treated.
SEPTIC PELVIC THROMBOPHLEBITIS (SPT)
Patients with SPT usually have spiking fever of obscure origin, together with chills and a pulse rate out of proportion to the degree of fever. Chest symptoms may result if pulmonary emboli occur. The combination of large pelvic veins, increased blood coagulability, and vessel injury (from infection or surgery) in the postpartum patient makes thrombosis a threat.37 A significantly increased rate of SPT occurs among women delivered by cesarean sections compared with those who deliver vaginally. Septic pelvic thrombophlebitis occurs in 1% to 2% of postpartum pelvic infections.13,24 Two clinically distinct syndromes of SPT exist. The most common syndrome is ovarian vein thrombosis. These patients have acute onset of abdominal pain and fever, usually 2 to 3 days post partum. They appear ill and usually have a palpable right-sided lower abdominal mass38 A second septic thrombosis syndrome develops later and is associated with an obscure, high spiking fever, minimal or no abdominal pain or tenderness, and usually no abdominal mass.39 It should be noted that most patients who develop persistent fever on antibiotic therapy do not have SPT but more commonly have persistent endometritis or less frequently another source of infection.
Organisms that have been related to SPT include facultative streptococci, S. aureus, Escherichia coli, anaerobic streptococci, and Bacteroides sp. The diagnosis can be made in patients with typical clinical features who have no other apparent source of fever. CT scan and an intravenous pyelogram may help demonstrate ovarian vein thrombosis. The diagnosis can be confirmed by the prompt resolution, usually within 24 hours, of fever and the signs and symptoms following the institution of heparin therapy to maintain clotting times or activated partial thromboplastin times two to three times longer than normal.40 Broad-spectrum antibiotics should be continued to cover common aerobic and anaerobic bacteria. Heparin is given for 7 to 10 days in usual cases. Prolonged heparin therapy is required only if pulmonary embolization occurs, in which case coumarin is given for 3 to 6 months. Surgery should be avoided if the diagnosis can be established prior to surgical exploration.37,41 Occasionally, surgical exploration will be needed to exclude adnexal torsion, degenerated leiomyomata, pelvic abscess, or appendicitis, and surgical removal or ligation may occasionally become necessary for patients who continue showering pulmonary emboli despite full heparinization.
PUDENDAL AND PARACERVICAL BLOCK INFECTIONS
Unusual pudendal and paracervical block infections are caused when the needle used for the nerve block carries bacteria from the vaginal surface into the retroperitoneal space. The large retroperitoneal space is limited by the pelvic diaphragm inferiorly, but infection can spread unchecked superiorly along the psoas muscle or laterally along the trochanter muscle into the hip. Infection is often advanced before recognition occurs. A variety of aerobic and anaerobic bacteria usually cause infection.
A clinical diagnosis can be made in patients who have had either a pudendal or a paracervical block. Hip tenderness is the characteristic and prominent feature of this infection. Patients are not able to place extensive weight on the leg of the affected hip.42 Physical examination reveals tenderness on motion of the hip and tenderness of the hip capsule and paravaginal and psoas muscles. Occasionally CT scan or MI will reveal an abscess in this space.
In recent experience, early institution of broad-spectrum antibiotic therapy that includes anaerobic coverage usually prevents serious abscess formation. However, early reports of pudendal and paracervical block infections included patients who had developed large abscesses.42 Surgical drainage is necessary if large abscesses occur.43 Retroperitoneal infection is often advanced because an extensive volume (several liters) of pus can collect in this large space. These infections are potentially serious because large abscesses have ruptured and caused paraplegia or resulted in extended hospitalization.
