Placental Protein Hormones
Authors
INTRODUCTION
The human placenta and fetal membranes have a large and diverse repertoire of secretory products including protein, glycoprotein, and steroid hormones as well as growth factors and cytokines.1 Many of the hormones produced by these extraembryonic tissues are identical or similar in structure and function to hormones secreted by the hypothalamus, pituitary, and ovaries. The maternal decidua also assumes important endocrine functions during gestation, secreting protein hormones, cytokines, growth factors, and growth factor-binding proteins. Therefore, many of the endocrine changes in the mother, intrauterine compartment, and fetus reflect the combined actions of secretions derived from the uterus, placenta, and fetal membranes. This chapter focuses on the key protein and glycoprotein hormones secreted by trophoblast cells of the placenta and chorion.
DIFFERENT TROPHOBLAST PHENOTYPES HAVE DIFFERENT ENDOCRINE FUNCTIONS
The trophoblast lineage, derived from the trophectoderm of the blastocyst, develops several distinct phenotypes during the process of implantation and placentation. Extravillous trophoblast cells, which invade into the endometrium during the first months of pregnancy, exist as mononucleated cells, variously called intermediate trophoblast cells, invasive trophoblast cells, X cells, or multinucleated giant wandering cells. Villous trophoblast cells are organized into arborizing chorionic villi with an outer layer of syncytiotrophoblast covering mononucleated cytotrophoblast (Langhans') cells.2 The trophoblast cells rest on a basement membrane surrounding the villous core, which contains mesenchymal cells, placental macrophages (Hofbauer cells), and fetal capillaries. The cytotrophoblast cells are the replicating trophoblast elements; the syncytium, which represents an end stage of trophoblast differentiation, is formed by the fusion of postmitotic cytotrophoblast cells. Early in pregnancy, the villi contain a continuous layer of cytotrophoblast cells, but as pregnancy advances, the cytotrophoblast layer becomes discontinuous. The syncytiotrophoblast is the major site of protein and steroid production and is in direct contact with maternal blood in the intervillous space. The chorion contains mononucleated cytotrophoblast cells. Each of these morphologic forms of trophoblast cells has a distinctive endocrine profile as assessed by immunohistochemistry and in situ hybridization histochemistry.
The endocrine activities of the different trophoblast forms are determined, in part, by differentiation pathways and the stage of gestation.3 The pattern of expression of the β subunit of human chorionic gonadotropin (hCG-β ) provides a good example of the functional variation of trophoblast cell phenotypes.4 hCG-β is produced initially by cytotrophoblast cells of the preimplantation blastocyst,5 and later at high levels by the syncytiotrophoblast of the first trimester placenta.3 The villous cytotrophoblast cells do not express hCG-β at this time. Later in pregnancy, secretion of hCG-β by the syncytiotrophoblast is markedly reduced.
The function of trophoblast cells is influenced by the extracellular matrix, endometrial products, trophoblast hormones/growth factors, the action of maternal hormones, and utero-placental perfusion, which also controls access to nutrients and oxygen.6, 7, 8, 9 Collectively, these and other factors determine the pathways of trophoblast cell differentiation, endocrine phenotype, and release of hormones into the maternal circulation.
PARACRINE INTERACTIONS BETWEEN VILLOUS TROPHOBLAST CELLS
The presence of hypothalamic hormones, such as gonadotropin-releasing hormone (GnRH), corticotropin-releasing hormone (CRH), growth hormone-releasing hormone (GH-RH), and somatostatin (somatotropin release-inhibiting factor ([SRIF]), in villous cytotrophoblast cells and of pituitary-like hormones, such as adrenocorticotropic hormone (ACTH), hCG, human chorionic somatomammotropin (hCS), and human growth hormone-variant (hGH-V) in the syncytiotrophoblast, and the ability of the “hypothalamic” polypeptides to stimulate or inhibit secretion of their respective pituitary-like hormones by placental tissue in vitro, have led to a model of paracrine interactions between cytotrophoblast and syncytiotrophoblast. In this model, cytotrophoblast cells are envisioned to regulate the secretory activity of the syncytiotrophoblast through paracrine interactions that mimic the hypothalamic-hypophyseal system.10 For example, GnRH is proposed to stimulate hCG secretion, GH-RH to stimulate hCS and hGH-V secretion, and SRIF to inhibit the release of hCS and hGH-V. Although this model is very attractive, the reader should be aware of the notable differences between trophoblast cells and pituitary cells. Syncytiotrophoblast cells, unlike anterior pituitary cells, do not have classic secretory granules in which preformed hormone is stored, ready for acute release in response to releasing factors.11 Placental hormones appear to be synthesized and rapidly secreted, possibly through a constitutive-like pathway. Therefore, many of the factors that influence trophoblast hormone production may act primarily to stimulate hormone synthesis or to control trophoblast cell differentiation.
PLACENTA-SPECIFIC PATTERNS OF GENE EXPRESSION
A number of protein hormones are synthesized only by cells of the trophoblast lineage or in small quantities by other cell types, particularly neoplastic cells.11, 12 The genetic elements that determine the trophoblast-specific pattern of expression of these genes have yet to be fully elucidated. However, numerous epigenetic phenomena, including histone modifications and DNA methylation, appear to influence gene expression significantly in the human placenta. Epigenetic modifications regulate trophoblast cell differentiation and invasion in the human placenta.13, 14, 15 Enzymes involved in these processes include DNA methyltransferases, histone deacetylases, histone acetylase, histone methyltranferases, and the methyl-binding domain protein. Moreover, the placenta has a large number of imprinted genes, which are methylated according to whether they are inherited from the mother or the father. Confined placental mosaicism of autosomal trisomies have been reported and must be considered when clinical geneticists evaluate chorionic villus samples. Most importantly, continued investigation of epigenetic regulation in the placenta at the earliest stages of gestation is warranted to elucidate key reproductive events that determine fetal outcomes.
Other mechanisms involving transcriptions factors that govern trophoblast gene expression are exemplified by regulation of the hCG-α and hCG-β genes and genes of the hCS/hGH cluster. The hCG-α gene is normally expressed in pituitary gonadotrophs and thyrotrophs and in primate trophoblast cells. The promoter of the hCG-α gene contains tandem cyclic adenosine monophosphate (cAMP) response elements (CREs), an upstream regulatory sequence, and a junctional response element (JRE), which specify trophoblast expression of the α subunit gene.16, 17, 18, 19 In trophoblast cells, the CREs bind CRE-binding proteins such as AP-1, the recruitment of which can be stimulated by epidermal growth factor and adenylate cyclase, and JREs bind placenta-specific trans-acting factors, such as the homeobox factor Dix 3.11, 16, 17, 18, 19 Together, this array of regulatory elements, in conjunction with tissue-specific expression of trans-acting factors, endows the promoter of the hCG-α gene with trophoblast specificity and maximal activity.17
The hCG-β genes evolved from the luteinizing hormone (LH) β subunit and have acquired new regulatory elements that favor their expression in the placenta rather than the pituitary gland. The β subunit genes also are transcriptionally regulated by cAMP. Unfortunately, less is known about the regulatory sequences within the β subunit genes, in part because highly differentiated cells are required to study their normal regulation; however, GnRH has been shown to regulate hCG expression from the placenta, and unique transcription factor-binding sites are expressed in the promoter region of the GnRH receptor gene in trophoblast cells, suggesting that paracrine regulation of hCG expression may be placenta-specific.20
The genes of four members of the hCS/hGH gene are expressed in the placenta, whereas hGH-normal (hGH-N) is expressed in the pituitary. Tissue-specific expression of these genes appears to be regulated by a locus control region located 15–32 kb upstream of the gene cluster.21 The placentally expressed genes contain novel sequences that are not present in the pituitary-expressed hGH-N gene.22 These unique sequences, when introduced into the promoter of the hGH-N gene, repress its expression in pituitary cells in an orientation-dependent manner. A nuclear protein binds specifically to these DNA sequences. These observations suggest that the expression of the placenta-specific hCS/hGH genes is silenced in nontrophoblast tissues by trans-acting factors binding to these novel gene sequences.
PLACENTAL CIRCULATION AND HORMONE SECRETION
The placenta has three circulations: maternal and fetal (active) and amniotic fluid (more or less static). The syncytiotrophoblast, a polarized epithelium, is in direct contact with maternal blood. This facilitates the introduction of syncytiotrophoblast products into the maternal circulation. Indeed, most of the hormones released by the syncytiotrophoblast enter the maternal blood with relatively little reaching the fetal circulation.
Placental, chorion laeve, and fetal hormones accumulate in amniotic fluid. The concentration of these hormones in amniotic fluid is determined by their rates of secretion and removal, the rates of metabolism of the hormones in situ, and changes in amniotic fluid volume. The active hormones that enter amniotic fluid can directly influence the fetus, particularly the fetal lungs, which are lavaged by amniotic fluid.
HUMAN CHORIONIC GONADOTROPIN
The Subunit Genes
hCG is the first hormone known to be elaborated by the conceptus.5 It is a glycoprotein heterodimer consisting of two dissimilar subunits, α and β , that are joined noncovalently. The single α subunit gene resides on the long arm of chromosome 6. The β subunit is encoded by a cluster of six transcriptionally active genes located on chromosome 19 (Fig. 1).12, 16 Three of the hCG-β genes are expressed at significant levels during pregnancy: gene 5 predominates, whereas genes 3 and 8 are transcribed at lower levels. There is very low expression of genes 1, 2, and 7. All six genes share extensive (> 80%) sequence homology with the LH-β gene, which is located in the same region on chromosome 19 and from which the hCG-β genes probably evolved.16, 17
Structure
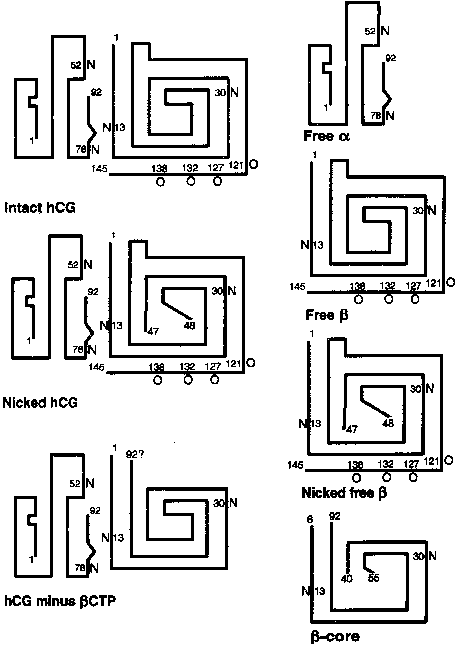
The α subunit is a 92-amino-acid polypeptide that is common to the pituitary gonadotropins and thyrotropin. The α subunit contains two asparagine-linked (N-linked) oligosaccharide side chains attached at amino acid residues 52 and 78 (Fig. 2). The trophoblast secretes two forms of the free α subunit, regular free α , which is the same as the α subunit of hCG, and a large free α subunit, which is hyperglycosylated with larger, more complex N-linked oligosaccharides that prevent its combination with the β subunit.18 Most of the free α subunit in maternal serum is large free α , although current immunoassays cannot discriminate between the two forms. The biological significance of these posttranslational modifications remains to be determined.
The 145-amino-acid hCG-β subunit is unique and distinguishes hCG from the other glycoprotein hormones. The major difference between LH-β (121 amino acids in length) and hCG-β is a 24-amino-acid C-terminal extension in hCG-β that results from a one base pair deletion and a two base pair insertion that removes a termination codon and produces an extended open reading frame. The C-terminal peptide of hCG-β contains four serine-linked (O-linked) oligosaccharide chains (see Fig. 2). The C-terminal peptide of hCG-β does not have a major impact on the biological activity of the hormone in vitro, but it markedly increases the half-life of holo-hCG in vivo.19 The β subunit also contains two N-linked oligosaccharides at amino acid positions 13 and 30.
The Role of Carbohydrates in the Function of hCG
hCG contains approximately 30% carbohydrate by weight. The carbohydrate chains usually terminate with sialic acid, the removal of which substantially reduces the half-life of hCG in the circulation by enhancing clearance of the hormone by the asialoglycoprotein receptor. There is considerable heterogeneity in glycosylation of hCG during normal pregnancy. Carbohydrate heterogeneity is also marked in hCG produced by the trophoblast of gestational trophoblast disease (moles and choriocarcinomas).
Removal of N-linked oligosaccharides on the α or β subunit, or both, has little effect on the binding of hCG to the LH/hCG receptor; however, the deletion of certain of these N-linked oligosaccharides affects intracellular assembly of the subunits and secretion of hCG.20 Additionally, the N-linked oligosaccharide at residue 52 of the α subunit is critical to signal transduction of the hCG hormone by stimulating cAMP accumulation and steroidogenesis in target cells.21, 22, 23
Synthesis and Secretion During Pregnancy
hCG is produced by trophectoderm in the preimplantation embryo.5 hCG can be detected in maternal serum 8–10 days after ovulation in a fertile cycle, which coincides with trophoblast formation and implantation of the blastocyst into the uterine wall (Fig. 3).16 As the trophoblast organizes into chorionic villi during the first trimester, hCG production is localized to the syncytiotrophoblast. Although hCG-β gene transcription can be detected in cytotrophoblast cells, synthesis of hCG-β and intact hCG (both subunits in their dimeric form) is restricted to the villous syncytiotrophoblast after 6 weeks of gestation.16 The factors responsible for coupling trophoblastic differentiation with the synthesis of hCG have not been identified.
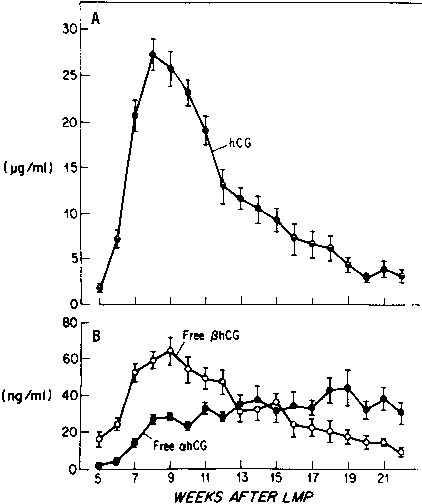
Most of the hCG produced by the syncytiotrophoblast is released into the maternal circulation. Maternal serum hCG levels increase progressively and reach a peak at 8–10 weeks' gestation. Initially, hCG concentrations double every 1.4 days; by the 5th week of pregnancy, the levels double more slowly (every 2.3 days).24, 25 After 10 weeks of pregnancy, placental hCG secretion declines to comparatively low levels, which are maintained until term. hCG concentrations in maternal blood are generally higher in multiple gestation pregnancies, reflecting a greater trophoblast mass.26 Higher hCG levels are also found in pregnancies complicated by chromosomal abnormalities, which permits clinicians to measure maternal serum hCG levels as one component of multiple-marker screening.27, 28, 29, 30 There is, however, no correlation between hCG levels in early pregnancy and fetal sex or birth weight.
Regulation of hCG Synthesis
Several models have been advanced to account for the biphasic pattern of placental hCG production during pregnancy, including differentiation-modulated gene expression, the sequential action of stimulating and inhibiting factors, and autocrine control by hCG itself. Boime and others have suggested that the formation of syncytia was linked to hCG production.16, 31 Observations consistent with this idea include the finding that isolated cytotrophoblast cells increase hCG secretion as they spontaneously fuse to form syncytial structures.32 However, the mononucleated trophectoderm of the blastocyst produces hCG before implantation, and the formation of syncytia and hCG expression can be dissociated in vitro.2 Therefore, the formation of a syncytium is not an absolute prerequisite for hCG expression, and the factors responsible for coupling trophoblast differentiation with synthesis of hCG remain obscure.16
hCG secretion may also be under the control of tropic factors. A number of substances have been reported to increase hCG production by trophoblast cells or placental explants, including (placental) GnRH,33 activin,1 epidermal growth factor,34 colony-stimulating factor-1 (CSF-1),35 retinoic acid,36 agents that stimulate adenylate cyclase,34, 37, 38 and the cytokines interleukin-1 and interleukin-6.39, 40 These factors act by increasing hCG subunit gene transcription or by increasing the stability of the hCG subunit mRNAs, and possibly by enhancing secretory mechanisms. As described above, cAMP, CRE-binding proteins, and other trans-acting factors control α subunit transcription by interacting with CREs, JREs, and upstream response elements in a combinatorial fashion.12, 34, 36, 37, 41 The hCG-β genes are also transcriptionally regulated by cAMP, but respond more slowly.42 The cAMP response elements in the hCG-β gene promoters are different from those of the hCG-α subunit gene. The activation of cAMP-dependent protein kinase, however, appears to be essential for the stimulation of both α and β subunit genes' cAMP-responsive enhancer elements.34 For example, the trans-acting factor CREB, which binds to the CREs, is phosphorylated in response to cAMP-dependent protein kinase activation. Glucocorticoids can also modify the response to cAMP, increasing the cAMP-stimulated hCG production in normal trophoblast cells.9 Another potential stimulator of trophoblast cAMP accumulation, which would lead to increased transcription of the α and β subunit genes, is hCG itself. hCG is known to activate placental adenylyl cyclase, and trophoblast cells express LH/CG receptors.43, 44, 45 Thus, a positive feedback might exist whereby low levels of hCG augment its own production in an autocrine fashion.44, 45 High levels of hCG, however, suppress trophoblast LH/CG receptor expression. This concentration-dependent effect of hCG on trophoblast cells may account, in part, for the initially rapid rise in hCG production through positive feedback effects of low levels of hCG on trophoblast cells in early pregnancy and the subsequent reduction in hCG expression after hCG levels rise to peak concentrations.
