Renal Disease and Pregnancy
Authors
INTRODUCTION
Marked improvement in maternal and perinatal outcome for pregnancies complicated by renal disease has occurred during the past 40 to 50 years. An understanding of the disease processes and improvements in obstetric care, with more successful and earlier intervention, have led to the improved outcomes. Renal disorders in pregnancy can range from asymptomatic bacteriuria to end-stage renal disease requiring dialysis, all being influenced by the physiologic changes of pregnancy. Women who have mild to moderate renal disease or a renal transplant are now challenging obstetricians and nephrologists with pregnancy. Thus, these physicians must understand renal diseases and their effect on pregnancy, and vice versa. This chapter reviews the physiology of renal changes during pregnancy and provides a summary of renal disorders.
RENAL CHANGES IN PREGNANCY
Anatomic changes involving the urinary tract begin in the first trimester of pregnancy and can persist up to 16 weeks postpartum. These changes include dilatation of the renal calyces, pelves, and ureters, as well as reduced ureteral peristaltic activity. Their precise etiology is unknown but can be attributed to a combination of mechanical and hormonal factors.1 Dilatation of the ureter is usually more prominent on the right secondary to dextrorotation of the gravid uterus. In addition, there is reduced ureteral peristalsis and a greater volume of residual urine compared with the nonpregnant state. These factors predispose to urinary stasis and to an increased risk of infection.
Much of the data relating to the effect of pregnancy on renal hemodynamics are derived from small studies in which measurements show considerable individual variation. Autoregulation maintains renal blood flow at a relatively constant level despite wide variations in perfusion pressure (mean renal artery pressure). Renal blood flow is usually assessed by p-aminohippurate clearance, which measures effective renal plasma flow (ERPF). The ERPF significantly increases during pregnancy. It reaches a peak increment in midtrimester of 50% to 85% and then shows a small decline during the third trimester that is unrelated to posture. The ERPF and glomerular filtration rate (GFR) in pregnancy are markedly affected by posture, being maximal when the pregnant woman lies on her side.2 Normal pregnancy is associated with plasma volume expansion and an increase in the GFR of 40% to 65% (measured by inulin clearance) and a decrease in GFR of approximately 15% to 20% late in the third trimester.1,3 The mechanisms responsible for the increase in GFR, plasma volume, and renal plasma flow rate are unknown. Nitric oxide, endothelin, and relaxin may play a role in renal vasodilation in human pregnancy.1 Changes in renal anatomy, hemodynamics, and tubular function are listed in Table 1.
Table 1. Physiologic Changes in Pregnancy
Renal
Increased renal size and volume
Increased glomerular size?
Dilation of collecting system
Altered glomerular membrane porosity
Ureteral smooth muscle hypertrophy
Ureteral connective tissue hyperplasia
Renal Hemodynamics
40–65% increase in GFR by second trimester
50–85% increase in renal plasma flow
Decreased renal vascular resistance
Initial decrease in filtration fraction
Increased GFR
Hyperfilteration of glucose and other sugars, amino acid, proteins and water-soluble vitamins
Increased urate and creatinine clearance
Resistance to kaliuresis
Positive sodium balance
Cardiovascular
Increased cardiac output as early as week 5 of gestation
Decreased systemic vascular resistance
Decreased blood pressure until midpregnancy; rises thereafter
Volume Hemostasis
Decreased plasma osmolality by 10 mOsm/kg (nadir at 10 weeks' gestation)
Regulation plasma osmolality within a narrow range
Increased total body water of at least 6.5 L at term
Increased plasma volume of 40–50%
Decreased plasma albumin
Acid-Base balance
Mild respiratory alkalosis
Decreased serum hydrogen ion
Increased blood pH 7.42–7.44
Decreased PCO2 (18–22 mEq/L)
Increased bicarbonate reabsorption
Hormonal
Decreased osmotic thresholds for vasopressin secretion and thirst
Increased metabolic clearance rate
Increased circulating vasopressinase
Increased circulating levels of antinatriuretic hormones, especially mineralocorticoids, aldosterone, and desoxycorticosterone
Increased plasma rennin substrate and PRA activity
Increased serum atrial natriuretic peptide levels
Increase renal kallikren excretion
GFR, glomerular filtration rate.
TESTS OF RENAL FUNCTION AND DISEASE IN PREGNANCY
Tests of renal function in pregnancy must be interpreted in relation to the changes in plasma volume, glomerular filtration, and tubular reabsorption that normally occur with advancing gestation. Many of the commonly used tests of function yield lower results in pregnancy than in the nonpregnant state. Consequently, values that may be regarded as normal in the nonpregnant state may well indicate renal dysfunction in pregnancy.
Uric acid, blood urea nitrogen (BUN), and serum creatinine levels are crude indices of renal function. In pregnancy, plasma uric acid usually decreases by 25% beginning in the first trimester and increases during the third trimester. Upper normal limits of plasma uric acid levels are 5 to 5.5 mg/dL in pregnancy.4 Levels are influenced by race, multiple gestation, and time of day sampled, with higher levels in the morning. An indicator of renal filtration, the BUN normally decreases from nonpregnant levels of 12 mg/dL (4.3 mmol/L) to 9 mg/dL (3.2 mmol/L), and plasma creatinine levels decline from a nonpregnant mean value of 0.7 mg/dL (62 mmol/L) to 0.5 mg/dL (44 mmol/L).1 If the plasma creatinine level exceeds 0.80 mg/dL (80 mmol/L) or the BUN is greater than 14 mg/dL at any stage in pregnancy, renal dysfunction should be suspected and more detailed investigation should be performed.
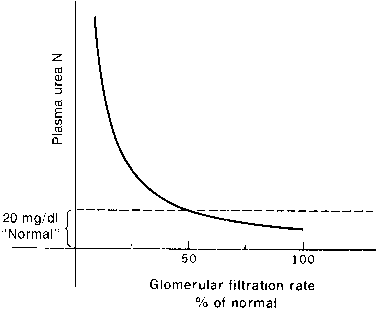
The kidney and liver affect serum urea levels. The liver synthesizes urea, which is influenced by protein intake, metabolism, and hepatic function. Urea reabsorption is by the kidneys and varies with hydration. Therefore, it is possible for renal function to be normal in the presence of an abnormal BUN or abnormal in the presence of a normal BUN. In addition, renal function must decline by at least 50% before either the BUN or the creatinine level becomes abnormal, where clinical signs of renal insufficiency become apparent (Figs. 1 and 2).
|
The 24-hour creatinine clearance is the best clinical measurement of GFR. By week 8 of pregnancy, the creatinine clearance rate normally increases by 45% and remains elevated during the second trimester. In the final weeks of pregnancy, creatinine clearance usually declines to near nonpregnant levels.
Error in the measurement of creatinine clearance in a pregnant woman can occur; the most common type of error is an incomplete 24-hour urine collection. Accurate timed urine collection is particularly difficult in pregnancy because significant volumes of urine may remain in the dilated collecting system. To avoid this error, patients should be well hydrated and should rest on their left side for 1 hour before starting and completing the 24-hour urine collection.1 In addition, the secretory component of creatinine excretion increases in moderate renal failure, and creatinine clearance rates tend to overestimate GFR. Despite these problems, creatinine clearance still remains the most useful measure of GFR in clinical practice.
Urinalysis is essentially unchanged during pregnancy. However, many variables can affect the results. Normal kidneys should be able to concentrate urine to a specific gravity of 1.026 or more and to dilute urine to a value less than 1.005. In pregnancy, posture affects urine concentration and specific gravity. Urine tends to be more dilute after a left lateral position is maintained compared with an upright position.2 The urine must be at room temperature for the dipsticks to be reliable. Dipsticks exposed to air will give false-positive results for glucose and false-negative results for blood.5 Observational error and training also affect the sensitivity of predicting proteinuria by dipstick.6 Saudan and colleagues7 showed that the use of an automated urinalysis device for the detection of proteinuria reduced the false-positive rate. Proteinuria diagnosed on dipstick should be confirmed with a 24-hour urine.
The method of collection is very important when collecting a urine specimen. It is difficult for the woman to obtain a satisfactory clean voided specimen by herself, especially when she is far along in pregnancy. In addition, the specimen must be collected before the pelvic examination; it may be collected by the examiner while the patient is in the dorsal lithotomy position.
The diagnosis of renal disease in pregnancy should begin by taking a careful history and performing a thorough physical examination. Particular attention should be directed toward any history of renal disorders, proteinuria, hypertension, collagen vascular diseases, or glycosuria affecting either the patient or close relatives. Physical examination includes inspection of the optic fundi. Signs of uremia are usually a late feature in the natural progression of renal disease and indicate significant dysfunction.