BREAST INFECTION
Approximately 2% of breast-feeding mothers develop breast infection. Staphylococcal colonization of the infant's oral cavity begins a few days after birth.44 Organisms can be introduced into the breast through fissures or by negative pressure during suckling. Mild milk stasis contributes to infection. Two forms of infection exist. The first and most common type is endemic mastitis typified by cellulitis in which fissures are frequent and fever with flulike symptoms occur.45 Some patients will develop fever or flulike symptoms even prior to breast tenderness. A second, less frequent, epidemic mastitis represents a hospital-acquired infection in which a primary ductal infection exists and pus may be present at the nipple but fissures are uncommon. Staphylococcus aureus and, less commonly, S. epidermidis are the usual organisms recovered in these infections. Diagnosis is based on the presence of redness and tenderness in an area of the breast or a fluctuant breast mass, usually together with fever. Therapy should include the administration of a broad-spectrum penicillinase-resistant antibiotic. Cloxacillin, dicloxacillin, or cephalexin, 500 mg given orally every 6 hours, is effective. Local measures should also be instituted to ensure milk drainage. Continued breast-feeding is helpful to prevent further breast milk stasis. The patient should be encouraged to begin breast-feeding on the infected breast first to promote adequate drainage. This may be difficult when fissures or excessive tenderness exists. An exception to continued breast-feeding should be made if a breast abscess is present, because neonatal lung abscesses have been associated with the presence of maternal breast abscesses .46 Although it is possible that an infant with lung abscesses infects the mother to cause breast infection, the alternative possibility is that a large concentration of bacteria in breast abscesses could be aspirated into the infant's lung, causing pulmonary abscesses. A breast pump should be used to ensure drainage when a breast abscess is present. Surgical drainage of breast abscesses is often necessary.47 Except for the rare development of toxic shock syndrome, patients with breast infection usually do not become seriously ill.
PSEUDOMEMBRANOUS COLITIS
Pseudomembranous colitis occurs in patients who received or are still receiving antibiotics. This potentially lethal infection is caused by an overgrowth of Clostridium difficile in the gastrointestinal tract related to the inhibition of normal intestinal flora by the antibiotic administered.48Clostridium difficile overgrowth produces sufficient toxin levels to cause intestinal mucosal sloughing and clinical manifestation. The severity of diarrhea is highly variable, ranging from mild diarrhea to severe megacolon. The possibility of pseudomembranous colitis needs to be considered when the patient on antibiotics develops a persistent fever together with other manifestations of this infection. The infection is associated with odorous, watery diarrhea, sometimes mixed with blood, and with leukocytes in the feces. High fever, leukocytosis, abdominal distention, and severe abdominal tenderness are common and herald severe illness in contrast to benign anti-biotic-associated diarrhea.49 Manifestations most frequently develop 4 to 9 days after starting antibiotics, but shorter and longer periods are not uncommon.