The placenta produces GnRH and possesses GnRH receptors, but these receptors have a lower affinity for GnRH than do pituitary GnRH receptors.33 Placental tissue responds to GnRH in vitro through a calcium-mediated second messenger system with increased hCG secretion.46 The immunodetection of GnRH in villous cytotrophoblast cells, the correspondence of the peak of placental hCG secretion with the greatest abundance of villous cytotrophoblast cells relative to syncytiotrophoblast, and the greater responsiveness to GnRH by first trimester placental tissue than by term placenta, are consistent with the idea that cytotrophoblast-derived GnRH governs syncytiotrophoblast hCG secretion.33 However, administration of GnRH to pregnant women does not increase serum hCG levels.47 The failure of exogenous GnRH to affect hCG levels in vivo may be attributed to the fact that placental GnRH receptors have a lower affinity for this releasing factor compared with pituitary GnRH receptors and would not be activated at the concentrations of GnRH administered or because the trophoblast GnRH response systems are saturated by endogenous hormone.
Placental hCG secretion is also subject to negative regulation. Specific transcription factors including OCT-4 and Jun suppress transcription of the α and β subunit genes,48, 49 and protein and steroid hormones as well as other substances (e.g. peroxisome proliferators) reduce hCG secretion.50 In fact, the ligand-dependent nuclear receptor peroxisome proliferator-activated receptor-gamma is expressed in trophoblast cell lines and has opposing, ligand-specific effects on placental differentiation, which likely affects the expression of the α and β subunit genes.50 Petraglia and colleagues proposed that inhibin and activin, also produced by the placenta, contribute to the biphasic pattern of hCG secretion.51In vitro studies carried out by these investigators indicate that inhibin and follistatin block GnRH stimulation of hCG secretion, whereas activin stimulates hCG secretion and may promote trophoblast differentiation. Others have reported that inhibin only suppresses hCG secretion by term placental tissue, but not in the first trimester.52 Progesterone reduces hCG release by placental explants, and it has been suggested that the decline in hCG secretion after the 10th week of gestation is coupled with increasing placental progesterone biosynthesis.53, 54 Finally, pro-inflammatory mediators also have been reported to inhibit hCG secretion,55 and pathogens that induce an inflammatory response may impair trophoblast invasion and placental function.56, 57
Integration of Factors Controlling hCG Secretion
Based on the existing literature, it is possible to construct a model in which stimulators working in a paracrine or autocrine mode such as GnRH, activin, and hCG itself increase hCG synthesis, whereas inhibin and progesterone suppress it. Early in pregnancy, placental GnRH and positive feedback effects of hCG on trophoblast function may cause increasing hCG secretion; as hCG levels peak and placental progesterone secretion rises, hCG secretion would be reduced. This concept, however, is based exclusively on in vitro studies and does not fully account for the differential patterns of hCG-α and hCG-β gene expression during pregnancy. Furthermore, only a fraction of newly synthesized hCG is stored in secretory granules and is responsive to secretagogues, whereas most hCG is released constitutively. Thus, the magnitude of induced hCG release is much less than GnRH-induced LH secretion by the pituitary gland, and construction of an integrated model for control of hCG synthesis/secretion remains elusive.16
Circulation of hCG Subunits in Maternal Blood
Free hCG-α and hCG-β subunits are present in maternal blood during pregnancy (see Fig. 3).58, 59, 60 These free subunits are secreted from trophoblast cells; they are not derived from the dissociation of holo-hCG. Over the course of pregnancy, placental hCG-α production is more than fivefold that of holo-hCG and hCG-α. Significant quantities of the hCG-α subunit are present throughout pregnancy, whereas concentrations of holo-hCG and free hCG-β subunit fall to relatively low levels during the second trimester. These observations suggest that hCG-β subunit synthesis limits holo-hCG formation.
When the hCG-α subunit does not combine intracellularly with hCG-β , it undergoes posttranslational modifications leading to a different pattern of N-glycosylation (more branched structures) and increased sialic acid content, which distinguishes secreted free hCG-α (large free α) from the α subunit of holo-hCG (regular α subunit).61, 62 The pattern of glycosylation of large free hCG-α subunit changes with increased incorporation of more highly branched fucosylated oligosaccharides as gestation progresses.63, 64 Although large free α will not combine with the β subunit to form holo-hCG, the biological significance of these glycosylation patterns remains to be determined.
Free hCG-β, as a percentage of total immunoreactive hCG, is high in very early pregnancy, accounting for as much as 50% of total immunoreactive hCG during the first few weeks of pregnancy, and 4–10% of total immunoreactive hCG between 4 and 6 weeks of gestation. However, from 8 weeks of pregnancy to term, free hCG-β is 1% or less of the total hCG-β immunoreactivity in serum.58, 59, 60
Metabolism and Clearance of hCG and Its Subunits
The clearance of holo-hCG from the circulation has two components: a fast component (approximately 7 hours) and a slow component (approximately 39 hours).65 The half-life of holo-hCG is considerably longer than that of LH, primarily because of the glycosylated C-terminal peptide of the β subunit. The metabolic clearance rate of holo-hCG, or volume of plasma cleared of hCG per unit of time, is approximately 4.4 L/day. Renal clearance, a major pathway of removal of hCG from maternal circulation, is unchanged during pregnancy. Approximately 30% of the cleared hormone appears more or less intact in the urine. The remainder is removed through nonrenal mechanisms. Because the rate of holo-hCG clearance does not change significantly during pregnancy, steady-state hCG levels in maternal blood are directly related to hCG synthesis and secretion.
The half-lives of free hCG-α β and hCG-β are short compared with that of holo-hCG.65 The fast and slow components for hCG-α and hCG-β subunits have been estimated to be 13 and 94 minutes and 43 and 239 minutes, respectively. Thus, the metabolic clearance rate for hCG-α is about threefold greater than that for hCG-β , but the free subunits are eliminated at a rate that is 10 to 30-fold faster than that of holo-hCG. Although the metabolic clearance rates of the free subunits are much more rapid than the clearance rate of holo-hCG, less than 1% of the free subunits are excreted in urine; thus, rapid renal excretion does not account for their shorter half-lives.
The hCG-β subunit is subject to proteolysis. Nicking of the hCG-β subunit between residues 47 and 48 or less commonly, between residues 43 and 44 or 44 and 45, markedly reduces biological activity (see Fig. 2).18 Nicked hCG molecules typically account for 10% to 20% of the hCG molecules present in maternal serum and urine. Further catabolism of the nicked β subunit in the maternal kidney produces the β core fragment. A unique form of the β subunit, consisting of hCG-β residues 6–40 with disulfide bond links to residues 55–92, the β core fragment accounts for as much as 90% of immunoreactive hCG-β in urine during pregnancy.66, 67 Although the β core fragment is a catabolite of hCG that is generated largely in the kidney, there is a possibility that some of this material originates from the placenta.68 In fact, placental macrophages may degrade hCG into nicked hCG and the β core fragment, thereby preventing intact hCG from entering the fetal circulation and deranging the developing fetal endocrine system.
hCG Actions: The Conceptus' Signal to the Ovary
The primary role of hCG in the maternal organism is to serve as a signal to the ovary to maintain the corpus luteum, which would regress if it were not rescued by hCG. hCG acts on the luteal cell LH/CG receptor, which activates adenylyl cyclase and possibly other signal transduction mechanisms that lead to maintenance of the steroid machinery. It appears that exponentially increasing amounts of hCG are required to prolong the functional lifespan of the corpus luteum, which explains why the corpus luteum survives early pregnancy but regresses during unfertilized menstrual cycles, despite the fact that LH and hCG have similar actions on the corpus luteum.69 Thus, administration of antisera to hCG in early gestation causes pregnancy termination in primate models, and immunization against hCG epitopes may be an effective contraceptive vaccine.70 Other roles for hCG have been suggested based on the discovery of LH/CG receptors in the fallopian tube, endometrium, and myometrium. Although hCG produced by the primate blastocyst appears to modulate decidualization of the maternal endometrium prior to implantation, the presence of LH/CG receptors in human endometrial cells in vitro is disputed, and treatment of these cells with hCG does not promote decidualization.71, 72 Thus, the signal transduction pathway by which hCG may affect decidualization remains to be elucidated. In the myometrium, hCG was reported to reduce the amplitude but increase the frequency of contractions of human myometrial strips collected during the proliferative phase of the cycle, providing evidence that the hCG receptors detected in myometrium are functional.73
There is abundant clinical evidence that hCG is a weak thyrotropin (TSH) agonist.74 The hormone, particularly certain glycosylated isoforms, binds to TSH receptors and stimulates thyroid adenylyl cyclase.74, 75 Hence, one of the factors contributing to increased maternal thyroxine secretion and decreased maternal thyroid stimulating hormone secretion during pregnancy may be hCG,76 which may cause hyperemesis gravidarum during pregnancy and clinical hyperthyroidism during molar pregnancies.74
Actions of hCG on Extraembryonic and Fetal Tissues
As noted above, hCG may be an autocrine/paracrine regulator of trophoblast function in a concentration-dependent fashion.43, 44, 45 In addition to the previously described effects of hCG on its own expression, there are several reports of hCG stimulation of placental progesterone synthesis.77 LH/CG receptors have also been detected in fetal membranes and umbilical cord, but the physiologic functions of the receptors in these locations are unknown.
hCG derived from the trophoblast or possibly produced by fetal kidneys or other fetal tissues may regulate fetal testicular testosterone secretion, which is needed to drive differentiation of the internal and external genitalia.78 hCG may also stimulate dehydroepiandrosterone production by the fetal adrenal gland.
A Biological Role for Free hCG Subunits?
Although it is generally believed that the free hCG subunits do not have important biological functions, experiments have linked the α subunit to growth of nontrophoblastic tumors.79 This is of particular interest, given the not uncommon expression of the α subunit gene by malignant cells. Neutralization of α subunits by antisera or suppression of hCG-α synthesis by anti-sense nucleic acid sequences reduces growth of lung tumor cells. The mechanisms by which free α subunit affects tumor cell growth have not been elucidated, and it is not known whether similar actions of free α subunit could influence proliferation of normal or malignant trophoblast cells.
Free hCG-α has been shown to function synergistically with progesterone to stimulate the differentiation of cultured human endometrial cells to decidualized cells80 and to be a potent stimulator of decidual prolactin secretion in vitro.81 Given the proximity of the trophoblast to the decidua and the substantial levels of free α subunit produced, it is reasonable to propose that free hCG-α could play a key role in controlling decidual formation and prolactin release in vivo.
Standardization of hCG Assays and Assay Specificity
hCG does not exist in biological fluids in a single molecular form (see Fig. 2). There is a spectrum of glycosylation patterns, and some of the circulating immunoreactive hCG undergoes proteolytic cleavage, including nicking.18, 82 The different molecular forms of hCG and its subunits bind to antibodies with different affinities. Most commercial hCG assays employ multiple antibodies to different sites on holo-hCG, free β subunit (nicked or non-nicked), and the β core fragment. In sandwich assays, one antibody is used to capture hCG at a specific site, whereas a second labeled antibody is used to detect or quantitate captured molecules. This technique improves the specificity of hCG assays, but the clinician must recognize that different kits use a wide variety of antibody combinations. Thus, discordant results can be obtained when two different immunoassays are carried out on a single specimen because the different antibodies detect various molecular forms of hCG that may be present in the sample with different efficiencies.
Because there is no single molecular form of hCG, assays must be calibrated to reference preparations of the hormone. Two different reference preparations have been widely employed for hCG assay standardization: the Third International Standard, formerly known as the First International Reference Preparation (IRP) of hCG for Immunoassay (1974), and the Second International Standard of hCG for Bioassay (1964).82, 83 These materials are not equivalent, having been characterized for immunoassays and bioassays, respectively. Both standards were made from the same large preparation of completely pure hCG, and 1 mg of the Second International Standard corresponds to 5.7 IU of the Third International Standard, whereas 1 mg of the Third International Standard equals 12 IU.83 Therefore, immunoassays standardized to the Third International Standard give values in international units that are approximately two times the value of immunoassays standardized to the Second International Standard.84 The hCG levels given in this chapter are based on the Third International Standard.
Although any of the hCG assays are excellent for the detection of normal pregnancy, the development of highly purified hCG immunoassays and standards (i.e. intact nick-free hCG, nicked hCG, or hCG subunits) may be more important for abnormal pregnancies.18 In some cases, such as gestational trophoblastic disease or Down syndrome pregnancies, only nicked hCG or only free β subunit is present in the circulation. Therefore, clinicians must be aware of what an assay is detecting, and immunoassays should be labeled appropriately.
Pregnancy Tests
The detection of hCG in serum or urine serves as the basis of contemporary pregnancy tests. Current serum hCG radioimmunoassays have a sensitivity as low as 2–10 IU/L and are able to detect pregnancy when implantation occurs, or approximately 8–10 days after ovulation. Urine hCG assays generally detect hCG concentrations as low as 25–50 IU/L, and results may be positive as early as 3–4 days after implantation or 10–14 days after ovulation. Ninety-eight percent are positive within 7 days after implantation.85
Monitoring Early Pregnancy
Quantitative hCG assays can provide an estimate of gestational age, and sequential monitoring of maternal serum hCG provides a biochemical index of growth of the conceptus.85 During the first 6 weeks of normal pregnancies, hCG titers in maternal serum double (100% increase) every 1.3–2 days. This pattern of hCG increase in maternal serum is abnormal in pregnancies that subsequently fail or when implantation of the embryo occurs at an ectopic site.24, 86 By determining maternal hCG levels and assessing the uterus and adnexa by transvaginal ultrasonography, one can possibly distinguish between intrauterine and ectopic pregnancies once hCG titers have reached certain threshold values.86, 87 The discriminatory zone is defined as the hCG level above which a gestational sac can be identified by ultrasound.87 At most institutions where transvaginal ultrasound is used, the discriminatory zone ranges from 1000–1500 IU/L. This level is achieved in a normal pregnancy approximately 1 week after the missed menses or 5 weeks after the last menstrual period. In a recent decision analysis, the most efficient method of diagnosing ectopic pregnancy (e.g. no ectopic pregnancy diagnoses missed, fewest intrauterine pregnancies interrupted) was determined to be transvaginal ultrasound followed by quantitative serum hCG measurements when ultrasound is nondiagnostic.87 In Table 1, a summary of events in early pregnancy observed by ultrasound is presented, together with the corresponding hCG levels.
Table 1. Events in early pregnancy observed by vaginal ultrasound and the corresponding mean human chorionic gonadotropin (hCG) levels
Author | Event | hCG [ IU/I] | |
Days | (Range) | ||
Cacciatore et al: Br J Obstet Gynaecol 97:899, 1990
| Gestational sac 1–3 mm | 31 | 730 (432–935) |
Yolk sac apparent | 36 | 4103 (1120–7280) | |
Heart action seen | 41 | 12,050 (9280–22,950) | |
Bernaschek et al: Am J Obstet Gynecol 158:608, 1988 | Gestational sac seen |
| > 600 |
Nyberg et al: Radiology 167:619, 1988
| Gestational sac seen | ||
20% of cases |
| < 1000 | |
80% of cases |
| 1000–2000 | |
100% of cases |
| > 1000 | |
Fossum et al: Fertil Steril 49:788, 1988
| Gestational sac seen | 34.8 ± 2.2 | 1398 ± 155 |
Fetal pole seen | 40.3 ± 3.4 | 5113 ± 298 | |
Fetal heart seen | 46.9 ± 6 | 17,208 ± 3772 | |
Daya et al: Can Med Assoc J 144:441, 1991
| Yolk sac first seen | 36 | 1900 |
Yolk sac always seen | 40 | 5800 | |
Heart action first seen | 41 | 9200 | |
Heart action always seen | 46 | 24,000 | |
(Modified from Chard T: Pregnancy tests: A review. Hum Reprod 7:701, 1992.)