Laboratory investigation begins with urinalysis of protein, glucose, ketones, specific gravity, and sediment. Glucosuria is usually detected with a glucose oxidase-impregnated dipstick. In pregnancy, the most common reasons for persistent glucose in the urine are physiologic glucosuria of pregnancy and diabetes. However, the possibility of primary renal disease with renal glucosuria should be considered. Conventional screening for proteinuria uses a dipstick that is sensitive to albumin. Five percent of healthy adults exhibit postural proteinuria, a benign condition; this can be ruled out by comparing protein levels in the first voided urine with a specimen obtained after the woman was upright for several hours. False-positive results for protein can be due to concentrated urine, many white blood cells in the urine, or vaginal secretions with epithelial cells. Fever, stress, and exercise can also cause transient proteinuria.
Proteinuria in pregnancy should be evaluated using a 24-hour urine collection and should not be considered pathologic until it exceeds 300 to 500 mg in 24 hours.1 Higby and coworkers8 showed that the upper limit of normal was 260 mg for urinary protein and 29 mg for albumin in a 24-hour period. Both increased after 20 weeks' gestation. Studies have shown that a 2-hour or 12-hour collection of urine correlates with creatinine clearance and protein measured in a 24-hour specimen.9,10 Evans and associates9 calculated total protein using a protein/creatinine ratio in the 2-hour group and compared the results with the 24-hour urine (1840.8 ± 786 and 1944 ± 1060 mg [mean ± SE], respectively, r2 = 0.95, p < 0.0001). The nephritic syndrome is characterized by greater than 3 to 3.5 g/24 hours. In assessing the significance of proteinuria in pregnancy, the clinician should remember that increasing protein excretion with advancing gestation associated with known renal disease does not necessarily indicate significant progression of the disease.
Examination of urinary sediment requires a fresh specimen, ideally the first voided morning specimen. The presence of cells within a cast indicates that the cells came from the renal parenchyma. As a general guide, leukocyte casts are associated with infections or inflammatory processes, erythrocyte casts with glomerular disease, and fatty casts with nephrosis. In addition, pyuria is a common finding in renal disease. White blood cells, casts, and pyuria may be present as reaction inflammation of the glomeruli or kidney substance. Pyuria in the absence of casts may be due to infection anywhere in the genitourinary tract. Finally, the nephritic syndrome is characterized by the presence of doubly refractile fat bodies (Maltese crosses), which are visible only under polarized light.
The role of renal biopsy in pregnancy is controversial. It is potentially dangerous, especially in the presence of hypertension. Further, it has been argued that the histologic changes of glomerular disease may be obscured by the changes from pregnancy itself. In the largest study of pregnant women, Packham and Fairley11 reported on 111 renal biopsies and concluded that renal biopsy in the first two trimesters of pregnancy did not appear to be associated with an increased rate of complications, and in most women it provides a positive diagnosis for undiagnosed hematuria and proteinuria. However, Kullner and coworkers12 reported 4 hematomas in 15 antepartum biopsies and 3 hematomas in 3 postpartum biopsies; 2 of these patients required transfusion. The occurrence of complications is similar to the major complication rate of 2.4% reported by Gonzales and associates13 in 1005 biopsies in the general population. Perirenal hematomas, perirenal abscess, and sepsis were considered major complications Lindheimer and associates14 reviewed the role of renal biopsy in pregnancy and believed that biopsy should be performed only if it would change management and should be postponed until after delivery.
Postpartum renal biopsy may be useful in determining the prognosis for patients with hypertension in pregnancy. In a study of 20 postpartum renal biopsies, Gaber and Spargo15 could distinguish between patients with glomerular endotheliosis, a reversible lesion with no long-term sequelae, and focal glomerulosclerosis, which is not reversible and indicates underlying hypertensive disease with nephrosclerosis.
It is possible to categorize almost all renal problems in pregnancy using the information gained from a detailed history, urinalysis, BUN, creatinine level, and creatinine clearance. In certain patients, renal ultrasound may provide useful additional information, particularly if renal calculi or a tumor is suspected, but may be limited secondary to the pregnancy. Renal radiographs are rarely indicated in pregnancy and pose a radiation hazard to the fetus. If they are necessary, limiting the number of films will minimize the risk to the fetus. Computed tomography scans may aid in the diagnosis of nephritic abscesses, and magnetic resonance imaging may be helpful to rule out tumor. Radiation exposure to the fetus should not exceed 5 rads. Cystoscopy can be performed for the usual indications.
ACUTE RENAL INSUFFICIENCY
With the legalization of abortions and the use of antibiotics, the incidence of acute renal failure (ARF) in pregnancy has markedly decreased in developed countries secondary to a decrease in the incidence of septic abortions. A further decrease is due to improvements in prenatal care, quick recognition of the condition, and initiation of treatment of abruptio placentae and preeclampsia. In industrialized countries, the rate of ARF is less than 0.004%.16 Preeclampsia, hemorrhage, and abortions account for 95% of the obstetric causes of ARF.17 Grunfeld and Pertuiset16 reviewed 57 patients presenting over a 22-year period. The most common precipitating factor was hemorrhage, which included 13 cases of abruptio placentae. Severe preeclampsia or eclampsia accounted for 12 cases, and infection was the cause in 8 patients. The overall maternal mortality rate was 14%, and the incidence of bilateral renal cortical necrosis was 33%.
Defined as a sudden decrease in renal function that results in retention of nitrogenous wastes, ARF can be classified as prerenal, intrinsic, or postrenal. The causes of ARF are listed in Table 2. Prerenal conditions are the most common causes of ARF and usually result from inadequate perfusion of the kidneys. Obstetric complications of ARF include hemorrhage from placenta previa, abruptio placentae, and uterine atony (Table 3). ARF from renal hypoperfusion is usually reversible within 24 to 36 hours with volume replacement and correction of the underlying cause.18 Without prompt treatment, acute tubular necrosis (ATN) may develop. Most of the deaths from ARF in pregnancy are due to the underlying disease rather than the renal failure itself.18
Table 2. Causes of Acute Renal Failure (ARF)
- Prerenal or azotemia results when there is a decrease in renal perfusion, which cannot maintain an adequate GFR.
- Hypovolemia and dehydration
- Vomiting of diarrhea
- Diuretic use
- Third spacing of fluids from burns, pancreatitis, and bowel obstruction
- Vomiting of diarrhea
- Reduced perfusion
- States of low cardiac output
- Obstruction of the renal vessels
- Renal artery or vein thrombosis
- Embolism, stenosis or dissection of the artery
- Renal artery or vein thrombosis
- States of low cardiac output
- Abnormal regulation
- Nephrotic syndrome
- Systemic inflammatory disease
- Sepsis
- Cirrhosis
- Drugs
- Aggressive antihypertensive theraphy, especially with afterload reduction
- Angiotensin-converting enzyme inhibitor use with bilateral renal artery stenosis
- Use of prostaglandin synthesis inhibitors with decreased renal profusion
- Aggressive antihypertensive theraphy, especially with afterload reduction
- Nephrotic syndrome
- Hypovolemia and dehydration
- Intrinsic or parenchymal renal
- Acute tubular necrosis: temporary loss of kidney function
- Diseases involving large renal vessels as listed above
- Diseases involving small vessels and glomeruli
- Glomerulonephritis/vasculitis
- Hemolytic uremic syndrome/thrombotic thrombocytopenic purpura
- Malignant hypertension
- Diseases involving small vessels and glomeruli
- ARF mediated by ischemia or toxins (acute tubular necrosis)
- Ischemia from recent hemorrhage, hypotension, cardiac arrest or septic shock
- Exogenous toxins (IV contrast)
- Endogenous toxins: rhabdomyolysis, hemolysis from blood transfusion
- Ischemia from recent hemorrhage, hypotension, cardiac arrest or septic shock
- Acute disease of tubulointerstitium
- Allergic interstitial nephritis (drug)
- Acute bilateral pyelonephriti
- Allergic interstitial nephritis (drug)
- Acute tubular necrosis: temporary loss of kidney function
- Postrenal ARF results when both ureters are obstructed (in a patient with two kidneys).
- Lithiasis
- Urethral stricture or tumor
- Malignancy
- Trauma
- Papillary necrosis in diabetes or sickle cell disease
- Lithiasis
GFR, glomerular filteration rate.
Table 3. Cause of Acute Renal Failure Unique to Pregnancy
Preeclampsia-eclampsia
HELLP syndrome
Severe peripartum hemorrhage
Abruptio placentae
Disseminated intravascular coagulation secondary to a prolonged fetal demise
Uterine atony
Uterine lacerations and perforations
Uterine dehiscence of cesarean scar
Septic shock
Chorioamnionitis
Septic abortion
Puerperal sepsis
Volume depletion
Hyperemesis gravidarum
Severe vomiting from pyelonephritis
Obstruction of gravid uterus
Hydramnios
Multiple gestations
Idiopathic postpartum renal failure
Amniotic fluid embolism
Acute fatty liver of pregnancy
HELLP, hemolysis, elevated liver enzymes, and low platelets.