Only a small portion of patients with antibiotic-associated diarrhea have pseudomembranous colitis. The more benign antibiotic-associated diarrhea without pseudomembranous colitis does not produce fever, bloody diarrhea, or abdominal signs. Although pseudomembranous colitis had initially been noted most commonly with the administration of clindamycin, virtually all antibiotics (especially ampicillin, cephalosporins, and tetracyclines) have since been associated with this infection. Pseudomembranous colitis has even been reported following a short course of cephalosporin antibiotic prophylaxis. Diagnosis can be made by finding mucosal ulcers and pseudomembranous plaques at sigmoidoscopy and demonstrating C. difficile toxin in diarrhea fluid. However, proximal cecitis may not be demonstrated at sigmoidoscopy. An increased number of leukocytes can be found in the stool in over 50% of cases. Although C. difficile can be recovered from these patients, it can also be recovered from approximately 5% of otherwise normal patients, and isolation of this organism without finding toxin is not sufficiently specific to diagnose pseudomembranous colitis. Other organisms associated with diarrhea (Salmonella, Shigella, Entamoeba histolytica, Campylobacter, and Yersinia) should also be sought with severe illness. Toxic megacolon, perforation, secondary sepsis, and severe hemorrhage are associated with high mortality. Treatment includes discontinuation of the antibiotic therapy, if possible, and fluid support. Medications that slow peristalsis and hence toxin elimination, such as diphenoxylate hydrochloride with atropine sulfate (Lomotil), should not be used. Oral vancomycin, 125 mg every 6 hours, or oral metronidazole can be used in patients with serious manifestations. These antibiotics inhibit C. difficile, and their administration decreases recurrent disease.50
REFERENCES
Swartz WH, Grolle K: Use of prophylactic antibiotics in cesarean section, a review of the literature. J Reprod Med 26: 595, 1981 |
|
Eschenbach DA, Wager GP: Puerperal infection. Clin Obstet Gynecol 23: 1003, 1980 |
|
Sachs BP, Brown DAJ, Driscoll SG et al: Maternal mortality in Massachusetts. N Engl J Med 316: 667, 1987 |
|
Hill GB, Eschenbach DA. Holmes KK: Bacteriology of the vagina. Scand J Urol Nephrol (Suppl)86:23, 1984 |
|
Wager GP, Martin DH, Koutsky L et al: Puerperal infectious morbidity: Relationship to route of delivery and to antepartum Chlamydia trachomatis infection. Am J Obstet Gynecol 138: 1028, 1980 |
|
Rosene K, Eschenbach DA, Tompkins L et al: Polymicrobial early postpartum endometritis with facultatively anaerobic and anaerobic bacteria, genital mycoplasmas and Chlamydia trachomatis. J Infect Dis 153: 1028, 1986 |
|
Filker R, Monif GRG: The significance of temperature during the first 24 hours postpartum. Obstet Gynecol 53: 358, 1978 |
|
Blanco JD, Gibbs RS, Castaneda YS et al: Correlation of quantitive fluid cultures with endometritis after cesarean section. Am J Obstet Gynecol 143: 897, 1982 |
|
Rehu M, Nilsson CG: Risk factors for febrile morbidity associated with cesarean section. Obstet Gynecol 56: 269, 1980 |
|
Gilstrap LC, Cunningham FG: The bacterial pathogenesis of infection following cesarean section. Obstet Gynecol 53: 545, 1979 |
|
Duff WP, Gibbs RS, Blanco JD et al: Endometrial culture techniques in puerperal patients. Obstet Gynecol 61: 217, 1983 |
|
Gibbs RS, Weinstein AJ: Puerperal infection in the antibiotic era. Am J Obstet Gynecol 124:769. 1976 |
|
Gibbs RS, Jones PM, Wilder CJ: Antibiotic therapy of endometritis following cesarean section: Treatment successes and failures. Obstet Gynecol 52: 31, 1978 |
|
Phillipson A: Pharmacokinetics of ampicillin during pregnancy. J Infect Dis 136: 370, 1977 |
|
Cunningham FG, Lucas M J, Hankins GDU: Pulmonary injury complicating antepartum pyelonephritis. Am J Obstet Gynecol 156: 797, 1987 |
|
Blanchard AC, Pastorek JG, Weeks T: Pelvic inflammatory disease during pregnancy. South Med J 80:1363, 1987 17. |
|
Freid MA, Vosti KL: The importance of underlying disease in patients with gram-negative bacteremia. Arch Intern Med 121: 418, 1968 |
|
Stamenkovic I, Lew PD: Early recognition of potentially fatal necrotizing fasciitis: The use of frozen-section biopsy. N Engl J Med 310:1689. 1984 |
|