The presence of hCG in the blood of a woman who is or has been pregnant reflects the presence of viable trophoblast tissue or the kinetics of hCG clearance, respectively. The time for clearance depends in part on the level of hCG. For example, hCG can be found in maternal serum or urine up to 24 days after normal delivery. However, because of the high levels of hCG in the maternal circulation during the first 13 weeks of pregnancy, hCG may be detected in maternal blood or urine for as many as 60 days after pregnancy termination in the first trimester.88
Prenatal Genetic Screening
Measurements of serum hCG and its subunits in conjunction with alpha-fetoprotein, inhibin-A, and plasma unconjugated estriol have been employed in the prenatal diagnosis of chromosomal abnormalities in so-called multiple marker screening (MMS).89, 90 In pregnancies in which the fetus has trisomy 21, maternal serum hCG and inhibin-A levels are frequently elevated and α-fetoprotein and unconjugated estriol levels are frequently reduced at or after 15 weeks of pregnancy. In pregnancies in which the fetus has trisomy 18, the maternal serum level of hCG, estriol, and α-fetoprotein are frequently lower than expected.91 Combined measurements of hCG, α-fetoprotein, inhibin-A, and unconjugated estriol are able to detect approximately 80% of fetuses with Down syndrome, with a false-positive rate of approximately 5%.82, 89 More recently, combined first and second trimester maternal serum markers including hCG, in combination with ultrasound screening measuring the fetal nuchal thickness at 11–14 weeks' gestation, has achieved 95% detection rates for Down syndrome.29, 30 The usefulness of these maternal serum markers may be a result of altered growth of the extraembryonic tissues when chromosomal abnormalities are present.
Because the maternal serum holo-hCG and free hCG-β levels are markedly elevated when the fetus has trisomy 21, hCG is considered the most sensitive individual marker for fetal Down syndrome.92 Higher than usual maternal serum proportions of nicked hCG and free β subunit, and β core fragment in maternal urine, also are associated with Down syndrome pregnancies, but there does not appear to be any benefit to using free hCG-β versus holo-hCG in MMS.93, 94, 95 Integration of other hCG molecules, such as urinary hyperglycosylated hCG, novel maternal serum markers (i.e. inhibin, pregnancy-associated plasma protein A [PAPP-A]), and ultrasound findings, with traditional MMS analytes is under investigation.96, 97
Gestational Trophoblastic Disease and Nontrophoblastic Tumors
hCG measurements are used to diagnose and monitor therapy of gestational trophoblastic disease as well as numerous hCG-secreting nontrophoblastic neoplasms (Table 2). Molar pregnancies are associated with extremely high levels of hCG that may be greater than 1,000,000 IU/L. These concentrations are usually much greater than those found in women with choriocarcinomas. Trophoblastic disease samples typically contain unduly high proportions of nicked hCG and free β subunit, and in many cases, nicked hCG persists longer than non-nicked hCG after successful treatment.98 Thus, serial monitoring of hCG with appropriate immunoassay kits can provide an index of success of tumor extirpation and chemotherapy.
Table 2. Immunoreactive human chorionic gonadotropin in sera of patients with cancer
Tumor Type/Site | No. | Positive (%) |
Islet cell | 104 | 39.4 |
Gynecologic | 2010 | 28.9 |
Ovary | 633 | 28.6 |
Endometrium | 348 | 16.7 |
Cervical | 976 | 33.8 |
Vulva/vagina | 37 | 27 |
Carcinoid | 41 | 26.8 |
Gastrointestinal | 2165 | 18 |
Oropharynx | 298 | 14.8 |
Esophagus | 124 | 17.7 |
Gastric | 232 | 20.7 |
Small intestine | 24 | 16.7 |
Colon/rectum | 693 | 9.8 |
Liver | 281 | 22.1 |
Gallbladder | 26 | 30.7 |
Pancreas | 200 | 20 |
Pulmonary | 1365 | 17.4 |
Breast | 3031 | 16.8 |
Melanoma | 244 | 13.9 |
Genitourinary | 658 | 11.8 |
Kidneys | 119 | 6.7 |
Bladder | 176 | 23.3 |
Prostate | 363 | 8 |
Sarcoma | 136 | 11.8 |
Hematopoietic | 544 | 6.1 |
Lymphoma | 339 | 5.3 |
Miscellaneous | 576 | 10.4 |
Total | 11,213 | 18.0 |
(Modified from Braunstein GD: Placental proteins as tumor markers. In Heberman RB, Mercer DW [eds]: Immunodiagnosis of Cancer, pp 673–701. New York, Marcel Dekker, 1991.)
Among testicular tumors, approximately 5% of seminomas and 66% of nonseminoma germ cell tumors secrete immunoreactive hCG.99 hCG is detectable in blood in 10–16% of subjects with other nontrophoblastic malignancies, including a high percentage of patients with pancreatic islet cell tumors, epithelial ovarian tumors, carcinoids, breast and gastrointestinal cancers, and nonovarian gynecologic malignancies.99, 100 The level of hCG found in these instances is usually modest, being in the range of 10–340 lU/L.
hCG in Normal Nonpregnant Subjects
Immunoreactive hCG has been found in low concentrations in normal men and women.100, 101 This hCG is probably produced by the pituitary gland, and its secretion may be under GnRH regulation.102, 103 Normal men are reported to have serum immunoreactive hCG levels of 0.02–0.8 lU/L, and normal premenopausal women are reported to have levels between 0.02–0.2 lU/L. Postmenopausal women have higher levels, ranging from less than 0.02–2.8 lU/L. Therefore, the presence of low levels of immunoreactive hCG in an apparently healthy nonpregnant subject does not necessarily indicate the presence of an occult malignancy.
HUMAN CHORIONIC SOMATOMAMMOTROPIN AND HUMAN GROWTH HORMONE-VARIANT
The hCS/hGH Gene Cluster
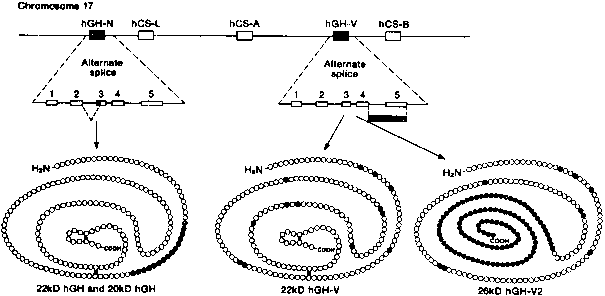
Human chorionic somatomammotropin (hCS, also referred to as human placental lactogen [hPL]), human growth hormone (hGH-N and hGH-V), and prolactin (hPRL) are members of a superfamily of protein hormones that regulate growth and development.104 The hCS, hGH, and hPRL precursor genes are closely related, but the hCS/hGH gene cluster is located on chromosome 17 (q22-24) whereas the hPRL gene is located on chromosome 6.105, 106 The hCS/hGH gene locus is composed of five linked genes that span 66 kb (Fig. 4). Each gene in the cluster is organized in the same transcriptional orientation and is composed of five exons and four introns.104
The hCS-A and hCSB genes (also referred to as hCS-1 [or hPL-A] and hCS-2 [or hPL-B] , respectively) are 98% identical at the nucleotide level, and their products differ in only one amino acid that resides in the signal peptide. Therefore, hCS-A and hCS-B encode an identical mature protein (hCS) that is 85% identical to hGH-N and 17% identical to hPRL. hCS is a 191-amino-acid single-chain protein that contains two disulfide bridges between cysteine residues, but no carbohydrate side chains. It is synthesized with a 26-amino-acid sequence that is cleaved co-translationally. A third hCS gene, called hCS-L (or hPL-L), gives rise to an mRNA that may encode a hCS-like protein of unknown function.107
The hGH-V gene is located between the hCS-A and hCS-B genes.104, 108 It is 93.5% identical to hCS at the nucleotide level and codes for a protein that shares identical amino acids with hCS at 167 of 191 positions. The hGH-V gene is expressed in the placenta, and hGH-V appears in maternal serum from the second trimester through term. The fifth gene, hGH-N, encodes pituitary hGH. It is not expressed in the placenta. The amino acid sequence of the hGHN protein differs from the hGH-V product in 13 residues (nonconservative substitutions) dispersed throughout the protein.
hCS Gene Regulation and Tissue Specificity
The hCS-A and hCS-B genes are expressed differentially during pregnancy.107, 108 They are transcribed at nearly equal levels at 8 weeks of pregnancy, but by term, hCS-A transcripts are approximately fivefold more abundant than hCS-B transcripts. Between 8 weeks and term, hCS-B mRNA increases 5 to 10-fold in villous tissue, whereas hCS-A mRNA rises 30-fold. The stability of hCS-B mRNA, however, is greater than that of hCS-A mRNA. Because the two genes encode identical mature proteins, the significance of this shift in expression is not clear. It may simply reflect the position of the genes in the hCS/hGH cluster and, therefore, their ability to respond to transcription activators.
Interactions of proteins with enhancer sequences most likely play an integral role in the high levels of placenta-specific hCS production. For example, the non-tissue-specific transcription factor Sp1 has an overlapping binding site with the pituitary-specific transcription factor Pit-1 in all genes of the hCS/hGH gene cluster.109 In the pituitary gland, where the Pit-1 concentration is high, it gains access to the hGH-N promoter by outcompeting Sp1. In the placenta, however, where the concentration of Pit-1 is minimal, Sp1 plays a crucial role in binding to upstream hCS promoters and stimulating gene transcription. Placental specificity of hCS expression may also be governed by an enhancer element located 2 kb downstream from the three hCS genes that is gradually activated as the syncytiotrophoblast is formed or by another 1-kb sequence that is located 2 kb upstream from the promoter regions of the hCS and hGH-V genes but is absent from the hGH-N gene.109, 110 This upstream sequence, “P,” is responsible for suppression of hCS gene expression in the pituitary gland.111 Finally, other transcription factors that appear to regulate the hCS-A or hCS-B genes include activator protein-2 and nuclear factor I (which is stimulated by mitogen-activated protein kinase), the latter of which binds to the “P” sequence and represses the hCS and hGH-V genes in pituitary gonadotropic cells.112, 113
Sites of hCS Synthesis
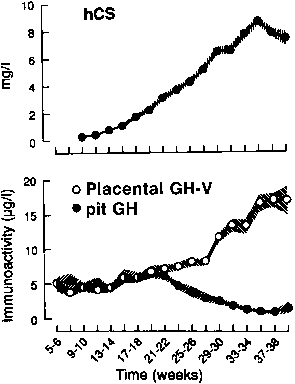
The syncytiotrophoblast and extravillous intermediate trophoblast cells, or X cells, are the sites of hCS synthesis, and hCS is one of the major secretory products of the trophoblast.104, 109 hCS can be detected by immunofluorescence in the syncytiotrophoblast by the second week after conception. At term, the placenta secretes 1–3 g of hCS per day, which is a rate considerably greater than any other polypeptide hormone and represents approximately 10% of total placental protein production (Fig. 5).114
Levels of hCS in Maternal and Fetal Blood and Amniotic Fluid
hCS is measurable in maternal serum by the third week after conception, and concentrations of hCS rise progressively until 34 weeks, reaching levels of 5–10 mg/L by term (see Fig. 5).115 The maternal serum levels of hCS parallel changes in placental mass. This is manifested as increased levels of hCS in multifetal gestations and an increased incidence of gestational diabetes in these pregnancies, owing to the diabetogenic effect of hCS.
hCS accumulates in amniotic fluid by routes that have not yet been clarified, but may include passive diffusion. The levels of hCS in amniotic fluid are similar to those of maternal serum in early pregnancy and increase gradually until term, but the increase is less than that observed in maternal serum. The concentrations of hCS in fetal blood are 300 to 1000-fold lower than those in maternal serum because the syncytiotrophoblast has closer contact with maternal serum.116 hCS has a short half-life in maternal serum (10–30 minutes), and it rapidly disappears from the maternal serum after delivery.
Syncytiotrophoblast Mass as a Major Determinant of hCS Production
The hormonal and metabolic factors that regulate hCS secretion are not completely understood but are likely to include the differentiation of trophoblast cells, transcriptional activation, and the action of secretogogues.104 Because hCS is not stored in classic secretory granules, regulatory factors are more likely to affect hCS synthesis than its secretion. As stated above, the increased capacity of the placenta to elaborate hCS is directly related to syncytiotrophoblast mass.115 However, the amount of hCS mRNA remains constant in individual syncytiotrophoblast cells throughout pregnancy.117 Instead, the expression of terminal placental differentiation markers, such as hCS-L mRNA, is decreased at term in placentas with modified differentiation patterns (i.e. placentas from diabetic pregnancies) compared with normal term placentas.118 Consequently, impaired placental differentiation or postdifferentiation impairment of normal placental function appears to affect hCS gene transcription.
Control of hCS Secretion by Human Growth Hormone-Releasing Hormone, Somatostatin, and Neurotransmitters
There is evidence suggesting that GH-RH, which is found in cytotrophoblast cells, stimulates hCS production and that SRIF, which is also found in cytotrophoblast cells, inhibits hCS release. Supporting this hypothesis are the findings that GH-RH stimulates hCS secretion by trophoblast cells in vitro, and that SRIF immunostaining in the placenta is most intense in early pregnancy when hCS production is lowest, and it declines as gestation progresses.119, 120 Thus, the inverse relationship between somatostatin-immunostaining intensity and hCS secretion raises the possibility of an inhibitory effect of SRIF on hCS production, although SRIF does not decrease hCS secretion by term placental cells.121 Neurotransmitters that regulate hPRL and hGH-N secretion do not appear to affect hCS production in vivo. The dopamine agonist, bromocriptine, which profoundly suppresses pituitary prolactin secretion, has no effect on hCS levels in early pregnancy.122 However, in vitro studies suggest roles for dopamine and β-adrenergic agonists in regulating hCS production.123, 124
Other Secretogogues Controlling hCS Secretion: Endocrine/Autocrine/Paracrine Factors and Lipoproteins
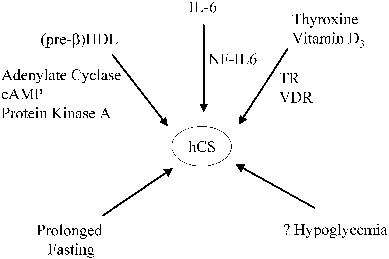
Several maternal and trophoblast products are known to regulate hCS secretion, and it is likely that other factors are involved as well (Fig. 6). For example, a positive thyroid hormone response element has been discovered in the promoter region of hCS, and maternal thyroid hormone appears to up-regulate hCS gene expression by trophoblast cells.110 Interleukin (IL)-1, IL-6, and 1,25-dihydroxyvitamin D are synthesized and secreted by the syncytiotrophoblast, and each of these factors stimulates the synthesis of hCS by trophoblast cells.104 Thyroid hormone and vitamin D stimulate hCS gene expression by the binding of thyroid hormone β receptor and vitamin D receptor, respectively, to the composite nuclear hormone receptor site on the hCS promoter.125 The action of IL-6 is mediated by the transcription factor NF-IL6, which binds to three consensus NF-IL6 elements in the hCS promoter.104, 113, 126
|
Recent studies suggest that high-density lipoproteins (HDL) also stimulate hCS synthesis and secretion.104 The stimulation appears to be attributed primarily to pre-β HDL, which is a minor component of the total HDL in the adult circulation but which contains a much higher apolipoprotein (apo) A-I/lipid ratio than the major circulating form (α -HDL). Apo A-I is the major apoprotein component of HDL, and HDL (with or without its lipid constituent) appears to increase hCS synthesis.127 hCS and pre-β HDL concentrations normally increase in parallel during pregnancy, during which time pre-β HDL concentrations increase from 3% to 4% of the total HDL in the first trimester to approximately 20% at term.104, 128 Conversely, pre-β HDL, apo A-I, and hCS concentrations are all decreased in cases of preeclampsia and fetal growth restriction.129 Apo A-I stimulates adenylate cyclase activity in trophoblast cell membranes, and cAMP and protein kinase pathways are involved in signal transduction of apo A-I-mediated hCS release.130, 131
Despite an extensive literature on the in vitro effects of all these agents, there is little evidence to date to support the idea that apolipoproteins or other factors have a physiologic role in the regulation of hCS secretion in vivo.
The Influence of Maternal Metabolic Factors on hCS Secretion
A number of observations indicate that placental hCS secretion is under maternal metabolic regulation. Prolonged fasting in the first half of pregnancy (in studies using patients scheduled for elective termination) has been reported to lead to an increase in hCS in maternal serum.132, 133 However, the specific mechanisms underlying the fasting-induced changes in maternal hCS are unknown, and these mechanisms do not seem to be operative during shorter periods of fasting.104 Conflicting data have been published on the effects of hyperglycemia and hypoglycemia on hCS levels. Some authors have concluded that intravenous infusions of glucose causing hyperglycemia are associated with a transient decline in hCS, but ingestion of protein- or glucose-rich meals has not been found to have a marked effect on maternal hCS levels. Moreover, in reports describing changes in hCS levels in response to glucose infusions, the changes have been modest, occurring in temporal patterns not fully consistent with the alterations in glucose concentrations.134, 135 In addition, acute and chronic changes in extracellular glucose concentrations have been shown to have no consistent effects on hCS secretion in vitro.104 Thus, the existing data collectively suggest that glucose, insulin, amino acids, and lipids are not major short-term regulators of hCS secretion, and the effects of maternal hypoglycemia or hyperglycemia on hCS secretion are less important than the effects of hCS on maternal glucose metabolism.