ACUTE TUBULAR NECROSIS
Vascular hyperactivity with preferential cortical ischemia, diminished glomerular permeability, intraluminal obstruction, back leak of luminal contents across damaged tubular epithelium, and abnormalities in prostaglandin metabolism all have been demonstrated in ATN.19 However, the mechanism precipitating renal shutdown is unknown. In ATN, the BUN and creatinine levels rise steadily for 2 to 10 days and then plateau. There follows a recovery phase characterized by a marked diuresis; in most patients, normal renal function resumes. Only about 50% of patients with ATN become oliguric, and in these patients proteinuria is rarely more than 1 g per day. The death rate ranges from 20% to 60%; sepsis and hemorrhage are the major causes of death. In addition, ATN can occur for the same reasons in the gravida as it occurs in the nonpregnant patient.
The management of ATN has been well described by Brenner and Lazarus.20 The first step is to identify and treat the underlying disease process, with the aim of preventing progression to parenchymal renal disease. It is important to distinguish between extrarenal azotemia and acute parenchymal renal failure. The former may result from congestive heart failure, hypovolemia, infection, trauma, hemorrhage, and urinary tract obstruction. Analysis of serum and urine samples may prove helpful if the diagnosis is not clear. The urine sodium concentration exceeds 25 mEq/L and urine sediment contains brownish pigmented casts and an increased number of tubular cells. In ARF, volume correction should always precede the use of diuretics, with the exception of mannitol, which is a volume expander and an osmotic diuretic. Mannitol may reduce swelling of the endothelial cells and thus improve renal blood flow. If the patient is volume expanded and not hypotensive, then mannitol is contraindicated and furosemide is the diuretic of choice.
During the oliguric phase, fluids should be restricted to avoid hypertension and pulmonary edema. Life-threatening hyponatremia, hyperkalemia, and acidosis may develop rapidly; thus, electrolyte and acid-base status must be carefully monitored. Acute hyperkalemia (potassium level more than 6 mEq/L) may be treated with sodium bicarbonate, insulin and glucose, ion exchange resins, or calcium gluconate. Dialysis is often the treatment of choice. During the diuretic phase of ATN, patients are at risk for electrolyte imbalance and hypovolemia.
ARF may be complicated by neurologic signs such as progressive lethargy, hyperreflexia, clonus, and a positive Babinski's sign. These signs disappear as renal function improves. Bacterial infection is a major risk and must be aggressively treated. Adequate nutritional status is important to combat infection, replace lost protein, and facilitate recovery of renal function. Standard recommendations include restriction of protein, sodium, and potassium; however, dietary requirements vary from patient to patient and according to the level of renal function. One of the benefits of dialysis is that it allows a more flexible diet.
ACUTE CORTICAL NECROSIS
Excluding death, the most serious complication of ARF is bilateral renal cortical necrosis (BRCN). The condition is characterized by the death of renal cortical tissue with sparing of the medulla. Its pathophysiology is uncertain. Lindheimer and colleagues21 suggested that endothelial damage by endotoxin is followed by the formation of thrombi. The incidence of BRCN is probably underestimated because patchy cortical necrosis with partial or almost complete recovery or renal function may be overlooked if a patient survives and the appropriate investigations are not undertaken. Abruptio placentae is the most common pregnancy complication associated with BRCN, whereas the incidence is relatively low in patients with severe preeclampsia. BRCN should be strongly suspected if ARF develops before 30 weeks of gestation and is associated with prolonged anuria or oliguria (of more than 10 days' duration). Anuria or oliguria is the rule, and urine is usually blood-stained.
Renal biopsy or selective arteriography can be used to confirm the diagnosis and distinguish between extensive and patchy cortical necrosis. Most patients with BRCN progress to chronic renal failure; before the availability of renal dialysis, the condition was usually fatal. Some patients with this disease have a slow recovery in renal function for up to 3 years after the onset and can achieve a satisfactory lifestyle without dialysis.22
PREECLAMPSIA
Preeclampsia is a syndrome characterized by hypertension and proteinuria. The disease process starts far before the appearance of any clinical signs or symptoms. Its etiology is unknown but is thought to be due to endothelial damage. A multisystem disorder, preeclampsia has multiple effects on the kidney. It is a frequent cause of proteinuria in pregnancy. Endotheliosis, swollen intracapillary endothelial cells in the glomeruli, is the hallmark lesion of preeclampsia in the kidney. In addition, preeclampsia may cause focal glomerular sclerosis. An increase in renal vascular resistance causes a reduction of renal blood flow. Therefore, GFR, ERPF, and the filtration fraction decrease in preeclampsia. The exact etiology for the decline in renal function is unknown. After delivery, the functional decrements usually reverse quickly but can progress to ATN if treatment is not initiated at an appropriate time.
Pregnancy outcomes complicated by preeclampsia and ARF are associated with high rates of morbidity and mortality. Sibai and colleagues18 reported outcomes in 31 pregnancies in 30 patients with renal failure; 18 women had preeclampsia and 12 had superimposed preeclampsia with existing hypertension, renal disease, or both. All the women in the preeclampsia-only group developed ARF 1 to 5 days postpartum, whereas seven women in the other group developed ARF postpartum. Overall, gestational age at delivery was less than 30 weeks in 37.5% of the pregnancies and 21 to 36 weeks in 47%. All 18 patients in the preeclampsia-only group had ATN, and 9 required dialysis. All patients had normal renal function within 8 weeks of follow-up. Two patients in this group died at 8 to 9 weeks postpartum after the ATN resolved. Autopsy results showed no residual renal disease. In the other group, 11 patients survived and 9 of these patients required dialysis. Before delivery, seven of the nine had abnormal renal function. One patient who had glomerulosclerosis and ARF died 3 days after termination of her pregnancy at 16 weeks' gestation.
The association of HELLP (hemolysis, elevated liver enzymes, and low platelets) syndrome complicated by acute renal failure does not significantly increase maternal morbidity or mortality rates. Selcuk and colleagues23 described 39 cases of pregnancy-related ARF. Fourteen (36%) of the patients had HELLP syndrome and 12 of the 14 required dialysis. Fourteen of the other patients without HELLP syndrome also required dialysis. Recovery rate, maternal death rate, and fetal death rate were similar in both groups. Another study compared adverse perinatal outcome in women with HELLP syndrome (n = 32) with women with severe preeclampsia without HELLP syndrome (n = 32) at less than 28.0 week's gestation.24 There were no significant differences between the groups with respect to ARF. There were no maternal deaths. An additional study revealed that subsequent pregnancies in patients who have had prior pregnancies complicated by ARF and HELLP syndrome tend to have favorable outcomes and long-term prognosis.24
In conclusion, ARF is infrequent in well-managed patients with severe preeclampsia. Maternal and perinatal mortality and morbidity rates increase with the association of ARF in these patients. Early identification and proper management of ARF in patients without associated medical or obstetric complications does not result in residual renal damage.
CHRONIC RENAL INSUFFICIENCY
Chronic renal failure is defined as a reduction of renal mass and loss of renal reserve from an insult to the kidney. Initially, surviving nephrons hypertrophy in number and function. This initial adaptation predisposes the remaining nephrons to sclerosis and unrelenting destruction, which can eventually lead to end-stage renal disease. Chronic renal failure has multiple etiologies; diabetes and hypertension have replaced glomerulonephritis as a major etiology.
Renal insufficiency is classified as mild, moderate, or severe. Patients with mild disease have a serum creatinine level of 1.4 mg/dL or less, or 125 umole/L or less, and no hypertension. Those with moderate renal insufficiency have a serum creatinine level of 1.5 to 2.5 mg/dL, or 125 to 250 umole/L, and those with severe disease have a creatinine level of 2.5 mg/dL or more, or 250 umole/L or more.25,26 Maternal and perinatal outcomes are usually not affected with mild renal insufficiency. Likewise, the effects of pregnancy do not worsen renal function when mildly impaired.27
Early studies have reported a significant deterioration of renal function in pregnant patients with moderate renal insufficiency.28,29. However, more recent studies have shown less deterioration.27,28,29,30,31,32 The largest study comprised 82 pregnancies in 67 women with primary renal disease and a serum creatinine level of more than 1.4 mg/dL.27 The underlying disorder was glomerulonephritis in 51% of the women and chronic tubulointerstitial disease in the remaining. Of these women, 72% had moderate renal insufficiency (serum creatinine level 1.4 to 2.4 mg/dL) and 18% had severe (2.5 mg/dL or more). In this study, end-stage renal disease was defined as a serum creatinine level of more than 6.0 mg/dL, and a change in renal function was defined as a 25% change in the equation 1/serum creatinine, which linearly correlates with GFR. Overall, the total rate of decline in renal function was 43%; renal function declined in 11 women during pregnancy and in 16 after delivery. Women with hypertension at the first prenatal visit had a significantly lower mean 1/Scr but not a decreased GFR in the third trimester compared with those who were normotensive. In contrast, women who developed hypertension in the third trimester had a decreased 1/Scr and GFR compared with women without hypertension. By 6 months postpartum, hypertension was no longer associated with a decline in the mean 1/Scr or in GFR.27 During pregnancy, a total of 43% of women experienced a decrease in the GFR, of these, 23% had a decline between delivery and 6 weeks postpartum, and the remaining 57% remained stable. At 6 month postpartum, 31% of the women experienced a pregnancy-related decline that persisted, 10% had a decline between 6 weeks postpartum and 6 months, 8% experienced a pregnancy-related decline but recovered, and the remaining 51% continued to be stable. Overall, the fetal survival rate was 93%, and 59% delivered prematurely. The rates of preterm delivery and intrauterine growth retardation (IUGR) for women with severe renal disease were 73% and 57%, respectively; the rates for women with moderate disease were 55% and 31%, respectively. The fetal survival rate in women with severe renal disease was 100%, and the outcome of pregnancy was not correlated with the presence of high-grade proteinuria. Women with hypertension and renal disease at the first prenatal visit were not at increased risk for preterm delivery, IUGR, or reduced fetal survival. However, hypertension in the third trimester was associated only with an increase in the rate of IUGR.27
END-STAGE RENAL DISEASE
Women with end-stage renal disease rarely become pregnant secondary to infrequent and irregular menstrual cycles, anovulation, and hormonal abnormalities. The frequency of conception has been reported to range from 0.3% to 2.2% per year; the rate of conception in patients undergoing hemodialysis is about twice that of patients undergoing peritoneal dialysis.33 Some conception rates may be incorrect as a result of biased data collection of surveys from dialysis centers. In addition, pregnancies may go unrecognized: early spontaneous abortion may be mistaken for heavy menses in women with end-stage renal disease with irregular menstruation.