Golde S, Ledger WJ: Necrotizing fasciitis in postpartum patients. Obstet Gynecol 50: 670, 1977 |
|
Stone HH, Martin JD: Synergistic necrotizing cellulitis. Am Surg 175: 702, 1972 |
|
Shy KK, Eschenbach DA: Fatal perineal cellulitis from an episiotomy site. Obstet Gynecol 54: 292, 1979 |
|
Felts SK, Schaffner W, Melly MA et al: Sepsis caused by contaminated intravenous fluids. Ann Intern Med 77:881. 1972 |
|
Maki DG, Weise CE, Sarafan HW: A semiquantitative culture method for identifying intravenous-catheterrelated infection. N Engl J Med 296:1305. 1977 |
|
Dizerega G, Yonekura L, Roy S et al: A comparison of clindamycin-gentamycin and penicillin-gentamycin in the treatment of post-cesarean section endomyometritis. Am J Obstet Gynecol 134: 238, 1979 |
|
Gerzof SG, Robbins AH, Johnson WC et al: Percutaneous catheter drainage of abdominal abscesses. N Engl J Med 305: 653, 1981 |
|
Reich H, McGlynn F: Laparoscopic treatment of tuboovarian and pelvic abscess. J Reprod Med 32:747. 1987 |
|
Gibbs RS, Blanco JD, St Clair PJ: A case-control study of wound abscess after cesarean delivery. Obstet Gynecol 62: 498, 1983 |
|
Cruse PJE, Foord R: A five-year prospective study of 23.649 surgical wounds. Arch Surg 107:206. 1973 |
|
Dineen P: A critical study of 100 consecutive wound infections. Surg Gynecol Obstet 113:91. 1961 |
|
Mead PB: Managing infected abdominal wounds. Contemp Obstet Gynecol 14: 69, 1979 |
|
Toxic-shock syndrome--United States. MMWR 29:229. 1980 |
|
Bracero L, Bowe E: Postpartum toxic-shock syndrome. Am J Obstet Gynecol 143:478. 1982 |
|
Bartlett P, Reingold AL, Graham DR et al: Toxic shock syndrome associated with surgical wound infections. JAMA 247:1448. 1982 |
|
Hoyme UB, Kiviat N, Eschenbach DA: Microbiology and treatment of late postpartum endometritis. Obstet Gynecol 68:226. 1986 |
|
Moore DE, Spadoni LR, Foy HM et al: Increased frequency of serious antibodies to Chhamydia trachomatimin women with distal tubal disease. Lancet 2: 574, 1982 |
|
Moller BR, Ahrons S, Lauren J et al: Pelvic infection after elective abortion associated with Chlamydia trachomatis. Obstet Gynecol 59: 210, 1982 |
|
Brown TK, Munsick RA: Puerperal ovarian vein thrombophlebitis: A syndrome. Am J Ohstet Gyneco1 109: 263, 1971 |
|
Derrick FC, Turner WR, House EE et al: Evidence of right ovarian vein syndrome in pregnant females. Obstet Gynecol 35: 37, 1970 |
|
Dunn LJ, Van Voorhis LW: Enigmatic fever and pelvic thrombophlebitis. N Engl J Med 276: 262, 1 1967 |
|
Duff P, Gibbs RS: Pelvic vein thrombophlebitis: Diagnostic dilemma and therapeutic challenge. Obstet Gynecol Surv 38: 365, 1983 |
|
Josey WE, Cook CC: Septic pelvic thrombophlebitis. Obstet Gynecol 35: 891, 1970 |
|
Hibbard LT, Snyder EN, McVann RM: Subgluteal and retropsoal infection in obstetrical practice. Obstet Gynecol 39: 137, 1972 |
|
Wegner DR, Gitcheil RG: Severe infections following pudendal block anesthesia: Need for orthopedic awareness. J Bone Joint Surg (Am) 55: 202, 1973 |
|
Duncan JT, Walker J: Staphylococcus aureus in the milk of nursing mothers and the alimentary canal of their infants. J Hyg 42: 474, 1942 |
|
Niebyl JR, Spence MR, Parmley TH: Sporadic (nonepidemic) puerperal mastitis. J Reprod Med 20: 97, 1978 |
|
Ravenholt RT, Wright P, Mulhern M: Epidemiology and prevention of nursery-derived staphylococcal disease. N Engl J Med 257: 789, 1957 |
|
Sherman AJ, Porter HC, Eisenberg GM: Puerperal breast abscess: II. Epidemiologic factors in hospital-acquired infection. Obstet Gynecol 8: 81, 1956 |
|
Bartlett JG, Chang TW, Gurwith M et al: Antibiotic-associated pseudomembranous colitis due to toxin-producing clostridia. 298: 531, 1978 |
|
Yonekura ML, DiZerega GS: Antibiotic-associated colitis. Obstet Gynecol Surv 35 (suppl): 743, 1980 |
|
George WL, Volpicelli NA, Stiner DB et al: Relapse of pseudomembranous colitis after vancomycin therapy. N Engl J Med 301: 414, 1979 |