The Biological Activities of hCS
The biological activities of hCS have been explored relative to hGH and hPRL. Most emphasis has been placed on the regulation by hCS of maternal lipid and carbohydrate metabolism, fetal somatotropic properties, and maternal mammary gland function.
Effects of hCS on Lipid Metabolism
The actions of hCS on lipolysis have been examined comprehensively. In short, hCS increases lipolysis and maternal serum levels of free fatty acids, which may be used by the mother as an energy source during times of fasting. When maternal fatty acids are used for fuel, ketone bodies are produced; these may cross the placenta and serve as an energy source for the fetus.
The majority of studies conducted in humans and animals have documented an increase in free fatty acids, ketones, and glycerol in serum after hCS administration.104 These findings are consistent with a stimulatory effect of hCS on lipolysis. Moreover, in vitro studies of human and rat adipose tissue and isolated adipocytes revealed stimulatory effects of hCS on lipolysis.136, 137, 138 hCS also has been shown to increase theophylline-stimulated lipolysis, suggesting that the hormone enhances adipocyte sensitivity to other lipolytic agents.138
The effects of hCS extend to increased glucose uptake by adipocytes and oxidation and incorporation of glucose carbon into fatty acids and glycerol.139, 140 These actions are insulin-like, and subsequent studies have indicated that hCS augments adipose sensitivity to insulin in rat models. It must be noted, however, that in many of the in vitro studies on adipose tissue, very high and in some cases unphysiologic concentrations of hCS have been used.
The net effect of the lipolytic activity of hCS, its actions to increase adipocyte sensitivity to other lipolytic agents, and its insulin-like effects on adipose tissue are to increase adipocyte basal metabolic activity, particularly turnover of free fatty acids. This is concordant with data indicating increased lipolysis and fatty acid esterification in human adipose tissue at the end of pregnancy. The consequences of these actions are blunted swings of energy source availability throughout pregnancy, increased fatty acid mobilization in the fasting state, and increased glucose uptake by adipocytes and subsequent fatty acid storage in the fed state.
Effects of hCS on Carbohydrate Metabolism
Adaptive changes that occur in pancreatic islet cells during normal pregnancy include an increase in β-cell division and a lowering of the threshold for glucose-stimulated insulin secretion.141 Most studies to date have been performed using rodent islets, but the available evidence strongly suggests that hCS and/or hPRL are responsible for up-regulating pancreatic islet cells, which results in enhanced insulin secretion during pregnancy.141, 142
Although hCS increases glucose uptake by adipocytes and enhances insulin secretion by pancreatic islet β-cells, the diabetogenic effect of pregnancy has been attributed to counterregulatory actions of hCS.104 Specifically, hCS induces glucose intolerance in diabetics and normal subjects, probably as a result of insulin resistance in the maternal liver, which is responsible for clearing a large fraction of plasma glucose for glycogen synthesis.143, 144 Thus, there may be tissue-specific responses to hCS. The mechanisms for insulin resistance have not been defined and may include direct effects of hCS or secondary effects of hCS mediated by nonesterified fatty acids.104, 144 However, hCS is unlikely to regulate the metabolism of glucose and lipids in response to short-term fluctuations in nutrient availability, because glucose, amino acids, and fatty acids exert little or no effect on maternal hCS levels.104, 145
In summary, the integrated role of hCS in modulating maternal intermediary metabolism is most likely of a long-term nature. It appears to dampen swings in maternal nutrient levels, providing for increased free fatty acids and ketones secondary to lipolysis and increased postprandial glucose levels secondary to peripheral insulin resistance. It must be remembered, however, that maternal metabolic changes during pregnancy are the result of the actions of many hormones, including sex steroids and glucocorticoids, and any of the proposed actions of hCS that have not been placed in the context of any changes in these other hormones may have to be reevaluated.
Is hCS a Fetal Growth Hormone?
Given the homology between hCS and hGH, there has been considerable interest in the potential role of hCS in fetal growth. Obviously, the metabolic effects of hCS in the maternal compartment could have important consequences for fetal growth. Moreover, hCS has been shown to induce angiogenesis in the placental microvasculature, which may have physiologic importance regarding the transfer of nutrients between the maternal and fetal circulation.146 However, whether hCS has a direct role in the fetal compartment is less certain.
Evidence that is compatible with a role for hCS as a growth hormone includes its anabolic effects in vivo and its in vitro effects in hGH bioassays. For example, hCS stimulates amino acid uptake, DNA synthesis, and insulin-like growth factor-I (IGF-I) production in human fetal myoblasts, fibroblasts, and hepatocytes in culture.104 These actions are blunted by an antiserum to IGF-I, suggesting that the somatotropic actions of hCS are mediated in part through the paracrine release of IGF-I. hCS also has been shown to stimulate DNA synthesis and insulin production in fetal pancreatic explants and to have growth-promoting effects in certain bioassays in rodents.147 hCS, however, is thought to be only 3% as potent as hGH in its somatotropic activity. Because levels of hCS in the fetus are quite low, fetal tissues would have to be very sensitive to hCS for this hormone to have a significant effect on fetal growth.
hCS and Mammary Gland Function
The role of hCS in mammary gland development and function in the perinatal period is unclear. In vitro, hCS acts on human breast epithelium to stimulate growth; it also stimulates DNA synthesis in epithelial cells in breast explants in nude mice.148, 149 hCS failed, however, to maintain established lactation in an in vitro system, whereas prolactin could be shown to do so. Thus, the effect of hCS on the mammary gland may be to stimulate cell proliferation, but not to enhance milk production.
Mutations in the hCS/hGH Gene Cluster
Several reports of normal gestation in the presence of low levels or absence of serum and placental hCS have been published. These cases were caused by partial or complete fetal hCS gene deletions.150, 151, 152, 153, 154 The women had normal glucose tolerance during pregnancy and normal placental function as assessed by measurements of hCG, estriol, and progesterone. They also experienced normal lactation after delivery, and their offspring were of normal size and had normal postnatal growth patterns. However, a more recent report describes children with severe intrauterine growth restriction who had deletions of the genes for hCS-A and -B and hGH-V.155 Therefore, these data suggest that a variable phenotype results when gene deletions within the hCS/hGH-V are present. Although hCS may not be required for normal pregnancy, fetal growth and development, and lactation, hormones with similar activities including hGH-V may have subsumed hCS functions. Indeed, in one well-studied case in which the hCS-A, hCS-B, and hGH-V genes were deleted, an hGH-hCS hybrid protein was detected that may have compensated for the absence of hCS and hGH-V. Moreover, the expression of hCS-L has not been explored.154, 156
Measurement of hCS as an Indicator of Fetal Well-Being
The placenta produces increasing amounts of hCS until 34 weeks of gestation, after which production plateaus. There is no circadian variation to hCS secretion, and it has a short half-life in the circulation. All of these factors make hCS attractive as a biochemical marker of placental function. In earlier studies, low levels of hCS were noted to be associated with intrauterine growth restriction and fetal distress; however, subsequent studies have found that hCS monitoring is not an effective screening test for potential fetal complications secondary to uteroplacental insufficiency.157, 158
Structure of the Human Growth Hormone-Variant Gene
Placental growth hormone (hGH-V) differs from pituitary growth hormone (hGH-N) by 13 amino acids. Because the amino acid differences are largely nonconservative, their biological properties may vary significantly.159 The hGH-N gene is alternatively spliced into mRNAs that produce the 22-kd major active hGH-N and a 20-kd variant that is lacking 15 amino acids from exon 3 (see Fig. 4). Two mature isoforms are also generated by alternative splicing of the hGH-V gene.109 The first isoform, the 22-kd hGH-V, consists of 191 amino acids and an N-glycosylation signal that yields two size variants, one that is glycosylated (25 kd) and the other that is nonglycosylated (22 kd).159 The second isoform, the 26-kd hGH-V2, is derived from alternative splicing of hGH-V pre-RNA that leads to the incorporation of its fourth intron into its fifth exon.109 This arrangement results in the loss of the N-glycosylation site and the formation of a hydrophobic tail that anchors hGH-V2 to the membrane of the syncytium.
hGH-V2 mRNA is expressed at levels 5–7% of hGH-V mRNA in the first trimester, but hGH-V2 mRNA increases to approximately 15–20% at term, suggesting a developmental pattern of alternative splicing of the hGH-V gene that could reflect changes in splice-site selection or mRNA stability.107, 108
Regulation of hGH-V Gene Expression
The hGH-V gene is expressed in the same pattern as are the hCS genes, but at levels that are several orders of magnitude lower than those of hCS (see Fig. 5).107, 108, 160 Although this parallel pattern suggests that similar regulatory mechanisms govern expression of these related genes, there is evidence from the analysis of hCS, hGH-V, and hCS-L gene expression in human trophoblast neoplasms that hGH-V and hCS-L are subject to different control mechanisms than hCS.161 Early in pregnancy, pituitary-derived hGH-N is measurable in maternal serum, and it is secreted in typical pulsatile fashion.162 hGH-V levels in maternal serum increase as pregnancy progresses, and it becomes the predominant hGH in maternal blood by the second trimester (see Fig. 5). hGH-V stimulates maternal IGF-I production, which inhibits hGH-N secretion through negative feedback action.159, 163 hGH-V appears to be secreted by the placenta at a relatively constant rate: levels do not fluctuate episodically or in a pulsatile fashion. Like hGH-N, hGH-V binds to a circulating hGH-binding protein, which is a secreted form of the hGH receptor. GRH has been detected in placental tissue, but it is not yet known whether hGH-V production is regulated by GRH.164
Biological Activities of Human Growth Hormone-Variant
hGH-V has high somatogenic and low lactogenic activities. It does not appear to have a direct effect on fetal growth, as hGH-V is not detectable in the fetal circulation and growth hormone receptors are sparse within the fetus.159 However, the somatogenic activities of hGH are mediated by maternal IGF-I, which is a peptide growth factor produced by most tissues that has multiple mitogenic effects on target cells, including increased amino acid transport, increased glucose uptake, enhanced nucleic acid and protein synthesis, and increased cell replication.164 Local and hepatic synthesis of IGF-I is stimulated by hGH-V, and increases in maternal hGH-V during pregnancy are paralleled by increases in IGF-I during the second half of gestation. Hence, hGH-V and IGF-I promote gluconeogenesis, lipolysis, and anabolism in the maternal liver and other organs, which augments nutrient availability for the fetoplacental unit. The physiologic role of hGH-V might also include a direct effect on placental development through an autocrine or paracrine mechanism, as suggested by the presence of specific growth hormone receptors on the syncytiotrophoblast.159, 160
The proposed somatogenic activities of hGH-V are supported by experimental and clinical evidence. HGH-V secretion is inhibited by glucose in vitro and in vivo, and significant increases in hGH-V levels can be induced during maternal hypoglycemia.145, 159 Additionally, preliminary studies suggest that maternal hGH-V levels are reduced in pregnancies complicated by fetal growth restriction.165
The role of hGH-V2 in pregnancy is not yet known. Its presumed location at the plasma membrane, however, raises the possibility that it functions in cell-to-cell communication.
LEPTIN
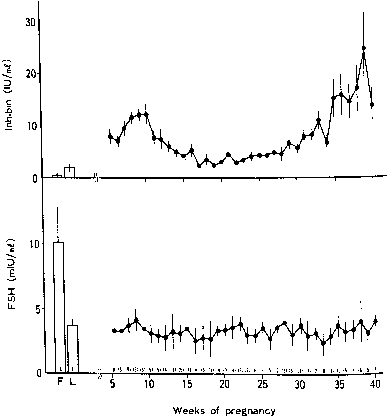
Leptin is the 16-kd protein hormone product of the obese (ob) gene that is secreted by adipose tissue. Leptin appears to be involved in energy homeostasis, and increased leptin levels have been correlated with adiposity in children and adults. Leptin mRNA has also been discovered in the human placenta, and it appears to be secreted primarily by the syncytiotrophoblast.166, 167 Most of the leptin produced by the placenta is released into the maternal circulation, but when compared with other placental hormones, a considerably higher proportion of leptin is released into the fetal circulation.168 Leptin levels are at least threefold higher in pregnant women than in nonpregnant obese women, and the rapid postnatal decrease in leptin levels in both the mother and the neonate is consistent with placental origin.167 Although autocrine regulation of leptin secretion from adipose tissue has been proposed, possibly through the inhibitory effects of tumor necrosis factor-α , regulation of leptin expression in the placenta has not been elucidated.169
Maternal leptin levels are correlated with measures of adiposity, and umbilical cord blood leptin levels are increased in large-for-gestational age neonates compared with average-for-gestational age and small-for-gestational age infants.167, 170, 171 In the mother, the role of placental leptin may be the accumulation of body fat, whereas in the fetus, insulin's anabolic actions may be mediated by leptin.170, 172 Although the importance of placental leptin production has not been clearly established, increased production of leptin appears to be involved in the adaptive response to placental hypoxia,173 and aberrations in maternal serum leptin levels have been associated with preeclampsia in singleton gestations and with growth discordance in twin pregnancies.174, 175
PLACENTAL CORTICOTROPIN-RELEASING HORMONE AND PROOPIOMELANOCORTINS
Structure and Sites of Synthesis of Placental CRH
The placenta produces a 41-amino-acid CRH molecule that is identical to hypothalamic CRH.176, 177 CRH mRNA is localized in syncytiotrophoblast, intermediate trophoblasts, amnion, the musculature of the umbilical vessels, and maternal decidua.178 However, the CRH peptide is not secreted until cytotrophoblast cells are fused together to form a syncytium, so the syncytiotrophoblast is the primary site of CRH production in late pregnancy.177
Patterns of CRH Secretion
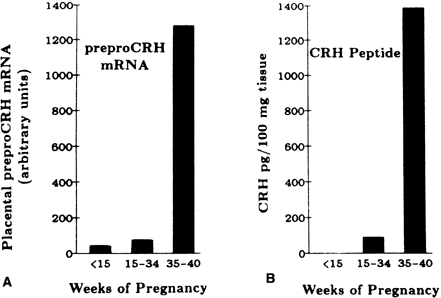
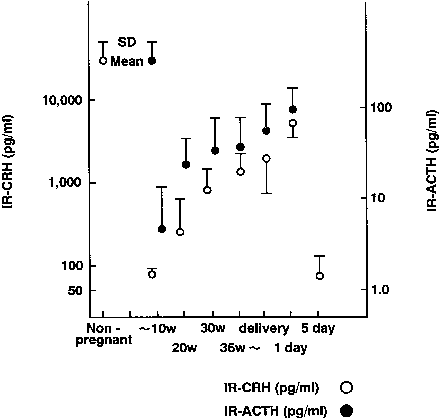
In nonpregnant women, plasma levels of CRH are low. Concentrations remain less than 10 pmol/L during the first trimester, but rise in the middle of the second trimester, reaching concentrations of approximately 55 pmol/L early in the third trimester.179, 180, 181 In the last weeks of pregnancy, CRH concentrations rise to between 250 and 300 pmol/L.
There is a 20-fold increase in CRH mRNA in the placenta during the last 5 weeks of pregnancy, which parallels the increase in placental CRH content and maternal serum CRH (Figs. 7 and 8).182, 183 Placental, maternal-fetal blood, and amniotic fluid CRH concentrations are highly correlated, with the exception that amniotic fluid CRH levels do not increase during labor, whereas maternal plasma CRH levels rise.184, 185, 186, 187 Within 20–30 minutes after delivery, maternal peripheral plasma and uterine venous plasma CRH levels decline to one half of the predelivery concentrations, and within 1–5 days after parturition, maternal plasma CRH concentrations are similar to those found in nonpregnant women.179, 182, 186
CRH-Binding Protein
Despite the increased levels of CRH in the maternal circulation, there is not a marked elevation in either maternal ACTH secretion or circulating glucocorticoids.177 The lack of CRH-induced ACTH secretion is the result of CRH inactivation by binding to a circulating 37-kd high-affinity binding protein (CRH-binding protein). The CRH-binding protein gene is expressed in liver, placenta, and brain, and it appears to protect the anterior pituitary preferentially from stimulation from placental but not hypothalamic CRH.188 CRH-binding protein and its mRNA have been localized in syncytiotrophoblast of term placenta, decidual cells, amniotic epithelial cells, and chorion cytotrophoblast cells.189 During the first half of gestation, maternal plasma concentrations of the CRH-binding protein are similar to those of nonpregnant women; however, they decline during the second half of pregnancy.190 Nevertheless, even with this decline, concentrations of the CRH-binding protein exceed those of CRH by approximately 10-fold in the third trimester (2700 pmol/L vs 270 pmol/L, respectively).191, 192 Over the final 5–6 weeks of normal pregnancy, and before the onset of spontaneous preterm labor, the concentration of CRH-binding protein falls by approximately 50% before returning to normal levels by 5 days postpartum.177 Thus, plasma CRH concentration increases and CRH-binding protein decreases at a time when the structural and functional changes that are associated with parturition are occurring in gestational tissue.193
Expression of CRH Receptors in the Placenta
CRH exerts its actions by binding to two different families of CRH receptors, termed CRH-R1 and CRH-R2.177 CRH-R1 and CRH-R2 are 415 and 411 amino acids in length, respectively, and each has several isoforms. In the placenta, CRH-binding sites are expressed almost exclusively by the syncytiotrophoblast, and only CRH-R1 is found.194 CRH receptors are also present in the fetal membranes and myometrium.