Okundaye and colleagues33 reported the largest registry of pregnant patients with end-stage renal disease. In 930 dialysis centers, 344 pregnancies occurred in 318 women. Limitations to this report are biased reporting, exclusion of 60% of the dialysis centers, and incomplete information. In these 318 women, 58 were started on dialysis after conception and 209 conceived while undergoing dialysis. The outcome in women who conceived before dialysis was better than in those who conceived after beginning dialysis. Seventy-three percent of women who started on dialysis after conception had surviving infants, compared with 40.2% of women who were receiving dialysis when they conceived. Overall, out of the 320 pregnancies, there were 42% surviving neonates, 6% stillborn infants, and 7.5% neonatal deaths. Spontaneous abortions occurred in 32%; of these, 38% occurred in the second trimester. The remaining 10.8% elected therapeutic abortion. The authors also concluded that a lower frequency of prematurity and low birthweight was associated with conception before dialysis. There was no statistical significance between the fetal survival rate and causes of renal failure. In addition, women who had fewer years of dialysis before conceiving tended to have a better outcome compared with those with more years of dialysis. Lastly, there was no statistical difference in infant survival or mean gestational age of live-born infants among women with different frequencies of dialysis. However, women who received more than 20 hours of dialysis per week had better infant survival rates and less prematurity than those receiving less than 20 hours per week.32
The pregnancy rate for women receiving dialysis ranges from 23% to 73.3%.32,33 Nakabayashi and co-workers34 reported 15 pregnancies in women receiving chronic hemodialysis from 1985 to 1997. Of these, 4 infants died shortly after birth from prematurity and the other 11 showed normal development at 1 year of age. One infant was diagnosed with retrolental fibroplasias of prematurity. There were no congenital anomalies. All showed signs of preterm labor and most were born prematurely, with the mean gestation age at delivery in the 11 surviving neonates of 33.0 ± 4.7 weeks (range 23 to 38 weeks). Survival of the neonate was associated with years of dialysis. Neonates who died of prematurity were born to women who had been receiving dialysis for more than 9 years, and the remaining who survived were born to women who had been receiving dialysis for less than 6 years.33
Dialysis management in a pregnant patient differs slightly from that in a nonpregnant one. The hours and frequency of dialysis are increased by 50% to maintain a BUN of less than 80 mg/dL, with the ideal range 50 to 60 mg/dL.26 BUN levels of more than 80 mg/dL are associated with an increased risk for fetal demise. During dialysis, it is important to avoid hypotension and rapid fluctuations in intravascular volume. Anemia is common in patients receiving dialysis and is exacerbated by pregnancy.35 Approximately 35% of patients need to be transfused before or at delivery. Giving packed red blood cells during dialysis prevents volume overload and aggravation of hypertension. Erythropoietin has been shown to be effective in treating anemia in pregnancy. In addition, patients receiving dialysis need a diet consisting of 70 g protein, 1500 mg calcium, 50 mmol potassium, and 80 mmol sodium, with supplements of dialyzable vitamins.35 Counseling of dialysis-dependent patients embarking on pregnancy should emphasize the poor fetal prognosis. Further, patients should be aware that they will have to contend with a high risk of pregnancy complications and an increased frequency and duration of dialysis.
PREGNANCY AFTER RENAL TRANSPLANT
Chronic renal failure is often accompanied by amenorrhea, but fertility returns rapidly after transplantation. During the past decade there has been a steady increase in the number of pregnancies after renal transplantation. Davison and Milne36 reviewed pregnancies in transplant patients. Fewer than 40% of conceptions did not continue beyond the first trimester; of the remainder, 94% were successful. In most patients, renal function improved during pregnancy, but in 15% there was a deterioration that could persist after delivery. The incidence of graft rejection appears to be no greater than in the nonpregnant population. Preeclampsia was the most common complication, affecting 30% of pregnancies.36 In transplant recipients, changes in urinary protein excretion, plasma uric acid, platelet count, or liver function tests seem to be less useful as markers of preeclampsia than in the normal population. Infection is an important consideration in any patient receiving immunosuppressive drugs. Aseptic technique should be used for even minor surgery and steroid therapy augmented. A transplanted kidney rarely obstructs labor, and delivery by cesarean section is required only for obstetric reasons.
In utero exposure to high-dose steroid and immunosuppressive agents does not seem to be associated with an increased incidence of congenital anomalies in the offspring of pregnant women with a renal transplant. Current data suggest that steroids and immunosuppressive agents (prednisone, cyclosporin A, and azathioprine) at the doses used to prevent graft rejection in transplant recipients are well tolerated by the fetus. In theory, these drugs could cause fetal growth retardation, adrenal and bone marrow suppression, and immunosuppression predisposing to intrauterine infection. However, more than 50% of live-born infants had no neonatal problems. Davison and Milne36 reported a 45% to 60% preterm delivery rate and an incidence of small-for-gestational-age infants of greater than 20%. Long-term studies are required to determine whether there may be other effects, particularly an increase in the incidence of malignancies or abnormalities in the subsequent generation.
Before any woman with a renal transplant embarks on a pregnancy, an obstetrician and nephrologist should counsel her. Lindheimer and colleagues37 proposed guidelines for transplant patients before pregnancy. At least 2 years should have elapsed since the transplant, and the woman should be in good health without severe hypertension, severe renal insufficiency, or persistent proteinuria. In addition, she should be receiving maintenance doses of immunosuppressive therapy. Pregnancy appears to have no effect on graft function or survival; however, an important concern is that a mother may not survive long after the pregnancy. Current data suggest that 10% of mothers die within 7 years of pregnancy. Eighty percent and 50% of recipients of kidneys from living donors and from cadavers, respectively, are alive 5 years after the transplant. If renal function is normal 2 years after transplantation, then the survival rate exceeds 80%.36
GLOMERULONEPHRITIS
Acute glomerulonephritis (the acute nephritic syndrome) is characterized by the abrupt appearance of red blood cells and red blood cell casts in the urine. Renal function is usually impaired, with sodium and water retention leading to edema and hypertension. The BUN and creatinine levels rise, and creatinine clearance declines. Proteinuria is common but is normally less than 3.5 g per 24 hours. Renal diseases presenting as acute glomerulonephritis include poststreptococcal glomerulonephritis, lupus glomerulonephritis, membranoproliferative glomerulonephritis, and Goodpasture's syndrome. Laboratory investigations may help to distinguish the different causes and should include urine microscopy, creatinine clearance, 24-hour urinary protein collection, serum IgA and complement determinations, streptozyme assay and antistreptolysin 0 titers, and evaluation of antinuclear antibody.38 Management problems include control of hypertension, electrolyte balance, and edema. Uremia may not respond to conservative measures and may require renal dialysis.
Chronic glomerulonephritis implies progressive loss of renal function, proteinuria, and diminishing renal size caused by primary or secondary glomerular disease that has failed to resolve or respond to treatment. End-stage renal failure eventually ensues, requiring hemodialysis. Jungers and associates39 observed 171 pregnancies in women with biopsy-proven primary chronic glomerulonephritis and concluded that pregnancy did not adversely affect the course of renal disease in patients who had normal renal function before pregnancy.