Regulation of Placental CRH Synthesis and Secretion
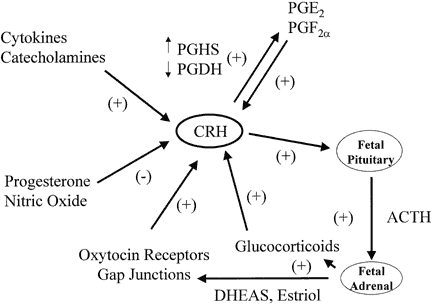
Placental CRH production is regulated by prostaglandins, oxytocin, glucocorticoids, and cytokines in a manner that implies a putative role for CRH in labor (Fig. 9).195, 196 Prostaglandins E and F stimulate CRH release from cultured human placental cells, and CRH stimulates prostaglandin release from amnion and chorion cells.196, 197, 198 More specifically, CRH appears to up-regulate prostaglandin synthase (PGHS), the activity of which stimulates the production of primary prostaglandins from arachidonic acid, and CRH down-regulates prostaglandin dehydrogenase (PGDH), which metabolizes prostaglandins in the fetal membranes.197, 199 Similarly, oxytocin stimulates placental cells to secrete CRH, which then increases the release of immunoreactive oxytocin from placental cells.200, 201 These observations suggest that placental CRH, prostaglandins, and oxytocin interact in a feed-forward regulatory loop that promotes labor. Furthermore, cytokines that have been associated with preterm labor caused by infection stimulate CRH secretion by cultured human placental cells.200
|
Glucocorticoids stimulate trophoblast CRH gene expression in vitro and CRH secretion by amnion, chorion, and decidual cells, whereas they inhibit the secretion of CRH in the hypothalamus.202, 203 The discovery of positive rather than negative feedback by dexamethasone on placental CRH expression led to the hypothesis that the increase in CRH near the end of pregnancy may be driven by glucocorticoids. In this proposed feed-forward system, glucocorticoids stimulate placental CRH production, which stimulates fetal pituitary and placental ACTH production, which subsequently increases glucocorticoid output from fetal adrenals, which in turn drives more placenta CRH secretion.195 This feed-forward system could help the fetus respond to stress or could be an antecedent to parturition. It remains to be established, however, whether this self-amplifying system functions in vivo. Indeed, the administration of betamethasone to women in premature labor at 28–32 weeks of pregnancy has not been observed to produce a consistent effect on maternal CRH levels, even though plasma ACTH and cortisol are suppressed.181, 204, 205 In experimental models of acute fetal hypoxemia induced in sheep by compression of the uterine artery or by infusion of adrenaline into the fetal circulation, CRH concentrations rise in the fetal and maternal plasma.195, 206 The increase in fetal CRH is probably from a reduction in uteroplacental blood flow, which causes the release of catecholamines that trigger CRH secretion in both the fetus and the placenta.207 A role for catecholamines is suggested by the fact that noradrenaline and adrenaline stimulate CRH release by human placental cells.200 The stimulatory actions of these catecholamines are mimicked by α1- and α2-adrenergic receptor agonists and are blocked by α1- and α2-receptor antagonists. Arginine vasopressin is increased in the fetal sheep circulation in response to hypoxemia, and arginine vasopressin also stimulates CRH production by cultured human placental cells.195, 208, 209 Besides being regulated by stimulatory factors, placental CRH secretion is also subject to inhibitory control by progesterone and nitric oxide.177, 203, 210
The Role of CRH in Labor
In addition to its association with prostaglandins, oxytocin, and glucocorticoids, CRH has biological activities within the fetus and myometrium that promote labor. As described above, placental CRH stimulates ACTH secretion from the fetal pituitary, which increases the production of dehydroepiandrosterone (DHEA) by the fetal zone of the adrenal gland. In turn, DHEA serves as the primary precursor for placental estriol, which increases myometrial oxytocin receptors and gap junction formation. CRH receptors are present in the myometrium, and stimulatory effects of placental CRH on myometrial contractility have been described. For example, exposure of myometrial strips, removed at the time of cesarean section, to CRH enhances the inotropic action of oxytocin.211 Finally, CRH appears to upregulate the activities of mediators such as matrix metalloproteinase-1 and -3 and IL-8, which promote cervical ripening preceding labor.212 Thus, CRH may have direct and indirect effects that promote parturition.
Placental Secretion of CRH in Response to Stress
The secretion of CRH by the placenta may reflect an adaptation by the fetus to hypoxemic stress, resulting in vasodilation of placental vessels, accelerated pulmonary maturation, and the initiation of parturition. CRH has significant vasodilatory actions on the mesenteric circulation, and in the experimental sheep models described above, a reduction in uteroplacental flow triggered the release of catecholamines and CRH in both the fetus and the placenta.206 In human gestations complicated by pregnancy-induced hypertension and fetal growth restriction, maternal plasma CRH levels were increased compared with normal controls.213, 214, 215 In these situations, placental CRH may ameliorate fetal hypoxemia by improving uteroplacental blood flow.216 Additionally, CRH stimulates the fetal adrenal glands to produce cortisol, which promotes lung maturation in preparation for extrauterine life, and as a final measure, CRH participates in the initiation of labor and delivery, which extricates the fetus from the stressful conditions.
Using Maternal CRH Levels to Predict Preterm Delivery
There has been much interest in the possibility that elevations of maternal plasma CRH levels may be used to predict women destined to deliver preterm.199 In at least three studies, elevated maternal plasma CRH levels were observed as early as 14–16 weeks' gestation among women who delivered preterm, and lower concentrations of CRH were discovered in women who delivered postterm.193, 217, 218, 219 In another study, maternal plasma CRH levels were elevated in preterm labor patients at 28–32 weeks' gestation who were destined to deliver within 48 hours.220 More recently, however, serum concentrations of CRH were shown to be not clinically useful for the prediction of preterm delivery among women with symptoms of preterm labor.181 Consequently, it seems unlikely that a single marker will prove to be reliable for predicting a multifactorial condition such as spontaneous preterm labor and delivery; however, a combination of biochemical tests, including CRH, salivary estriol, and fetal fibronectin, may be sufficiently sensitive and specific to warrant clinical application.199
Urocortin
Urocortin is a neuropeptide that has approximately 50% sequence homology with CRH. Urocortin binds to CRH receptors and is more potent than CRH in causing vasodilation. Immunoreactive urocortin has been localized in the syncytiotrophoblast and fetal membranes, and it is 25 times more potent than CRH in causing vasodilation of the fetal placental vasculature in vitro.221 However, the importance of urocortin in modulating placental vascular resistance in vivo and in the fetal response to stress has not been elucidated.
Chorionic Proopiomelanocortin Peptides
The placenta contains ACTH, β-lipotropin, β-endorphin, and three forms of dynorphin.222, 223, 224 Maternal plasma ACTH concentrations increase only slightly during pregnancy, presumably reflecting placental production of CRH, the effects of which are limited by an excess of CRH-binding protein (see Fig. 8).186, 225 The placental content of dynorphin is relatively high at term, and high levels of dynorphin have been found in amniotic fluid and umbilical venous plasma.224, 226 Dynorphin concentrations in maternal plasma in the third trimester and at delivery are higher than those found in nonpregnant women. β-endorphin levels in maternal plasma are relatively low during pregnancy, but rise in late labor and increase even further during delivery.227 The physiologic roles played by chorionic ACTH and other placental proopiomelanocortin peptides during pregnancy have not been elucidated. Chorionic ACTH, β-endorphin, and other proopiomelanocortin-derived peptides function in fetal responses to stress (e.g. hypoxia, acidosis) and in the initiation of labor.228 Dynorphin binds to the kappa opiate receptors, which are abundant in the placenta, raising the possibility that dynorphin may have a paracrine role in modulating placental function.229
OTHER PROTEIN HORMONES SECRETED BY THE PLACENTA
Neuropeptide Y
Neuropeptide Y is a 36-amino-acid peptide that is mainly found co-located with norepinephrine in a subset of sympathetic neurons. It is a potent vasoconstrictor that is released after sympathetic stimulation. Immunohistochemistry techniques have demonstrated neuropeptide Y in the cytotrophoblast and intermediate trophoblast cells.230 Maternal levels of neuropeptide Y rise in early pregnancy and remain elevated at term, increasing further during labor, but not in women who are delivered by cesarean section.231 Neuropeptide Y levels fall precipitously after delivery.231 Maternal plasma levels are greater in women with preeclampsia than in pregnant controls, which suggests that neuropeptide Y may play a role in the development of pregnancy-induced hypertension.232 Neuropeptide Y receptors have been detected in the placenta, and the peptide has been reported to stimulate release of CRH from placental cells.230, 233
Inhibins, Activins, and Follistatin
Inhibin and activins are glycoproteins that belong to the transforming growth factor-β family and participate in “fine-tuning” the hypothalamic-pituitary-gonadal secretion of gonadotropins.234 Inhibins and activins are encoded by three different genes (α , β A. and β B), which form dimers held together by disulfide bridges. Inhibin is composed of an α subunit and a β A or β B subunit, whereas activins are homodimers or heterodimers of β A or β B, or both. Follistatin is a single-chain glycoprotein that is present in several isoforms and is structurally unrelated to the inhibins and activins.
Inhibins were initially discovered in the gonads and shown to have an inhibitory effect on pituitary follicle-stimulating hormone (FSH) release. Later, activins and follistatin were discovered in the gonads; activins stimulate FSH release from the pituitary, whereas follistatin, which is an activin-binding protein, inhibits pituitary FSH release. Specific mRNAs for the inhibins, activins, and follistatin are also expressed in the human placenta, and the respective immunoreactive proteins are localized in small amounts within cytotrophoblastic cells and abundantly within the syncytiotrophoblast.235, 236, 237
α-inhibin subunit mRNA can be detected in placenta in early pregnancy.235, 236 Expression of the β gene is also detectable, but at much lower levels than the α-inhibin subunit gene. Both β-inhibin subunit and β A subunit mRNA levels increase in the placenta during gestation. Low levels of β B subunit mRNA are found only in the third trimester. Immunoreactive protein for all three subunits was localized in the syncytiotrophoblast, but the β A subunit appeared to be more widely expressed.237 The β A homodimer, activin-A, is present in cytotrophoblast cells and syncytiotrophoblast throughout gestation, in Hofbauer cells in first- and second-trimester chorionic villi, and in the amniotic epithelium and cytotrophoblast cells of the chorion.237 Consequently, activin-A is the major form of activin in the human placenta.238
Maternal serum α-inhibin levels increase in pregnancy, probably as a result of placental production of the hormone, and decline rapidly after delivery (Fig. 10).51 Interestingly, the ratio of inhibin bioactivity to immunoreactivity in pregnancy serum is much lower than in the nonpregnant state, which raises questions about the relative proportions of inhibin and activins produced during pregnancy. The reduced inhibin bioactivity could be due to the contradictory actions of activins.239 The regulation of inhibin and activin production by the placenta is not understood. In vitro, trophoblast cells secrete inhibin as well as activins. GnRH, calcium ionophores, phorbol esters, and epinephrine increase inhibin secretion, whereas activin secretion is responsive only to phorbol esters.239 These observations provide a basis for the hypothesis that differential regulation of inhibin and activin production occurs during pregnancy.
Inhibin secreted by the placenta could play a role in modulating maternal pituitary FSH secretion during pregnancy (see Fig. 10), participating in the suppression of this gonadotropin and thus follicular development. As noted in the discussion on the regulation of hCG production, the inhibin/activin peptides have also been postulated to have a role in governing trophoblast function. Thus, neutralization of inhibin with antibodies has been shown to increase hCG secretion. Conversely, exogenous activins have been shown to increase GnRH and progesterone secretion by placental cells. These actions of activins are blocked by inhibin and by binding to follistatin.
In addition to their effects on hCG secretion, immunomodulator and embryogenic roles have been proposed for inhibins, activins, and follistatin.51 Inhibins decrease the release of cytokines from lymphocytes, which may be an important function at the site of implantation, whereas activins play a role in stem cell differentiation and promote mesodermal differentiation in various animals.51, 240 Abnormal maternal serum levels of inhibins and activins have been associated with various pregnancy complications. Pregnant women with preeclampsia and spontaneous preterm labor have elevated serum levels of activin-A.241 However, activin-A does not appear to play an acute role in the initiation or regulation of parturition.242 During the second trimester of Down syndrome pregnancies, maternal serum inhibin-A (α , β A) levels are approximately twice the normal value, and these elevated levels are from the increased production in the trophoblast layer of the placental villi.243 Using two multiples of the median as the discriminatory value, univariate inhibin-A analysis can detect 45% of Down syndrome cases with a 5% false-positive rate.244 Most recently, a combination of second trimester maternal serum markers, including inhibin-A, and first trimester markers was shown to be more sensitive and specific than the standard multiple marker screen.245
Pregnancy-Specific β1 -Glycoproteins
The pregnancy-specific β1-glycoprotein (PSG), which is also known as Schwangerschafts protein-1 (SP1), family of glycoproteins is encoded by a cluster of closely linked genes on chromosome 19q13.2–13.3.246, 247 There are at least 11 distinct PSG genes that have marked sequence similarities. As many as five different transcripts can be derived from a single PSG gene through alternative splicing. If each gene gives rise to this number of transcripts, the number of PSG proteins would be substantial.
The PSGs are related to carcinoembryonic antigens, which are in turn similar to the immunoglobulins. They are primarily produced by the syncytiotrophoblast, but other placental cell types are capable of producing PSG isoforms. PSG immunoreactivity is detectable in maternal serum by 1–2 weeks after conception; concentrations increase as pregnancy progresses, reaching levels of 200–400 mg/mL by term, a pattern that is similar to that of hCS.248
The roles of PSG in pregnancy are not known, but in animal models, PSGs appear to have an important function in the preimplantation embryo. Specifically, monkey embryos were more likely to abort subsequent to treatment with antibodies to PSG, and two-cell stage mouse embryos co-cultured with Chinese hamster ovary cells transfected with PSG demonstrated enhanced cleavage and maturation rates compared with control subjects.249 PSG has been proposed to be a carrier protein for hormones and iron, a regulator of carbohydrate metabolism, and a factor preventing rejection of the fetus. It also is considered an extracellular matrix molecule because of the presence of the tripeptide RGD in the amino termini of some PSG forms.240, 250 Although the expression of PSG is more or less pregnancy specific, qualitative or quantitative assays of these proteins have not proved to be superior to the assay of hCG as a biochemical test of pregnancy. Assay of PSG has, however, been applied to the monitoring of therapeutic response in patients treated for choriocarcinoma and other malignancies.251 Additionally, first trimester maternal serum levels of PSG were lower in Down syndrome pregnancies than in unaffected pregnancies, but the difference was not large enough for PSG to be a useful marker in screening.252
Pregnancy-Associated Plasma Protein A
Pregnancy-associated plasma protein A (PAPP-A) is a 750-kd glycoprotein that is structurally related to α 2 macroglobulin, which binds and inactivates serine proteases.253, 254, 255 It is isolated as a dimer of homologous subunits. PAPP-A is produced primarily by the syncytiotrophoblast, but it also is synthesized by ovarian granulosa cells as well as other tissues of the reproductive tract. PAPP-A is present in the serum of nonpregnant women, and levels rise progressively through pregnancy with an accelerated increase after 30 weeks until term. PAPP-A is rapidly lost from maternal blood after parturition, declining with an estimated half-life of 55–73 hours.
PAPP-A has been identified as the IGF-dependent IGF-binding protein-4 protease.256, 257 Intact IGF-binding protein is a potent inhibitor of IGF action in vitro, and cleavage of IGF-binding protein-4 has been shown to abolish its ability to inhibit IGF stimulatory effects in a variety of systems. Thus, PAPP-A may act as a positive regulator of IGF bioavailability in the maternal serum. In addition to its role as an IGF-binding protein protease, PAPP-A may also modulate coagulation because of its ability to bind heparin and to block thrombin-induced coagulation of fibrin.258, 259 Hence, PAPP-A could participate in the maintenance of blood flow in the intervillous space by discouraging coagulation.
In the past several years, maternal serum levels of PAPP-A have been incorporated into first trimester screening tests for Down syndrome. Serum concentrations of PAPP-A are lower in the presence of a fetus with Down syndrome, and PAPP-A levels have been combined with risk factors based on maternal age. Other first trimester markers including elevated serum levels of hCG-β and thickened fetal nuchal translucency (as detected by ultrasound); and second trimester markers including maternal serum α-fetoprotein, hCG, and unconjugated estriol detect approximately 95% of Down syndrome fetuses with a false-positive rate of 5%.29, 30
Placenta Growth Factor
Placenta growth factor (PLGF) is a homodimeric glycoprotein that is similar in structure and function to vascular endothelial growth factor. PLGF exists as two isoforms, PLGF-1 and -2, which range in size from 46–50 kd in size. The site of primary expression of PLGF is the villous and extravillous trophoblast.