POSTSTREPTOCOCCAL GLOMERULONEPHRITIS
Acute poststreptococcal glomerulonephritis is very uncommon in pregnancy. The diagnosis usually depends on a history of streptococcal infection within the previous weeks and an elevated antistreptolysin titer. Fetal loss is almost invariable. Renal function returns to normal after delivery. Patients who have a history of poststreptococcal glomerulonephritis and whose renal function has returned to normal are not at risk of fetal loss or preeclampsia.40
MESANGIAL IgA NEPHROPATHY
In most countries, mesangial IgA nephropathy is the most common type of glomerulonephritis, and yet there are surprisingly few accounts of pregnancy and IgA nephropathy. Kincaid-Smith and Fairley41 summarized the outcome of 102 pregnancies in 65 patients with mesangial IgA nephropathy. The outcome in individual patients with IgA nephropathy may vary from no change in renal function or biopsy features during 25 years to a fulminating progression to end-stage renal failure in a matter of 2 months. It is therefore difficult to be certain whether renal function that deteriorates during pregnancy was precipitated by the pregnancy or was the natural course of the disease. The prognosis for fetal outcome is generally good in patients with normal renal function and without pre-existing or early developing hypertension.41
FOCAL AND SEGMENTAL HYALINOSIS AND SCLEROSIS
Jungers and colleagues42 studied 10 pregnancies in six patients with focal and segmental hyalinosis and sclerosis and found a high rate of fetal complications with one abortion, four preterm deliveries (including one stillbirth), and two neonates with severe growth retardation. Proteinuria recurred or increased in four pregnancies. None of the patients studied were in renal failure at conception, and none progressed to failure during pregnancy. Similar findings were reported by Kincaid-Smith and Fairley.41 Again it is difficult to determine the effect of pregnancy on the course of this condition, but most reports suggest an adverse effect,40 particularly if renal failure and hypertension are present at conception.42,43,44
REFLUX NEPHROPATHY
Kincaid-Smith and Fairley41 analyzed data from 345 pregnancies complicated by reflux nephropathy and examined the effect of renal function on outcome. Abnormal renal function, defined as a serum creatinine level greater than 1.25 mg/dL, was associated with significantly higher rate of fetal loss and a greater chance of preeclampsia and a decline in renal function. They also observed a marked and rapid decline in renal function in every patient with a serum creatinine level greater than 2.3 mg/dL. Proteinuria proved to be a good indicator of prognosis because it reflected the development of progressive secondary glomerular lesion. Abe and colleagues45 retrospectively studied the influence of antecedent renal disease on pregnancy in 72 women with primary glomerular disease. The incidence of normal delivery and live births was highest in cases of membranous glomerulonephritis. There was no difference in the fetal outcome for IgA nephropathy and non-IgA proliferative glomerulonephritis. Outcome was unfavorable in cases associated with hypertension (more than 140/90 mm Hg) or impaired renal function (glomerular filtration rate less than 70 mL per minute) or when biopsy specimens showed arteriosclerosis or cortical tubulointerstitial changes. Although renal function deteriorated in 11 patients, the authors could not be certain whether this had been precipitated by the pregnancy. They concluded that a combination of clinical and histologic parameters should be used to assess the prognosis for pregnancy in women with primary glomerular disease.
INTERSTITIAL NEPHRITIS
Interstitial nephritis implies primary damage to the tubulointerstitial system of the kidneys with secondary glomerular damage. The acute form usually presents with rapid deterioration in renal function, especially if caused by a drug or an infectious agent. Chronic interstitial disease may occur as a consequence of any disorder that produces chronic damage to the renal interstitium—for example, chronic hypertension, diabetes mellitus, chronic pyelonephritis, and drug abuse, particularly a combination of phenacetin and aspirin. Hematuria and proteinuria are not characteristic of chronic interstitial nephritis, and the condition is typically insidious in onset and progression. It is often associated with an inability to concentrate urine. Pregnancy outcome is less than optimal. The fetal loss rate is particularly high (10% perinatal mortality) if interstitial nephritis is complicated by hypertension or preeclampsia.
COLLAGEN VASCULAR DISEASES
The collagen vascular diseases are systemic disorders of unknown cause characterized by multiorgan inflammation and unpredictable remissions and exacerbations. Chronic inflammatory changes are usually found in the microvasculature, although larger vessels may also be involved. Renal involvement is generally an unfavorable prognostic sign.
Systemic Lupus Erythematosus
Investigation has predominantly focused on systemic lupus erythematosus (SLE), which is by far the most common collagen vascular disorder encountered in obstetric practice. The condition is associated with overproduction of circulating autoantibodies to a wide variety of antigens, particularly nuclear antigens. The autoantibodies may damage tissue directly or indirectly by forming antigen-antibody immune complexes, as in lupus glomerulonephritis.
Bobrie and colleagues46 reviewed 213 pregnancies (73 women) associated with lupus nephritis. Renal biopsy was performed in 66 women, showing proliferative glomerulonephritis in 48. After exclusion of therapeutic abortions (24/213), the overall percentage of live births was 87%. Fetal loss was higher if the onset of SLE occurred during pregnancy or in the immediate postpartum period. Fetal prognosis when pregnancy occurs after the onset of SLE depends not only on the disease activity but also on other factors such as the presence of nephrotic syndrome, hypertension, or impaired renal function.47 The fetal mortality rate appears to be independent of the presence or absence of lupus anticoagulant.48 The latter is closely associated with anticardiolipin antibodies. A high anticardiolipin level has been reported to be a sensitive predictor of fetal growth retardation or intrauterine fetal death in pregnant patients with SLE.49 Although most offspring of patients with lupus are normal, it has been known for many years that some neonates have transient skin rashes and positive results of antinuclear antibody tests. A neonatal lupus syndrome has been described,50 the predominant features of which are discoid skin rashes, congenital heart block, and antinuclear antibodies in maternal and neonatal serum. The cutaneous manifestations usually resolve within 3 months. Congenital heart block may occur in isolation or may be associated with other congenital cardiac anomalies or with skin rashes. The heart block is caused by fibrosis of the conducting system and is permanent. There is a close correlation between isolated congenital heart block and the presence of maternal antibody to soluble tissue ribonucleoprotein antigens anti-Ro (SS-A).
The activity status at conception provides no guide to the course of lupus nephropathy. This is particularly true in patients with a history of a severe form of lupus nephritis.51 Proteinuria may increase during pregnancy, and the serum creatinine level may either rise or not fall normally. Exacerbations of lupus nephropathy are usually moderate and can easily be controlled by steroid therapy. However, the course of maternal lupus nephritis is especially poor when SLE presents during pregnancy.46,51,52 SLE with superimposed preeclampsia may present with signs and symptoms indistinguishable from a flare-up of lupus nephritis. Antibody assays may help to differentiate these conditions. Rising titers of anti-DNA antibodies or falling levels of complement C3 or C4 are compatible with an exacerbation of SLE.53,54 Patients with SLE in pregnancy should receive steroid therapy if clinical or immunologic signs or symptoms of disease activity develop. Hypertension should be treated with antihypertensive agents. Bobrie and colleagues46 favor routine administration of steroids postpartum to prevent a flare-up.
In the past, lupus nephritis was one of the most common reasons for recommending therapeutic abortion in SLE. This procedure was not always followed by a remission and sometimes resulted in significant maternal morbidity and even death. Therapeutic abortion is now rarely indicated. A favorable fetal outcome may be anticipated even in the presence of severe lupus nephritis, provided the disease is stable and in remission and renal function and blood pressure are normal at conception. Fetal wastage is higher when the disease is active. There is no evidence to suggest that pregnancy has an adverse effect on SLE, and most exacerbations of lupus nephropathy during pregnancy represent the effect of hypertension or superimposed preeclampsia. Even in the presence of nephrotic syndrome during pregnancy, fetal and maternal outcome may be good if hypertension and renal insufficiency are not severe. The successful management of a pregnancy complicated by lupus nephritis requires close cooperation between obstetrician, nephrologist, and rheumatologist.
Scleroderma
Scleroderma usually presents in the fourth and fifth decades of life and is rarely encountered in pregnant women. Complications are most likely to arise in patients with renal involvement. Some would consider scleroderma to be a contraindication to pregnancy and recommend sterilization, particularly if there is widespread organ involvement or renal disease.55 However, the prognosis is not uniformly grim, and pregnancy can progress without evidence of renal disease and result in a healthy infant. There is no satisfactory treatment for scleroderma.