Like vascular endothelial growth factor, PLGF possesses potent angiogenic and mitogenic activities that are capable of inducing proliferation, migration, and activation of endothelial cells.260 In in vitro experiments, PLGF was found to stimulate the proliferation of extravillous trophoblast cells, which are the cells that invade the maternal uterine wall during the first and second trimester of pregnancy.260, 261 Not surprisingly, therefore, maternal serum levels of PLGF are reduced in women with preeclampsia, a condition whose cause is widely believed to be inadequate trophoblastic invasion into the maternal spiral arterioles.262 However, maternal serum levels of PLGF are not decreased before other clinical signs and symptoms of preeclampsia develop, suggesting that lower concentrations of PLGF may be a result of preeclampsia rather than a causal factor.263
REFERENCES
Parry S, Strauss JF III: Placental hormones. In DeGroot LJ, Jameson JL (ed): Endocrinology, 4th ed, Chap 178, p 2379. Philadelphia, WB Saunders, 2001 |
|
Ringler GE, Strauss JF III: In vitro systems for the study of human placental endocrine function. Endocr Rev 11: 105, 1990 |
|
Sasagawa M, Yamazaki T, Endo M et al: Immunohistochemical localization of HLA antigens and placental proteins ahCG, bhCG, CTP, hPL and SPI in villous and extravillous trophoblast in normal human pregnancy: A distinctive pathway of differentiation of extravillous trophoblast. Placenta 8: 515, 1987 |
|
Handschuh K, Guibourdenche J, Tsatsaris V et al: Human chorionic gonadotropin expression in human trophoblasts from earlyplacenta: comparative study between villous and extravillous trophoblastic cells. Placenta. 2007 Feb-Mar;28(2-3):175-84. Epub 2006 Apr 11. |
|
Fishel SB, Edwards RG, Evans CHJ: Human chorionic gonadotropin secreted by pre-implantation embryos cultured in vitro. Science 223: 816, 1984 |
|
Castellucci M, Kaufmann P, Bischof P: Extracellular matrix influences hormone and protein production by human chorionic villi. Cell Tissue Res 262: 135, 1990 |
|
Cross JC, Werb Z, Fisher SJ: Implantation and the placenta: Key pieces of the development puzzle. Science 266: 1508, 1994. |
|
Fox H: Effects of hypoxia on trophoblast in organ culture. Am J Obstet Gynecol 107: 1058, 1970 |
|
Ringler GE, Kallen CB, Strauss JF III: Regulation of human trophoblast function by glucocorticoids: Dexamethasone promotes increased secretion ofchorionic gonadotropin. Endocrinology 124: 1625, 1989 |
|
Petraglia F, Volpe A, Genazzani AR et al: Neuroendocrinology of the human placenta. Front Neuroendocrinol 11: 6, 1990 |
|
Morrish DW, Marusyk H, Siy O: Demonstration of specific secretory granules for human chorionic gonadotropin in placenta. J Histochem Cytochem 36: 93, 1988 |
|
Knoll BJ: Gene expression in the human placental trophoblast: A model for developmental gene regulation. Placenta 13: 311, 1992 |
|
Rahnama F, Shafiei F, Gluckman PD et al: Epigenetic regulation of human trophoblastic cell migration and invasion. Endocrinology. 2006 Nov;147(11):5275-83. Epub 2006 Aug 3. |
|
Dokras A, Coffin J, Field L et al: Epigenetic regulation of maspin expression in the human placenta. Mol Hum Reprod. 2006 Oct;12(10):611-7. Epub 2006 Aug 26. |
|
Chiu RW, Chim SS, Wong IH et al: Hypermethylation of RASSF1A in human and rhesus placentas. Am J Pathol. 2007 Mar;170(3):941-50. |
|
Muyan M, Boime I: Secretion of chorionic gonadotropin from human trophoblast. Placenta 18: 237, 1997 |
|
Talmadge K, Vamvakopoulos NC, Fiddes JC: Evolution of the genes for the &b.beta; subunits of human chorionic gonadotropin and luteinizing hormone. Nature 307: 37, 1984 |
|
Cole LA: Immunoassay of human chorionic gonadotropin, its free subunits, and metabolites. Clin Chem 43: 2233, 1997 |
|
Fares FA, Suganuma N, Nishimori K et al: Design of a long-acting follitropin agonist by fusing the C-terminal sequence of the chorionic gonadotropin subunit to the follitropin &b.beta; subunit. Proc Natl Acad Sci USA 89: 4304, 1992 |
|
Feng W, Matzuk MM, Mountjoy K et al: The asparagine-linked oligosaccharides of the human chorionic gonadotropin beta subunit facilitate correct disulfide bond pairing. J Biol Chem 270: 11851, 1995 |
|
Matzuk MM, Boime I: The role of the asparagine-linked oligosaccharides of the &b.alpha; subunit in the secretion and assembly of human chorionic gonadotropin. J Cell Biol 106: 1049, 1988 |
|
Matzuk MM, Boime I: Site-specific mutagenesis defines the intracellular role of the asparagine-linked oligosaccharides of chorionic gonadotropin b subunit. J Biol Chem 263: 17106, 1988 |
|
Matzuk MM, Keene JL, Boime I: Site-specificity of the chorionic gonadotropin N-linked oligosaccharides in signal transduction. J Biol Chem 264: 2409, 1989 |
|
Kadar N, Romero R: Observations on the log human chorionic gonadotropin-time relationship in early pregnancy and its practical implications. Am J Obstet Gynecol 157: 73, 1987 |
|
Pittaway DE, Reish RL, Wentz AC: Doubling times of human chorionic gonadotropin increase in early viable intrauterine pregnancies. Am J Obstet Gynecol 152: 299, 1985 |
|
Jovanovic L, Landesman R, Saxena BB: Screening for twin pregnancies. Science 198: 738, 1977 |
|
Bogart MH, Pandian MR, Jones OW: Abnormal maternal serum chorionic gonadotropin level in pregnancies with fetal chromosome abnormalities. Prenat Diagn 7: 623, 1987 |
|
Lambert-Messerlian GM, Canick JA: Endocrine analytes in multiple-marker screening. Clin Perinatol 25: 963, 1998 |
|
Malone FD, Canick JA, Ball RH et al: First-trimester or second-trimester screening, or both, for Down's syndrome. N Engl J Med. 2005 Nov 10;353(19):2001-11. |
|
Wapner R, Thom E, Simpson JL et al: First-trimester screening for trisomies 21 and 18. N Engl J Med. 2003 Oct 9;349(15):1405-13. |
|
Boime I: Human placental hormone production is linked to the stage of trophoblast differentiation. Trophoblast Res 5: 57, 1991 |
|
Kliman HJ, Nestler JE, Sermasi E et al: Purification, characterization and in vitro differentiation of cytotrophoblasts from human term placentae. Endocrinology 118: 1567, 1986 |
|
Siler-Khodr TM, Kang IA, Khodr GS: Effects of chorionic GNRH on intrauterine tissues and pregnancy. Placenta 12: 91, 1991 |
|
Roberson MS, Ban M, Zhang T et al: Role of the cyclic AMP response element binding complex and activation of mitogen-activated protein kinases in synergistic activation of the glycoprotein hormone alpha subunit by epidermal growth factor and forskolin. Mol Cell Biol 20: 3331, 2000 |
|
Saito S, Saito M, Motoyoshi K et al: Enhancing effects of human macrophage colony-stimulating factor on secretion of human chorionic gonadotropin by human chorionic villous cells and tPA 30-1 cells. Biochem Biophys Res Commun 178: 1099, 1991 |
|
Budworth PR, Quinn PG, Nilson JH: Multiple characteristics of a pentameric regulatory array endow the human &b.alpha; -subunit glycoprotein hormone promoter with trophoblast specificity and maximal activity. Mol Endocrinol 11: 1669, 1997 |
|
Strauss JF III, Kido S, Sayegh R et al: The cAMP signalling system and human trophoblast function. Placenta 13: 389, 1992 |
|
Nulsen JE, Woolkalis MJ, KopfGS et al: Adenylate cyclase in human cytotrophoblasts: Characterization and its role in modulating chorionic gonadotropin secretion. J Clin Endocrinol Metab 66: 258, 1988 |
|
Masuhiro K, Matsuzaki N, Nishino E et al: Trophoblast-derived interleukin-1 (IL-1) stimulates the release of human chorionic gonadotropin by activating IL-6 and IL-6 receptor system in first trimester human trophoblasts. J Clin Endocrinol Metab 72: 594, 1991 |
|
Nishino E, Matsuzaki N, Masuhiro K et al: Trophoblast-derived interleukin-6 (IL-6) regulates human chorionic gonadotropin release through IL-6 receptor on human trophoblasts. J Clin Endocrinol Metab 71: 436, 1990 |
|
Roberson MS, Meermann S, Morasso MI et al: A role for the homeobox protein distal-less m3 in the activation of the glycoprotein hormone alpha subunit gene in choriocarcinoma cells. J Biol Chem 30: 10016, 2001 |
|
Jameson JL, Hollenberg AN: Regulation of chorionic gonadotropin gene expression. Endocr Rev 14: 203, 1993 |
|
Lei ZM, Rao ChV, Ackerman DM et al: The expression of human chorionic gonadotropin/human luteinizing hormone receptors in human gestational trophoblastic neoplasms. J Clin Endocrinol Metab 74: 1236, 1992 |
|
Licht P, Cao H, Lei ZM et al: Novel self-regulation of human chorionic gonadotropin biosynthesis in term human placenta. Endocrinology 133: 3014, 1993 |
|
Shi QJ, Lei ZM, Rao ChV: Novel role of human chorionic gonadotropin in differentiation of human cytotrophoblasts. Endocrinology 132: 1387, 1993 |
|
Belisle S, Petit A, Bellbarba D et al: Ca2+ but not membrane lipid hydrolysis, mediates human chorionic gonadotropin production by LHRH in human term placenta. J Clin Endocrinol Metab 69: 117, 1989 |
|
Tamada T, Akabori A, Konuma S et al: Lack of release of human chorionic gonadotropin by gonadotropin-releasing hormone. Endocrinol Jpn 23: 531, 1976 |
|
Pestell RG, Hollenberg AN, Albanese C et al: c-Jun represses transcription of the human chorionic gonadotropin &b.alpha; and &b.beta; genes through distinct types of CREs. J Biol Chem 269: 31090, 1994 |
|
Matsuo H, Strauss JF III: Peroxisome proliferators and retinoids affect JEG-3 choriocarcinoma cell function. Endocrinology 135: 1135, 1994 |
|
Schaiff WT, Carlson MG, Smith SD et al: Peroxisome proliferator-activated receptor-gamma modulates differentiation of human trophoblast in a ligand-specific manner. J Clin Endocrinol Metab 85: 3874, 2000 |
|
Petraglia F: Inhibin, activin and follistatin in the human placenta: A new family of regulatory proteins. Placenta 18: 3, 1997 |
|
Mersol-Barg MS, Miller KF, Choi CM et al: Inhibin suppresses human chorionic gonadotropin secretion in term, but not first trimester, placenta. J Clin Endocrinol Metab 71: 1294, 1990 |
|
Wilson EA, Jawad MJ, Powell DE: Effect of estradiol and progesterone on human chorionic gonadotropin secretion in vitro. Am J Obstet Gynecol 149: 143, 1984 |
|
Maruo T, Matsuo H, Ohtani T et al: Differential modulation of chorionic gonadotropin (CG) subunit messenger ribonucleic acid levels and CG secretion by progesterone in normal placenta and choriocarcinoma cultured in vitro. Endocrinology 119: 855, 1986 |
|
Leisser C, Saleh L, Haider S et al: Tumour necrosis factor-alpha impairs chorionic gonadotrophin beta-subunitexpression and cell fusion of human villous cytotrophoblast. Mol Hum Reprod. 2006 Oct;12(10):601-9. Epub 2006 Aug 8. |
|
Chou D, Ma Y, Zhang J et al: Cytomegalovirus infection of trophoblast cells elicits an inflammatory response:a possible mechanism of placental dysfunction. Am J Obstet Gynecol. 2006 Feb;194(2):535-41. |
|
Arechavaleta-Velasco F, Ma Y, Zhang J et al: Adeno-associated virus-2 (AAV-2) causes trophoblast dysfunction, and placental Am J Pathol. 2006 Jun;168(6):1951-9. |
|
Cole LA, Kroll TG, Ruddon RW et al: Differential occurrence of free beta and free alpha subunits of human chorionic gonadotropin (hCG) in pregnancy sera. J Clin Endocrinol Metab 58: 1200, 1980 |
|
Hay DL: Discordant and variable production of human chorionic gonadotropin and its free a- and b-subunits in early pregnancy. J Clin Endocrinol Metab 61: 1195, 1985 |
|
Ozturk M, Bellet D, Manil L et al: Physiological studies of human chorionic gonadotropin (hCG), ahCG and bhCG as measured by specific monoclonal immunoradiometric assays. Endocrinology 120: 549, 1987 |
|
Blithe DL: N-linked oligosaccharides on free &b.alpha; interfere with its ability to combine with the human chorionic- &b.beta; subunit. J Biol Chem 265: 21951, 1990 |
|
Blithe DL, Nisula BL: Variations in the oligosaccharides on free and combined &b.alpha; subunits of human chorionic gonadotropin in pregnancy. Endocrinology 117: 2218, 1995 |
|
Blithe DL: Carbohydrate composition of the &b.alpha; subunit of human chorionic gonadotropin (&b.alpha; hCG) and the free &b.alpha; molecule produced in pregnancy: Most free &b.alpha; and some hCG-&b.alpha; molecules are fucosylated. Endocrinology 126: 2788, 1990 |
|
Skarufis MC, Webmann RE, Nisula BC et al: Glycosylation changes in human chorionic gonadotropin and free alpha subunit as gestation progresses. J Clin Endocrinol Metab 75: 91, 1992 |
|
Nisula BL, Wehmann RE: Distribution, metabolism and excretion of human chorionic gonadotropin and its subunits in man. In Segal SJ (ed): Chorionic Gonadotropin, pp 199-212. New York, Plenum Press, 1980 |
|
Kato Y, Braunstein GD: b-Core fragment is a major form of immunoreactive urinary chorionic gonadotropin in human pregnancy. J Clin Endocrinol Metab 66: 1197, 1988 |
|
Wehmann RE, Blithe DL, Akar AH et al: Disparity between b-core levels in pregnancy urine and serum: Implications for the origin of urinary b-core. J Clin Endocrinol Metab 70: 371, 1990 |
|
de Medeiros SFG, Amato F, Bacich D et al: Distribution of the b-core human chorionic gonadotropin fragment in human body fluids. J Endocrinol 135: 175, 1992 |
|
Zeleznik AJ: In vivo responses of the primate corpus luteum to luteinizing hormone and chorionic gonadotropin. Proc Natl Acad Sci USA 95: 11002, 1998 |
|
Stevens VC: Potential control of fertility in women by immunization with hCG. Res Reprod 7: 1, 1985 |
|
Fazleabas AT, Donnelly KM, Srinivasan S et al: Modulation of the baboon ( Papio anubis ) uterine endometrium by chorionic gonadotrophin during the period of uterine receptivity. Proc Natl Acad Sci USA 95: 2543, 1999 |
|
Kasahara K, Takakura K, Takebayashi K et al: The role of human chorionic gonadotropin on decidualization of endometrial stromal cells in vitro. J Clin Endocrinol Metab 86: 1281, 2001 |
|
Rao E, Ambrus G, Rao CV: Direct regulation of human myometrial contractions by human chorionic gonadotropin. J Clin Endocrinol Metab 79: 1582, 1994 |
|
Hershman JM: Human chorionic gonadotropin and the thyroid: Hyperemesis gravidarum and trophoblastic tumors. Thyroid 9: 653, 1999 |
|
Tomer Y, Huber GK, Davies TF: Human chorionic gonadotropin (hCG) interacts directly with recombinant human TSH receptors. J Clin Endocrinol Metab 74: 1477, 1992 |
|
Haddow JE, McClain MR, Lambert-Messerlian G et al: Variability in thyroid-stimulating hormone suppression by human chronicgonadotropin during early pregnancy. J Clin Endocrinol Metab. 2008 Sep;93(9):3341-7. Epub 2008 Jun 10. |
|
Bhattacharyga S, Chaudhary J, Das C: Antibodies to hCG inhibit progesterone production from human syncytiotrophoblast cells. Placenta 13: 135, 1992 |
|
McGregor WG, Kuhn RW, Jaffe RB: Biologically active chorionic gonadotropin: Synthesis by the human fetus. Science 220: 306, 1983 |
|
Kumar S, Talwar GP, Biswas DK: Necrosis and inhibition of growth of human lung tumor by anti-a-human chorionic gonadotropin antibody. J Natl Cancer Inst 84: 42, 1992 |
|
Moy E, Kimzey LM, Nelson LM et al: Glycoprotein hormone &b.alpha; -subunit functions synergistically with progesterone to stimulate differentiation of cultured human endometrial stromal cells to decidualized cells: A novel role for free &b.alpha; -subunit in reproduction. Endocrinology 137: 1332, 1996 |
|
Blithe DL, Richards RG, Skarulis MC: Free alpha molecules from pregnancy stimulate secretion of prolactin from human decidual cells: A novel function for free alpha in pregnancy. Endocrinology 129: 2257, 1991 |
|
O'Connor JF, Birken S, Lustbader JW et al: Recent advances in the chemistry and immunochemistry of human chorionic gonadotropin: Impact on clinical measurements. Endocr Rev 15: 650, 1994 |
|
Bangham DR, Grab B: The second international standard for chorionic gonadotropin. Bull World Health Organ 31: 111, 1964 |
|
Paul M, Schaff E, Nichols M: The roles of clinical assessment, human chorionic gonadotropin assays, and ultrasonography in medical abortion practice. Am J Obstet Gynecol 183: S34, 2000 |
|
Chard T: Pregnancy tests: A review. Hum Reprod 7: 701, 1992 |
|
Batzer FR, Schlaff S, Goldfarb AF et al: Serial beta-subunit human chorionic gonadotropin doubling time as a prognosticator of pregnancy outcome in an infertile population. Fertil Steril 35: 307, 1981 |
|
Gracia CR, Barnhart KT: Diagnosing ectopic pregnancy: Decision analysis comparing six strategies. Obstet Gynecol 97: 464, 2001 |
|
Ren GS, Braunstein GD: Human chorionic gonadotropin. Semin Reprod Endocrinol 10: 95, 1992 |
|
Wald NJ, Cuckle HS, Densem JW et al: Maternal serum screening for Down's syndrome in early pregnancy. BMJ 297: 883, 1988 |
|
Spencer K, Wallace EM, Ritoe S: Second-trimester dimeric inhibin-A in Down's syndrome screening. Prenat Diagn. 1996 Dec;16(12):1101-10. |
|
Canick JA, Palomaki GE, Osathanondh R: Prenatal screening for trisomy 18 in the second trimester. Prenat Diagn 10: 546, 1990 |
|
Bogart MH, Jones OW, Felder RA et al: Prospective evaluation of maternal serum human chorionic gonadotropin levels in 3428 pregnancies. Am J Obstet Gynecol 165: 663, 1991 |
|
Macri JN, Kasturi RV, Krantz DA et al: Maternal serum Down syndrome screening: Free beta-protein is a more effective marker than human chorionic gonadotropin. Am J Obstet Gynecol 163: 1248, 1990 |
|
Cuckle HS, Iles RK, Chard T: Urinary &b.beta; -core human chorionic gonadotropin: A new approach to Down's syndrome screening. Prenat Diagn 14: 953, 1994 |
|
Spencer K, Coombes EJ, Mallard AS et al: Free beta human choriogonadotropin in Down's syndrome screening: A multicentre study of its role compared with other biochemical markers. Ann Clin Biochem 29: 506, 1992 |
|
Bahado-Singh RO, Oz U, Shahabi S et al: Comparison of urinary hyperglycosylated human chorionic gonadotropin concentration with the serum triple screen for Down syndrome detection in high-risk pregnancies. Am J Obstet Gynecol 183: 1114, 2000 |
|
Haddow JE, Palomaki GE, Knight GJ et al: Screening of maternal serum for fetal Down's syndrome in the first trimester. N Engl J Med 338: 955, 1998 |
|
Cole LA: hCG, its free subunits and its metabolites. J Reprod Med 43: 3, 1998 |
|
Braunstein GD, Thompson R, Prinder SL et al: Trophoblastic proteins as tumor-markers in nonseminomatous germ cell tumors. Cancer 57: 1842, 1986 |
|
Borkowski A, Pattaert V, Gyling M et al: Human chorionic gonadotropin-like substances in plasma of normal nonpregnant subjects and women with breast cancer. J Clin Endocrinol Metab 58: 1171, 1984 |
|
Odell WD, Griffin J: Pulsatile secretion of chorionic gonadotropin during the normal menstrual cycle. J Clin Endocrinol Metab 69: 528, 1989 |
|
Odell WD, Griffin J, Bashey HM et al: Secretion of chorionic gonadotropin by cultured human pituitary cells. J Clin Endocrinol Metab 71: 1318, 1990 |
|
Dalter E, Braunstein GD, Synder PJ et al: Gonadotropin-releasing hormone-dependent chorionic gonadotropin secretion in a menopausal woman. J Clin Endocrinol Metab 781: 1293, 1994 |
|
Handwerger S, Freemark M: The roles of placental growth hormone and placental lactogen in the regulation of human fetal growth and development. J Pediatr Endocrinol Metab 13: 343, 2000 |
|
Geoge DL, Phillips J, Francke U et al: The genes for growth hormone and chorionic somatomammotropin are on the long arm of human chromosome 17 in the region q21 to pter. Hum Genet 57: 138, 1981 |
|
Harper ME, Barrera-Saldana HA, Saunders GF: Chromosomal localization of the human placental lactogen growth hormone gene cluster to 17q22-24. Am J Hum Genet 34: 227, 1982 |
|
MacLeod JN, Lee AK, Liebhaber A et al: Developmental control and alternative splicing of the placentally expressed transcripts from the human growth hormone gene cluster. J Biol Chem 267: 14219, 1992 |
|
Cooke NE, Emery JG, Ray J et al: Placental expression of the human growth hormone-variant gene. Troph Res 5: 61, 1991 |
|
Barrera-Saldana HA: Growth hormone and placental lactogen: Biology, medicine, and biotechnology. Gene 211: 11, 1998 |
|
Eberhardt NL, Jiang SW, Shepard AR et al: Hormonal and cell-specific regulation of the human growth hormone and chorionic somatomammotropin genes. Prog Nucleic Acid Res Mol Biol 54: 127, 1996 |
|
Su Y, Liebhaber SA, Cooke NE: The human growth hormone gene cluster locus control region supports position-independent pituitary- and placenta-specific expression in the transgenic mouse. J Biol Chem 275: 7902, 2000 |
|
Richardson BD, Langland RA, Bachurski CJ et al: Activator protein-2 regulates human placental lactogen gene expression. Mol Cell Endocrinol 160: 183, 2000 |
|
Brar AK, Richards RG, Cheng YH et al: Mitogen-activated protein kinase activates human placental lactogen-B enhancer by an NF-IL6-dependent pathway. Endocrine 12: 47, 2000 |
|
Norquay LD, Jin Y, Surabhi RM et al: A member of the nuclear factor-1 family is involved in the pituitary repression of the human placental growth hormone genes. Biochem J 354: 387, 2001 |
|
Sciarra JJ, Sherwood LM, Varma AA et al: Human placental lactogen (HPL) and placental weight. Am J Obstet Gynecol 101: 413, 1968 |
|
Houghton DJ, Shackleton P, Obiekwe BC et al: Relationship of maternal and fetal levels of human placental lactogen to the weight and sex of the fetus. Placenta 5: 455, 1984 |
|
Hoshina M, Boothby M, Boime I: Cytological localization of chorionic gonadotropin and placental lactogen mRNA during development of the human placenta. J Cell Biol 93: 190, 1982 |
|
Hu L, Lytras A, Bock ME et al: Detection of placental growth hormone and chorionic somatomammotropin-L RNA expression in normal and diabetic pregnancy by reverse transcriptase-polymerase chain reaction. Mol Cell Endocrinol 131: 42, 1999 |
|
Hochberg Z, Bick T, Perlman R: Two pathways of placental lactogen secretion by cultured human trophoblast. Biochem Med Metab Biol 39: 111, 1988 |
|
Walkins WB, Yen SSC: Somatostatin in cytotrophoblast of the immature human placenta: Localization by immunoperoxidase cytochemistry. J Clin Endocrinol Metab 50: 969, 1980 |
|
Macaron C, Kynel M, Rutsky L: Failure of somatostatin to affect human chorionic somatomammotropin and human chorionic gonadotropin secretion in vitro. J Clin Endocrinol Metab 47: 1141, 1978 |
|
Yikorkala O, Kivinen S, Ronnberg L: Bromocryptine treatment during early human pregnancy: Effects on the levels of prolactin, sex steroids and placental lactogen. Acta Endocrinol (Copenh) 95: 412, 1980 |
|
Macaron C, Famuyiwa O, Singh SP: In vitro effects of dopamine and pimozide on human thorionic somatomammotropin (hCS) secretion. J Clin Endocrinol Metab 47: 168, 1978 |
|
Shu-ronj Z, Bremme K, Eneroth P et al: The regulation in vitro of placental release of human chorionic gonadotropin, placental lactogen and prolactin: Effects of an adrenergic beta-receptor agonist and antagonist. Am J Obstet Gynecol 143: 444, 1982 |
|
Stephanou A, Handwerger S: Retinoic acid and thyroid hormone regulate placental lactogen expression in human trophoblast cells. Endocrinology 136: 933, 1995 |
|
Stephanou A, Handwerger S: The nuclear factor NF-IL6 activates human placental lactogen gene expression. Biochem Biophys Res Commun 206: 215, 1995 |
|
Handwerger S, Quaffordt S, Barrett J et al: Apolipoproteins AI, All, and CI stimulate placental lactogen release from human placental tissue. J Clin Invest 79: 625, 1987 |
|
Handwerger S, Datta G, Richardson B et al: Pre-beta-HDL stimulates placental lactogen release from human trophoblast cells. Am J Physiol 276: 384, 1999 |
|
Rosing U, Samsloe G, Oluna A et al: Serum levels of apolipoprotein A-I, A-II and HDL-cholesterol in second half of normal pregnancy and in pregnancy complicated by preeclampsia. Horm Metab Res 21: 76, 1989 |
|
Wu YQ, Jorgensen EV, Handwerger S: High density lipoproteins stimulate placental lactogen release and adenosine 3',5'-monophosphate (cAMP) production in human trophoblast cells: Evidence for cAMP as a second messenger in human placental lactogen release. Endocrinology 123: 1879, 1988 |
|
Kanda Y, Richards RG, Handwerger S: Apolipoprotein A-I stimulates human placental lactogen release by activation of MAP kinase. Mol Cell Endocrinol 143: 125, 1998 |
|
Tyson JE, Austin KL, Farinholt JW: Prolonged nutritional deprivation in pregnancy: Changes in chorionic somatomammotropin and growth hormone secretion. Am J Obstet Gynecol 109: 1080, 1971 |
|
Kim YJ, Felig P: Plasma chorionic somatomammotropin levels during starvation in midpregnancy. J Clin Endocrinol Metab 32: 864, 1971 |
|
Burt RL, Leake NH, Rhyne AL: Human placental lactogen and insulin-blood glucose homeostasis. Obstet Gynecol 36: 233, 1970 |
|
Gaspard U, Sandront H, Luyckz A: Glucose-insulin interaction and the modulation of human placental lactogen (HPL) secretion during pregnancy. J Obstet Gynaecol Br Commonw 81: 201, 1974 |
|
Williams C, Coltart TM: Adipose tissue metabolism in pregnancy: The lipolytic effect of human placental lactogen. Br J Obstet Gynaecol 85: 43, 1978 |
|
Turtle JR, Kipnis DM: The lipolytic action of human placental lactogen on isolated fat cells. Biochim Biophys Acta 144: 583, 1967 |
|
Genazzani AR, Benuzzi-Badoni M, Felber JP: Human chorionic somatomammotropin (hCSM): Lipolytic action of a pure preparation of isolated fat cells. Metabolism 18: 593, 1969 |
|
Genazzani AR, Zaragoza N, Neri P et al: Human chorionic somatomammotropin (hCS): Effects on glucose metabolism in rat epididymal adipose tissue. Ital J Biochem 19: 230, 1970 |
|
Felber JP, Zaragoza N, Benuzzi-Badoni M et al: The double effect of human thorionic somatomammotropin (hCS) and pregnancy in lipogenesis and on lipolysis in the isolated rat epididymal fat pad and fat pad cells. Horm Metab Res 4: 293, 1972 |
|
Sorenson RL, Brelje TC: Adaptation of islets of Langerhans to pregnancy: &b.beta; -cell growth, enhanced insulin secretion and the role of lactogenic hormones. Horm Metab Res 29: 301, 1997 |
|
Nielsen JH: Effects of growth hormone, prolactin and placental lactogen on insulin content and release and deoxyribonucleic acid synthesis in cultured pancreatic islets. Endocrinology 110: 600, 1982 |
|
Samaan N, Yen SCC, Gonzalez D et al: Metabolic effects of placental lactogen (HPL) in man. J Clin Endocrinol Metab 28: 485, 1968 |
|
Sladek CD: The effects of human chorionic somatomammotropin and estradiol on g!uconeogenesis and hepatic glycogen formation in the rat. Horm Metab Res 7: 50, 1975 |
|
Bjorklund AO, Adamson UK, Carlstrom KA et al: Placental hormones during induced hypoglycaemia in pregnant women with insulin-dependent diabetes mellitus: Evidence of an active role for placenta in hormonal counter-regulation. Br J Obstet Gynaecol 105: 649, 1998 |
|
Struman I, Bentzien F, Lee H et al: Opposing actions of intact and N-terminal fragments of the human prolactin/growth hormone family members on angiogenesis: An efficient mechanism for the regulation of angiogenesis. Proc Natl Acad Sci USA 96: 1246, 1999 |
|
Brelje TC, Scharp DW, Lacy PE et al: Effect of homologous placental lactogens, prolactins, and growth hormones on islet B-cell division and insulin secretion in rat, mouse, and human islets: Implication for placental lactogen regulation of islet cell function during pregnancy. Endocrinology 132: 879, 1993 |
|
Welsch CW, McManus MJ: Stimulation of DNA synthesis by human placental lactogen or insulin in organ cultures of benign human breast tumors. Cancer Res 37: 2257, 1977 |
|
McManus MJ, Dembroske SE, Pienkowski MM et al: Successful transplantation of human benign breast tumors into the athymic nude mouse and demonstration of enhanced DNA synthesis by human placental lactogen. Cancer Res 38: 2343, 1978 |
|
Parks JS, Nielsen PV, Sexton LA et al: An effect of gene dosage on production of human chorionic somatomammotropin. J Clin Endocrinol Metab 60: 994, 1985 |
|
Hubert C, Descombey D, Mondon F et al: Plasma human chorionic somatomammotropin deficiency in a normal pregnancy is the consequence of low concentration of messenger RNA coding for human chorionic somatomammotropin. Am J Obstet Gynecol 147: 676, 1983 |
|
Simon P, Decoster C, Brocas H et al: Absence of human chorionic somatomammotropin during pregnancy associated with two types of gene deletion. Hum Genet 74: 235, 1986 |
|
Nielsen PV, Pedersen H, Kampmann EM: Absence of human placental lactogen in an otherwise uneventful pregnancy. Am J Obstet Gynecol 135: 322, 1976 |
|
Wurzel JM, Parks JS, Herd JE et al: A gene deletion is responsible for absence of human chorionic somatomammotropin. DNA 1: 251, 1982 |
|
Rygaard K, Revol A, Esquivel-Escobedo D et al: Absence of human placental lactogen and placental growth hormone (HGH-V) during pregnancy:PCR analysis of the deletion. Hum Genet 102: 87, 1998 |
|
Frankenne F, Hennen G, Parks JS et al: A gene deletion in the hGH/hCS gene cluster could be responsible for the placental expression of hGH and/or hCS like molecules absent in normal subjects. Endocrinology 118 (suppl 1): 128, 1986 |
|
Spellacy WN, Buhi WC, Birk SA: The effectiveness of human placental lactogen measurements as an adjunct in decreasing perinatal deaths. Am J Obstet Gynecol 121: 835, 1975 |
|
Polin JI, Frangipane WL: Current concepts in management of obstetric problems for pediatricians: I. Monitoring the high-risk ferns. Pediatr Clin North Am 33: 621, 1986 |
|
Alsat E, Guibourdenche J, Luton D et al: Human placental growth hormone. Am J Obstet Gynecol 177: 1526, 1997 |
|
Frankenne F, Rentier-Delrue F, Scippo ML et al: Expression of the growth hormone variant gene in human placenta. J Clin Endocrinol Metab 64: 633, 1987 |
|
Lytras A, Bock ME, Dodd JG et al: Detection of placental growth hormone variant and chorionic somatomammotropin ribonucleic acid expression in human trophoblastic neoplasms by reverse transcriptase-polymerase chain reaction. Endocrinology 134: 2461, 1994 |
|
Eriksson L, Frankenne F, Eden S et al: Growth hormone 24-hour serum profiles during pregnancy: Lack of pulsatility for the secretion of the placental variant. Br J Obstet Gynaecol 96: 949, 1989 |