Polyarteritis Nodosa
Polyarteritis nodosa involves medium-sized muscular arteries and is characterized by necrotizing inflammation of the arterial wall that may result in vascular occlusion. The disease may progress slowly, with exacerbations and remissions, or may progress rapidly and relentlessly. The most serious complications in pregnancy are severe hypertension, preeclampsia, and deterioration of renal function. Prognosis is greatly improved if the disease is diagnosed and under good medical control before conception.56 Fetal outcome is often good, although there is a high risk of premature delivery. High doses of steroids are the mainstay of management. In selected cases in which maternal condition is not improving, cyclophosphamide may be indicated
DIABETIC NEPHROPATHY
Diabetic nephropathy complicates 4% to 10% of pregnancies in women with diabetes. The characteristic pathology of diabetic nephropathy is glomerulosclerosis, either diffuse or nodular. This disorder usually presents with heavy proteinuria, azotemia, and hypertension. The exact cause of diabetic nephropathy is unknown but has been attributed to glycemic control, hypertension, an increase in GFR, or an increase in protein intake and excretion, all of which are affected by pregnancy.57 It is unclear whether diabetic nephropathy is accelerated by pregnancy. A few studies have shown that pregnancy has an adverse affect on diabetic nephropathy, increasing proteinuria and creatinine clearance.58,59,60 Other studies have not observed any progression or development of nephropathy in women with diabetes.61,62,63,64,65 Most studies involve a small number of patients and are observational.
Kitzmiller and colleagues61 reported on 26 pregnancies complicated by White class F diabetes. All attained at least 24 weeks of gestation. Seventy-five percent had proteinuria before conception, 30% were hypertensive at the onset of pregnancy, and 30% had renal insufficiency in the first trimester. Follow-up was 9 to 35 months after delivery. Creatinine clearance remained stable throughout pregnancy in the eight patients with a clearance of less than 70 mL per minute in the first trimester. Overall, there was a marked rise in proteinuria during the third trimester, and in 58% it exceeded 6 g per 24 hours. Thirty-seven percent of those pregnant women starting with a normal blood pressure were complicated by hypertension. After delivery, proteinuria declined spontaneously in most patients, and mean creatinine clearance was unchanged compared with first-trimester levels. Three women progressed to severe renal failure 1 to 2 years after pregnancy. In this study, the perinatal survival rate for diabetic women with nephropathy was 89%. Thirty percent of the pregnancies were delivered before 34 weeks, and 31% were complicated by fetal growth retardation.
Reports by Reece and colleagues,66 Jovanovic and Jovanovic,67, and Grenfell and associates62 have substantiated the findings of Kitzmiller and coworkers.61 Changes in creatinine clearance during pregnancy are variable in diabetic nephropathy, and even patients with severe reductions in creatinine clearance (less than 60 mL per minute) usually show no further decline during pregnancy. It may be concluded that in the absence of severe hypertension or severe renal insufficiency, pregnancy does not alter the natural course of diabetic nephropathy. However, this complication is associated with higher perinatal rates of mortality and morbidity than in pregnant diabetic women without renal involvement. Current evidence suggests that optimal outcome depends on maintaining good control of maternal glucose levels and blood pressure. About 20% of women with diabetic nephropathy during pregnancy will progress to renal failure by 5 years postpartum.
Women with diabetic nephropathy are at increased risk for adverse maternal and fetal outcome, especially preterm delivery, IUGR, preeclampsia, and hypertensive complications. The rates of preterm delivery at less than 34 weeks' gestation range from 16% to 31%,60,61 and the rates of IUGR range from 9% to 22%.63,64,65,66,67,68 In addition, the rate of preeclampsia may be a high as 50%.60,68 In pregnancy, hypertension may exist before or may occur during pregnancy. Pregnancy-induced hypertension affects 10% to 15% of women with diabetes. In addition, women with diabetes alone are at increased risk for preeclampsia. Those with hypertension are at risk for superimposed preeclampsia and long-term complications from hypertension. Therefore, it is very important to maintain the blood pressure at less than 140 mm Hg systolic and less than 90 mm Hg diastolic. Angiotensin-converting enzymes are antihypertensive agents that are renoprotective but should not be used in pregnancy. They are contraindicated in the second and third trimesters secondary to the risk of fetal renal failure. Calcium-channel blockers seem to have no major adverse effects on the fetus and may be renoprotective.
NEPHROTIC SYNDROME
The nephrotic syndrome is a collection of signs and symptoms commonly associated with certain glomerular diseases that are characterized by increased capillary wall permeability to serum proteins. The hallmark of nephrotic syndrome is proteinuria greater than 3 g per 24 hours. Hypoalbuminuria, edema, hyperlipidemia, and lipiduria may occur secondary to proteinuria. Nephrotic syndrome usually is insidious in onset, and renal function is often normal at the time of presentation. In two thirds of adults and most children, the nephrotic syndrome is a manifestation of one of three forms of primary glomerular disease: minimal-change nephrotic syndrome, membranous nephropathy, or membranoproliferative glomerulonephritis. The nephrotic syndrome may accompany acute or chronic disease, particularly when caused by collagen vascular disease. The nephrotic syndrome of pregnancy is a rare condition in which proteinuria appears during pregnancy, disappears after delivery, and recurs in subsequent pregnancies.69
The most important complications of nephrotic syndrome are severe protein malnutrition, hypercoagulability leading to thromboembolic complications, and ARF. Renal vein thrombosis has been reported in association with nephrotic syndrome in pregnancy, resulting in a deterioration of renal function. This is uncommon, however, and does not justify prophylactic anticoagulation. Diuretics may be indicated if edema is very debilitating but should be used with great caution and not at all if the vascular volume is decreased. Opinions concerning the value of steroids vary, and it is advisable to discuss the use of these agents with a nephrologist in the context of a specific patient.
Prognosis for a successful pregnancy without deterioration in renal function depends on the cause of nephrotic syndrome. It is generally optimistic, especially if renal function is adequate and hypertension absent. When the syndrome is associated with azotemia, a successful pregnancy is unlikely.
HEREDITARY RENAL DISEASE
There have been very few reports describing the outcome of pregnancy complicated by hereditary renal disease. The most frequent congenital problem encountered is polycystic kidney disease, although the disorder usually presents after age 35 years (i.e., toward the end of the childbearing years). The major problems to be expected from polycystic kidney disease are infection, hematuria, hypertension, and atypical pain. In retrospective studies,70,71 patients without hypertension or azotemia did well. When hypertension or azotemia was present, the incidence of preeclampsia and perinatal mortality was increased. Intracranial aneurysms are found in approximately 20% of patients with polycystic kidney disease, obviously posing important implications for pregnancy. The question of inheritance must also be discussed (autosomal dominant trait with high penetrance), and adoption may be an acceptable and safer option for some couples.
TUMORS OF THE URINARY TRACT
Malignant tumors of the urinary tract are rare in pregnancy. A survey of the literature between 1890 and 1969 revealed 32 renal tumors, the majority being adenocarcinoma.72 Hematuria is the most common presenting sign. Associated complaints include dysuria, frequency, loin pain, a renal mass, and symptoms of urinary tract obstruction. Renal or ovarian cysts, hydronephrosis, or polycystic kidney disease should be considered in the differential diagnosis. Urinalysis and cytology may provide a clue to the diagnosis, but more sophisticated investigations are usually required. Cystourethroscopy can identify a tumor of the lower urinary tract, but intravenous pyelography is necessary to reveal tumors of the ureter and renal pelvis. Renal tumors may be shown by ultrasonography, but magnetic resonance imaging, with its apparent freedom from fetal side effects, may soon become the investigation of choice in pregnancy.
The treatment of urinary tract malignancy in pregnancy may involve surgery, radiation, or chemotherapy. The risks to the fetus of preterm delivery or the hazards of radiation and chemotherapy must be balanced against the risk to the mother of delaying treatment. Management should be individualized according to the pregnancy gestation, character of the tumor, and wishes of the parents. Carcinoma of the kidney and ureter is resistant to chemotherapy and radiotherapy, so surgical excision is the therapy of choice. Tumors of the lower urinary tract usually require a combination of wide excision and radiotherapy.
DEVELOPMENTAL DEFECTS
Developmental abnormalities of the kidneys such as horseshoe kidneys, ectopic pelvic kidneys, or ectopic or bifid ureters are relatively rare and have usually been diagnosed before pregnancy. These conditions are associated with an increased risk of infection. An abnormal location of the kidneys may obstruct labor or restrict access to the uterus if cesarean section is required.
MANAGEMENT OF RENAL DISEASE IN PREGNANCY
Women with known renal disease should be encouraged to seek advice before they embark on a pregnancy. Apart from discussing the effects of pregnancy on the renal disease, preconception counseling provides an opportunity to assess the severity of the renal disease and if necessary implement treatment to control hypertension and optimize renal function.
Pregnancy outcome is likely to be good if renal function and blood pressure are normal at conception. Fetal and maternal morbidity and mortality rates relate directly to the development of superimposed preeclampsia and renal failure.
General care should include frequent antenatal visits to assess blood pressure, weight gain, and renal function, as well as to rule out urinary tract infection. Blood pressures greater than 140/90 mm Hg should be treated because fetal and maternal outcomes appear to be better if hypertension is corrected.73 Renal function should be assessed monthly to 28 weeks and then every 2 weeks until term by creatinine clearance, BUN, serum creatinine level, and electrolytes.
Fetal well-being and growth must be carefully monitored. Nonstress testing is indicated from 26 weeks until delivery. The interval of testing depends partly on the severity of renal disease and hypertension but as a rule should be at least weekly from 26 weeks and twice weekly from 32 weeks. Ultrasonographic measurements of fetal growth should be performed at 26, 32, and 36 weeks. The biophysical profile provides additional information about fetal condition.74 It should be used in conjunction with nonstress testing and performed at similar intervals.
Most patients with renal disease require some form of medication during pregnancy. In patients with renal disease, the excretion of medications may be reduced and the drug dosage should be frequently reviewed to avoid toxicity. The potential complications of immunosuppressive agents and steroids have already been discussed. Patients with renal disease are susceptible to infection. Antibiotics that are reported to be nephrotoxic (particularly the aminoglycosides, cephaloridine, and methicillin) should be avoided. This is sometimes not possible, but whatever drug is chosen, its effect on renal function must be carefully monitored.
The most widely used antihypertensive drug in pregnancy is methyldopa. It stimulates central alpha-2-receptors and may also act as an alpha-2-peripheral blocker by a false neurotransmitter effect. Data from studies of infants exposed to methyldopa in utero suggest no long-term harmful effects. Reported maternal complications include sedation and postural hypotension. Hemolytic anemia affects 5% of patients and is an indication to discontinue therapy. In addition, elevated liver enzymes may be a side effect that requires discontinuation of the drug.
Beta-adrenergic blocking agents are also effective in controlling hypertension in pregnancy. It has been suggested that they may cause IUGR, fetal distress, hypoglycemia, and respiratory depression; however, most of these reports are anecdotal. Atenolol, when used chronically, is associated with severe IUGR.75,76 Angiotensin-converting enzyme inhibitors are contraindicated in the second or third trimester.77,78,79 Hydralazine, a potent vasodilator, may be used alone or in combination with another antihypertensive agent, usually methyldopa. Maternal side effects include headache, tachycardia, palpitations, and fluid retention. Chronic doses exceeding 200 mg per day have been associated with a lupus-like syndrome. Maternal side effects are significantly lessened when hydralazine is used in combination with methyldopa. Fetal effects have not been fully evaluated.
If maternal blood pressure is well controlled, renal function is not deteriorating, and fetal growth and well-being are satisfactory, then pregnancy can be safely prolonged until term. In the absence of premature delivery or evidence of fetal distress, vaginal delivery may be anticipated.
MATERNAL AND FETAL PROGNOSIS FOR PREGNANCY WITH RENAL DISEASE
Women who have chronic renal disease with well-preserved renal function and normal blood pressure can be reassured the pregnancy is likely to be associated with a good fetal outcome and no permanent adverse effect on maternal renal function.80,81 Fewer studies have been conducted on the risks of pregnancy when renal function is more than mildly impaired. Hou and colleagues44 reported 25 pregnancies associated with a serum creatinine level of 1.4 mg/dL or more before or at the onset of pregnancy. In one third of the cases, there was a decline in renal function greater than expected from the natural history of this disease. A decline in function occurred mainly in women with glomerular disease. Fetal outcome was good (23 live births and 21 long-term survivors), although the incidence of preterm delivery was high (14 of 23).
The question of maternal mortality has become less of an issue in recent years, and it is now recognized that although pregnancy may be associated with a severe decline in renal function and may hasten the need for dialysis, it rarely carries a risk to maternal life that mandates termination of pregnancy. In the absence of superimposed preeclampsia, it is unusual for patients with renal diseases to be delivered prematurely for fetal compromise. However, regular assessment of fetal well-being with nonstress tests and ultrasonographic measurements of growth and fetal activity is central to successful management.
REFERENCES
Linheimer MD, Katz AI: Renal physiology and disease in pregnancy. In Seldin DW, Giebisch G (eds): The Kidney: Physiology and Pathophysiology, pp 3d ed. Philadelphia, Lippincott Williams and Wilkins, 2000 |
|
Davison JM, Vollotton MB, Lindheimer MD: Plasma osmolality and urinal concentration and dilution during and after pregnancy: Evidence that lateral recumbency inhibits maximal urinary concentrating ability. Br J Obstet Gynaecol 88: 472, 1981 |
|
Davison JM, Dunlop W: Changes in renal hemodynamics and tubular function induced by normal pregnancy. Semin Nephrol 4: 198, 1984 |
|
Cohen HT, Spiegel DM: Air-exposed urine dipsticks give false-positive results for glucose and false-negative results for blood. J Clin Pathol 96: 398– 400, 1991 |
|
Lim KH, Friedman SA, Ecker JK et al: The clinical utility of serum uric acid measurements in hypertensive diseases of pregnancy. Am J Obstet Gynecol 178: 1067, 1998 |
|
Bell SC, Halligan AW, Martin A et al: The role of observer error in antenatal dipstick proteinuria analysis. Br J Obstet Gynaecol 106: 1113, 1999 |
|
Saudan PJ, Brown MA, Farrell T, Shaw L: Improved methods of assessing proteinuria hypertensive pregnancy. Br J Obstet Gynaecol 104: 1159, 1997 |
|
Higby K, Suiter CR, Phelps JY et al: Normal values of urinary albumin and total protein excretion during pregnancy. Am J Obstet Gynecol 171: 984, 1994 |
|
Evans W, Lensmeyer JP, Kirby RS et al: Two-hour urine collections for evaluating renal function correlates with 24-hour urine collection in pregnant patients. J Maternal Fetal Med 9: 233, 2000 |
|
Rinehart BK, Terrone DA, Larmon JE et al: A 12-hour urine collection accurately assesses protein in the hospitalized hypertension gravida. J Perinatol 19: 556, 1999 |
|
Packham D, Fairley KF: Renal biopsy: Indication and complications in pregnancy. Br J Obstet Gynaecol 94: 935, 1987 |
|
Kullner JA, D'Andrea NM, McMahan MJ: Renal biopsy and pregnancy. Am J Obstet Gynecol 184: 1093, 2001 |
|
Gonzalez M, Chew W, Soltero L et al: Percutaneous kidney biopsy analysis of 26 years: Complication rate and risk factors. Rev Invest Clin 52: 125, 2000 |
|
Lindheimer MD, Davison JM: Renal biopsy during pregnancy: ‘To b… or not to b..?’ Br J Obstet Gynaecol 94: 932, 1987 |
|
Gaber LW, Spargo BH: Pregnancy-induced nephropathy: The significance of focal segmental glomerulosclerosis. Am J Kidney Dis 9: 317, 1987 |
|
Grunfeld JP, Pertuiset N: Acute renal failure in pregnancy. Am J Kidney Dis 9: 359, 1987 |
|
Turney JH, Ellis CM, Parsons FM: Obstetric acute renal failure 1956-1987. Br J Obstet Gyaecol 96: 679, 1989 |
|
Sibai BM, Villar MA, Mabie BD: Acute renal failure in hypertensive disorders of pregnancy. Pregnancy outcome and remote prognosis in thirty-one consecutive cases. Am J Obstet Gynecol 162: 777, 1990 |
|
Gabow PA, Anderson RJ, Schreier RW: Acute renal failure. Cardiovasc Med 2: 1161, 1977 |
|
Brenner BM, Lazarus JM (eds): Acute renal failure. In Management of Acute Renal Failure, p 1762. Philadelphia, WB Saunders, 1983 |
|
Lindheimer MD, Katz Al, Ganeval D et al: Acute renal failure in pregnancy. In Brenner BM, Lazara JM (eds): Acute Renal Failure, p 510. Philadelphia, WB Saunders, 1983 |
|
Leinknecht S, Grunfeld JP, Cia Gomez P et al: Diagnostic procedures and long-term prognosis in bilateral renal cortical necrosis. Kidney Intl 4: 390, 1973 |
|
Selcuk NY, Obabas AR, Cetinkaya R et al: Outcome of pregnancies with HELLP syndrome complicated by acute renal failure (1989-1999). Renal Failure 22: 319, 2000 |
|
Sibai BM, Ramadan MK: Acute renal failure in pregnancies complicated by hemolysis, elevated liver enzymes, and low platelets. Am J Obstet Gynecol 168: 1682, 1993 |
|
Davison JM, Lindheimer MD: Renal disorders. In Maternal-Fetal Medicine, p Philadelphia, WB Saunders, 1999 |
|
Jones DC: Pregnancy complicated by chronic renal disease: Perinatal care for chronic maternal conditions. Clin Perinatol 24: 483, 1997 |
|
Jones DC, Hayslett JP: Outcome of pregnancy in women with moderate or severe renal insufficiency. N Engl J Med 335: 226, 1996 |
|
Kincaid-Smith P, Fairley KF, Bullen M: Kidney disease and pregnancy. Med J Aust 2: 115, 1967 |
|
Bear RA: Pregnancy in patients with renal disease: A study of 44 cases. Obstet Gynecol 48: 13, 1976 |
|
Cunningham FG, Cox SM, Harstad TW et al: Chronic renal disease and pregnancy outcome. Am J Obstet Gynecol 163: 453, 1990 |
|
Hou SH, Grossman SD, Madias NE: Pregnancy in women with renal disease and moderate renal insufficiency. Am J Med 78: 185, 1985 |
|
Jungers P, Shauveau D, Choukroun G et al: Pregnancy in women with impaired renal function. Clin Nephrol 47: 281, 1997 |
|
Okundaye I, Abrinko P, Hou S: Registry of pregnancy in dialysis patients. Am J Kidney Dis 31: 766, 1998 |
|
Nakabayashi M, Adachi T, Itoh S et al: Perinatal and infant outcome of pregnant patients undergoing chronic hemodialysis. Nephron 82: 27, 1999 |
|
Davison JM: Dialysis, transplantation, and pregnancy. Am J Kidney Dis 17: 127, 1991 |
|
Davison JM, Milne JEC: Pregnancy and renal transplantation. Br J Urol 80: S29, 1997 |
|
Lindheimer MD, Grunfeld JP, Davison JM: Renal disorders. In Baarron WM, Lindheimer MD (eds): Medical Disorders During Pregnancy, p 39. St. Louis, Mosby, 2000 |
|
Couser WG: Glomerular disorders. In Wyngaarden JB, Smith LH (eds): Textbook of Medicine, p 578. Philadelphia, WB Saunders, 1985 |
|
Jungers P, Houillier P, Forget D et al: Influence of pregnancy on the course of primary chronic glomerulonephritis. Lancet 346: 1122, 1995 |
|
Kaplan AL, Smith JP, Tillman AJB: Healed acute and chronic nephritis in pregnancy. Am J Obstet Gynecol 83: 1519, 1962 |
|
Kincaid-Smith P, Fairley KF: Renal disease in pregnancy. Three controversial areas: Mesangial IgA nephropathy, focal glomerular sclerosis (focal and segmental hyalinosis and sclerosis), and reflux nephropathy. Am J Kidney Dis 9: 328, 1987 |
|
Jungers P, Forget D, Houillier P et al: Pregnancy in IgA nephropathy, reflux nephropathy, and focal glomerular sclerosis. Am J Kidney Dis 9: 334, 1987 |
|
Taylor J, Novak R, Christiansen R et al: Focal sclerosis glomerulopathy with adverse effects during pregnancy. Arch Intern Med 138: 1695, 1978 |
|
Hou SH, Grossman SD, Madias NE: Pregnancy in women with renal disease and moderate renal insufficiency. Am J Med 78: 185, 1985 |
|
Abe S, Amagasaki Y, Konishi K et al: The influence of antecedent renal disease on pregnancy. Am J Obstet Gynecol 153: 508, 1985 |
|
Bobrie G, Liote F, Houillier P et al: Pregnancy in lupus nephritis and related disorders: Am J Kidney Dis 9:339, 1987 |
|
Cameron JS, Hicks J, Frampton G et al: The outcome of pregnancy in patients with lupus nephritis: Proceedings of the European Dialysis Transplant Association and the European Renal Association 22:487, 1985 |
|
Loite F, Hannedouche T, Meyer O et al: Recurrent thrombosis and lupus anticoagulant in systemic lupus erythematosus. Arch Intern Med 146: 1247, 1986 |
|
Lockshin MD, Druzin ML, Goei S et al: antibody to cardiolipin as a predictor of fetal distress or death in pregnant patients with systemic lupus erythematosus. N Engl J Med 313: 152, 1985 |
|
Abruzzo JL: The neonatal lupus syndrome, autoantibodies and connective tissue disease. Ann Intern Med 99: 716, 1983 |
|
Imbasciati E, Surian M, Bottino S et al: Lupus nephropathy and pregnancy. A study of 26 pregnancies in patients with systemic lupus erythematosus and nephritis. Nephron 36: 46, 1984 |
|
Hayslett JP, Lynn RI: Effect of pregnancy in patients with lupus nephropathy. Kidney Int 18: 207, 1980 |
|
Weinstein A, Bordwell B, Stone B et al: Antibodies to native DNA serum complement (C3) levels. Application to the diagnosis and classification of systemic lupus erythematosus. Am J Med 74: 206, 1983 |
|
Zulman JI, Talal N, Hoffman GS et al: Problems associated with the management of pregnancies in patients with systemic erythematosus. J Rheumatol 7: 37, 1980 |
|
Karlsen JR, Cook WAS: Renal scleroderma and pregnancy. Obstet Gynecol 44: 349, 1974 |
|
Burkett G, Richards R: Periarteritis nodosa and pregnancy. Obstet Gynecol 59: 252, 1982 |
|
Rosenn BM, Miodovnik M: Medical complications of diabetes mellitus in pregnancy. Clin Obstet Gynecol 43: 17, 2000 |
|
Biesenbach G, Stoger H, Zazgornik J: Influence of pregnancy on progression of diabetic nephropathy and subsequent requirement of renal replacement therapy in female type I diabetic patients with impaired renal function. Nephrol Dial Transplant 7: 105, 1992 |
|
Purdy LP, Hantsch C, Molitch ME et al: Effect of pregnancy of renal function in patients with moderate-to-severe diabetic renal insufficiency. Diabetes Care 19: 1067, 1996 |
|
Gordon M, Landon MB, Samuels P et al: Perinatal outcome and long-term follow-up associated with modern management of diabetic nephropathy. Obstet Gynecol 87: 407, 1996 |
|
Kitzmiller JL, Brown ER, Phillippe M et al: Diabetic nephropathy and perinatal outcome. Am J Obstet Gynecol 141: 741, 1981 |
|
Grenfell A, Brudenell JM, Doddridge MC et al: Pregnancy in diabetic women who have proteinuria. Q J Med 59: 379, 1986 |
|
Kimmerle R, Zass RP, Cupisti S et al: Pregnancies in women with diabetic nephropathy: Long-term outcome for mother and child. Diabetologia 38: 227, 1995 |
|
Mackie A, Doddridge MC, Gamsu HR et al: Outcome of pregnancy in patients with insulin-dependent diabetes mellitus and nephropathy with moderate renal impairment. Diabetes Med 13: 90, 1996 |
|
Hemachandra A, Lloyd CE, Ellis D: The influence of pregnancy on IDDM complications. Diabetes Care 18: 950, 1995 |
|
Reece E, Coustan DR, Hayslett JP et al: Diabetic nephropathy: Pregnancy performance and fetomaternal outcome. Am J Obstet Gynecol 159: 56, 1998 |
|
Jovanovic R, Jovanovic L: Obstetric management when normoglycemia is maintained in diabetic pregnant women with vascular compromise. Am J Obstet Gynecol 149: 617, 1984 |
|
Reece E, Leguizamon G, Homko C: Stringent control in diabetic nephropathy associated with optimization of pregnancy outcomes. J Matern Fetal Med 7: 213, 1998 |
|
Schreiner GE: Diseases of the Kidney, p 390. Boston, Little, Brown & Co, 1963 |
|
Landesman R, Scherr L: Congenital polycystic kidney diseases in pregnancy. Obstet Gynecol 8: 673, 1956 |
|
Dalgaard OZ. Polycystic disease of the kidneys. In Strauss MB, Walt LG (eds): Diseases of the Kidney, p 1223. 3d ed. Boston, Little, Brown & Co, 1971 |
|
Ney C, Posner A, Ehrbek JC: Tubular adenoma of the kidney during pregnancy. Obstet Gynecol 37: 267, 1971 |
|
Ferris TF: Renal diseases. In Burrow GN, Ferris TF (eds): Medical Complications During Pregnancy, p 289. Philadelphia, WB Saunders, 1988 |
|
Manning FA, Platt LD, Sipos L: Antepartum fetal evaluation: Development of a fetal biophysical profile. Am J Obstet Gynecol 136: 787, 1980 |
|
Lip GYH, Beevers M, Churchill D et al: Effect of atenolol on birth weight. Am J Cardiol 70: 146, 1997 |
|
Lydakis C, Lip GYH, Beevers M et al: Atenolol and fetal growth in pregnancies complicated by hypertension. Am J Hypertens 12: 541, 1999 |
|
Briggs GG, Freeman RK, Yaaffee SJ: Drugs in Pregnancy and Lactation: A Reference Guide to Fetal and Neonatal Risk. 5th ed. Baltimore, Williams & Wilkins, 1988 |
|
Piper JM, Ray WA, Rosa FW: Pregnancy outcome following exposure to angiotensin-converting enzyme inhibitors. Obstet Gynecol 80: 429, 1992 |
|
Easterling TR, Carr DB, Davis C et al: Low-dose, short acting, angiotensin-converting enzyme inhibitors as rescue therapy in pregnancy. Obstet Gynecol 96: 956, 2000 |
|
Katz, AI, Davison JM, Hayslett JP et al: Pregnancy in women with kidney disease. Kidney Int 18: 192, 1980 |
|
Jungers P, Forget D, Henry-Amar M et al: Chronic kidney disease and pregnancy. Adv Nephrol 15: 103, 1986 |