|
Daughaday WH, Trivedi B, Winn HN et al: Hypersomatotropism in pregnant women, as measured by a human liver radioreceptor assay. J Clin Endocrinol Metab 70: 215, 1990 |
|
Styne DM: Fetal growth. Clin Perinatol 25: 917, 1998 |
|
Mirlesse V, Frankenne F, Alsat E et al: Placental growth hormone levels in normal pregnancy and in pregnancies with intrauterine growth retardation. Pediatr Res 34: 439, 1993 |
|
Masuzaki H, Ogawa Y, Sagawa N et al: Nonadipose tissue production of leptin: Leptin as a novel placenta-derived hormone in humans. Nat Med 3: 1029, 1997 |
|
Hassink SG, de Lancey E, Sheslow DV et al: Placental leptin: An important new growth factor in intrauterine and neonatal development? Pediatrics 100: E1, 1997 |
|
Linnemann K, Malek A, Sager R et al: Leptin production and release in the dually in vitro perfused human placenta. J Clin Endocrinol Metab 85: 4298, 2000 |
|
Yamaguchi M, Murakami T, Tomimatsu T et al: Autocrine inhibition of leptin production by tumor necrosis factor-alpha (TNF-alpha) through TNF-alpha type-I receptor in vitro. Biochem Biophys Res Commun 244: 30, 1998 |
|
Wolf HJ, Ebenbichler CF, Huter O et al: Fetal leptin and insulin levels only correlate in large-for-gestational age infants. Eur J Endocrinol 142: 623, 2000 |
|
Helland IB, Reseland JE, Saugstad OD et al: Leptin levels in pregnant women and newborn infants: Gender differences and reduction during the neonatal period. Pediatrics 101: E12, 1998 |
|
Stock SM, Bremme KA: Elevation of plasma leptin levels during pregnancy in normal and diabetic women. Metab Clin Exp 47: 840, 1998 |
|
Trollmann R, Klingmuller K, Schild RL et al: Differential gene expression of somatotrophic and growth factors in response to in vivo hypoxia in human placenta. Am J Obstet Gynecol. 2007 Dec;197(6):601.e1-6. |
|
Mise H, Yura S, Itoh H et al: The relationship between maternal plasma leptin levels and fetal growthrestriction. Endocr J. 2007;54(6):945-51. Epub 2007 Nov 14. |
|
Hauguel-de Mouzon S, Lepercq J, Catalano P: The known and unknown of leptin in pregnancy. Am J Obstet Gynecol. 2006 Jun;194(6):1537-45. Epub 2006 Apr 21. |
|
Sasaki A, Tempst P, Liotta AS et al: Isolation and characterization of a corticotropin-releasing hormone-like peptide from human placenta. J Clin Endocrinol Metab 67: 768, 1988 |
|
Karteris E, Grammatopoulos DK, Randeva HS et al: The role of corticotropin-releasing hormone receptors in placenta and fetal membranes during human pregnancy. Mol Genet Metab 72: 287, 2001 |
|
Riley SC, Walton JC, Herlick JM et al: The localization and distribution of corticotropin-releasing hormone in the human placenta and fetal membranes throughout gestation. J Clin Endocrinol Metab 72: 1001, 1991 |
|
Goland RS, Wardlaw SL, Stark RI et al: High levels of corticotropin-releasing hormone immunoreactivity in maternal and fetal plasma during pregnancy. J Clin Endocrinol Metab 63: 1199, 1986 |
|
Sasaki A, Shinkawa O, Margioris AN et al: Immunoreactive corticotropin-releasing hormone in human plasma during pregnancy, labor, and delivery. J Clin EndocrinoI Metab 64: 224, 1987 |
|
Coleman MAG, France JT, Schellenberg JC et al: Corticotropin-releasing hormone, corticotropin-releasing hormone-binding protein, and activin A in maternal serum: Prediction of preterm delivery and response to glucocorticoids in women with symptoms of preterm labor. Am J Obstet Gynecol 183: 643, 2000 |
|
Frim DM, Emanuel RL, Robinson BG et al: Characterization and gestational regulation of corticotropin-releasing hormone messenger RNA in human placenta. J Clin Invest 82: 287, 1988 |
|
Okamoto E, Takagi T, Sath H et al: Immunoreactive corticotrophin-releasing hormone, adrenocorticotrophin and cortisol in human plasma during pregnancy, delivery and post partum. Horm Metab Res 21: 566, 1989 |
|
Economides D, Linton E, Nicolaides K et al: Relationship between maternal and fetal corticotropin-releasing hormone-41 and ACTH levels in human mid-trimester pregnancy. J Endocrinol 114: 497, 1987 |
|
Maser-Gluth C, Lorenz U, Vecsei P: In pregnancy, corticotropin-releasing factor in maternal blood and amniotic fluid correlates with the gestational age. Horm Metab Res 16 (suppl): 42, 1987 |
|
Laatikainen T, Virtanen T, Raisanen I et al: Immunoreactive corticotrophin-releasing factor and cordcotrophin in plasma during pregnancy, labor and puerperium. Neuropeptides 10: 343, 1987 |
|
Petraglia F, Giardino L, Coukos G et al: Corticotropin-releasing factor and parturition: Plasma and amniotic fluid levels and placentai binding sites. Obstet Gynecol 75: 784, 1990 |
|
Linton EA, Behan DP, Saphier PW et al: Corticotropin-releasing hormone (CRH)-binding protein: Reduction in adrenocorticotropin-releasing activity of placental but not hypothalamic CRH. J Clin Endocrinol Metab 70: 1574, 1990 |
|
Petragtia F, Potter E, Cameron VA et al: Corticotropin-releasing factor-binding protein is produced by human placenta and intrauterine tissues. J Clin Endocrinol Metab 77: 919, 1993 |
|
Suda T, Iwashita M, Tozawa F et al: Characterization of corticotrophin-releasing hormone binding protein in human plasma by chemical cross-linking and its binding during pregnancy. J Clin Endocrinol Metab 67: 1278, 1988 |
|
Orth DN, Mount CD: Specific high-affinity binding protein for human corticotropin-releasing hormone in normal human plasma. Biochem Biophys Res Commun 143: 411, 1987 |
|
Linton EA, Wolfe CDA, Behan DP et al: A specific carrier substance for human corticotrophin-releasing factor in late gestational maternal plasma which could mask the ACTH-releasing activity. Clin Endocrinol (Oxf) 28: 315, 1988 |
|
McLean M, Smith R: Corticotrophin-releasing hormone and human parturition. Reproduction 121: 493, 2001 |
|
Karteris E, Grammatopoulos DK, Dai Y et al: The human placenta and fetal membranes express the CRH-R1&b.alpha; and the CRH-C variant receptor. J Clin Endocrinol Metab 83: 1376, 1998 |
|
Whittle WL, Patel FA, Alfaidy N et al: Glucocorticoid regulation of human and ovine parturition: The relationship between fetal hypothalamic-pituitary-adrenal axis activation and intrauterine prostaglandin production. Biol Reprod 64: 1019, 2001 |
|
Challis JRG, Matthews SG, Gibb W et al: Endocrine and paracrine regulation of birth at term and preterm. Endocr Rev 21: 514, 2000 |
|
Challis JR, Matthews SG, Van Meir C et al: Current topic: The placental corticotrophin-releasing hormone-adrenocorticotrophin axis. Placenta 16: 481, 1995 |
|
Dibbs KI, Anteby E, Mallon MA et al: Transcriptional regulation of human placental corticotropin-releasing factor by prostaglandins and estradiol. Biol Reprod 57: 1285, 1997 |
|
Challis JRG: Understanding preterm birth. Clin Invest Med 24: 60, 2001 |
|
Petraglia F, Sutton S, Vale W: Neurotransmitters and peptides modulate the release of immunoreactive CRH from cultured human placental cells. Am J Obstet Gynecol 160: 247, 1989 |
|
Florio P, Lombardo M, Gallo R et al: Activin A, corticotropin-releasing factor and prostaglandin F2 alpha increase immunoreactive oxytocin release from cultured human placental cells. Placenta 17: 307, 1996 |
|
Robinson BG, Emanuel RL, Frim DM et al: Glucocorticoids stimulate expression of corticotropin releasing hormone gene in human placenta. Proc Natl Acad Sci USA 85: 5244, 1988 |
|
Jones SA, Brooks AW, Challis JRG: Steroids modulate corticotrophin-releasing factor production in human fetal membranes and placenta. J Clin Endocrinol Metab 68: 825, 1989 |
|
Troper PJ, Goland RS, Wardlaw SL et al: Effects of betamethasone on maternal plasma corticotrophin-releasing factor, ACTH and cortisol during pregnancy. J Perinatol Med 15: 221, 1987 |
|
Marinoni E, Korebrits C, DiIorio R et al: Effect of betamethasone in vivo on placental corticotropin-releasing hormone in human pregnancy. Am J Obstet Gynecol 178: 770, 1998 |
|
Jones CT, Gu W, Parer JT: Production of corticotrophin-releasing hormone by the sheep placenta in vivo. J Dev Physiol 11: 97, 1989 |
|
Potter E, Bahan DP, Fischer WH et al: Cloning and characterization of the cDNAs for human and rat corticotropin-releasing hormone, ACTH and prostaglandin interactions in the amnion and placenta of early pregnancy in man. J Endocrinol 125: 153, 1970 |
|
Stark RI, Wardlaw SL, Daniel SS et al: Vasopressin secretion induced by hypoxia in sheep: Developmental changes and relationship to b-endorphin. Am J Obstet Gynecol 143: 204, 1982 |
|
Oosterbaan HP, Swaab DF: Amniotic oxytocin and vasopressin in relation to human fetal development and labour. Early Hum Dev 10: 253, 1989 |
|
Sun K, Smith R, Robinson PJ: Basal and KCl-stimulated corticotropin-releasing hormone release from human placental syncytiotrophoblasts is inhibited by sodium nitroprusside. J Clin Endocrinol Metab 79: 519, 1994 |
|
Quartero HWP, Fry CH: Placental corticotrophin-releasing factor may modulate human parturition. Placenta 10: 439, 1989 |
|
Dubicke A, Akerud A, Sennstrom M et al. Different secretion patterns of matrix metalloproteinases and IL-8 and effect of corticotropin-releasing hormone in preterm and term cervical fibroblasts. Mol Hum Reprod. 2008 Nov;14(11):641-7. Epub 2008 Oct 15 |
|
Wolfe CD, Patel SP, Campbell EA et al: Plasma corticotropin-releasing factor (CRF) in normal pregnancy. Br J Obstet Gynaecol 95: 997, 1989 |
|
Wolfe CD, Patel SP, Campbell EA et al: Plasma corticotropin-releasing factor (CRF) in abnormal pregnancy. Br J Obstet Gynaecol 95: 1003, 1989 |
|
Lockwood CJ: Stress-associated delivery: The role of corticotropin-releasing hormone. Am J Obstet Gynecol 180: 264, 1999 |
|
Clifton VL, Read MA, Leitch IM et al: Corticotropin-releasing hormone-induced vasodilatation in the human fetal placental circulation. J Clin Endocrinol Metab 79: 666, 1994 |
|
Holzman C, Jetton J, Siler-Khodr T et al: Second trimester corticotropin-releasing hormone levels in relation to preterm delivery and ethnicity. Obstet Gynecol 97: 657, 2001 |
|
Leung TN, Chung TK, Madsen G et al: Rate of rise in maternal plasma corticotrophin-releasing hormone and its relation to gestational length. Br J Obstet Gynaecol 108: 527, 2001 |
|
Erickson K, Thorsen P, Chrousos G et al: Preterm birth: Associated neuroendocrine, medical, and behavioral risk factors. J Clin Endocrinol Metab 86: 2544, 2001 |
|
Korebrits C, Ramirez MM, Watson L et al: Maternal corticotropin-releasing hormone is increased with impending preterm birth. J Clin Endocrinol Metab 83: 1585, 1998 |
|
Leitch IM, Boura ALA, Botti C et al: Vasodilator actions of urocortin and related peptides in the human perfused placenta in vitro. J Clin Endocrinol Metab 83: 4510, 1998 |
|
Liotta AS, Houghten R, Krieger DT: Identification of a b-endorphin-like peptide in cultured human placental cells. Nature 195: 593, 1982 |
|
Odagiri ED, Sherrell BJ, Mount CD et al: Human placental immunoreactive corticotropin, lipotropin and b-endorphin: Evidence for a common precursor. Proc Natl Acad Sci USA 76: 2027, 1977 |
|
Lemalre S, Valetie A, Chouinard L et al: Purification and identification of multiple forms of dynorphin in human placenta. Neuropeptides 3: 18l, 1983 |
|
Carr BR, Parker CR, Madden JD et al: Maternal plasma adrenocorticotrophin and cortisol relationships through human pregnancy. Am J Obstet Gynecol 139: 416, 1981 |
|
Valetie A, Desprat R, Cros J et al: Immunoreactive dynorphine in maternal blood, umbilical vein and amniotic fluid. Neuropeptides 7: 145, 1986 |
|
Coland RS, Wardlaw SL, Stark RI et al: Human plasma b-endorphin during pregnancy, labor and delivery. J Clin Endocrinol Metab 52: 74, 1981 |
|
Wardlaw SL, Stark RI, Baxi L et al: Plasma bendorphin and b-lipotropin in the human fetus at delivery: Correlation with arterial pH and pO2. J Clin Endocrinol Metab 49: 888, 1979 |
|
Belisle S, Petit A, Gallo-Payet N et al: Functional opioid receptor sites in human placentas. J Clin Endocrinol Metab 66: 283, 1988 |
|
Petraglia F, Calza L, Giardino L et al: Identification of immunoreactive neuropeptide Y in human placenta: Localization, secretion and binding sites. Endocrinology 124: 2016, 1989 |
|
Petragtia F, Coukos G, Battaglia C et al: Plasma and amniotic fluid immunoreactive neuropeptide-Y level changes during pregnancy, labor and at parturition. J Clin Endocrinol Metab 69: 324, 1989 |
|
Khatun S, Kanayama N, Belayet H et al: Increased concentrations of plasma neuropeptide Y in patients with eclampsia and preeclampsia. Am J Obstet Gynecol 182: 896, 2000 |
|
Robidoux J, Simoneau L, St-Pierre S et al: Characterization of neuropeptide Y-mediated corticotropin-releasing factor synthesis and release from human placental trophoblasts. Endocrinol 141: 2795, 2000 |
|
Lockwood GM, Muttukrishna S, Ledger WL: Inhibins and activins in human ovulation, conception and pregnancy. Hum Reprod Update 4: 284, 1998 |
|
Petraglia F, Garuti GC, Calza L et al: Inhibin subunits in human placenta: Localization and messenger ribonucleic acid levels during pregnancy. Am J Obstet Gynecol 165: 750, 1991 |
|
Mayo KE, Cerelli GM, Speiss J et al: Inhibin A-subunit cDNAs from porcine ovary and human placenta. Proc Natl Acad Sci USA 83: 5849, 1986 |
|
Rabinovici J, Goldsmith PC, Librach CL et al: Localization and regulation of the activin-A dimer in human placental cells. J Clin Endocrinol Metab 75: 571, 1992 |
|
Yokoyama Y, Nakamura T, Nakamura R et al: Identification of activins and follistatin proteins in human follicular fluid and placenta. J Clin Endocrinol Metab 80: 915, 1995 |
|
Keelan J, Song Y, France JT: Comparative regulation of inhibin activin and human chorionic gonadotropin production by placental trophoblast cells in culture. Placenta 15: 803, 1994 |
|
Petraglia F, Calza L, Gartuti GC et al: Presence and synthesis of inhibin subunits in human decidua. J Clin Endocrinol Metab 71: 487, 1990 |
|
Fraser RF II, McAsey ME, Coney P: Inhibin-A and pro-alpha C are elevated in preeclamptic pregnancy and correlate with human chorionic gonadotropin. Am J Reprod Immunol 40: 37, 1998 |
|
Schneider-Kolsky M, D'Antona D, Evan LW et al: Maternal serum toptal activin A and follistatin in pregnancy and parturition. Br J Obstet Gynaecol 107: 995, 2000 |
|
Dalgliesh GL, Aitken DA, Lyall F et al: Placental and maternal serum inhibin-A and activin-A levels in Down's syndrome pregnancies. Placenta 22: 227, 2001 |
|
Wald NJ, Kennard A, Hackshaw AK et al: Antenatal screening for Down's syndrome. J Med Screen 4: 181, 1997 |
|
Wald NJ, Watt HC, Hackshaw AK: Integrated screening for Down's syndrome based on tests performed during the first and second trimesters. N Engl J Med 341: 461, 1999 |
|
Teglund S, Olsen A, Khan WN et al: The pregnancy specific glycoprotein (PSG) gene cluster on human chromosome 19: Five new genes forming a third subgroup within the carcinoembryonic antigen (CEA) family. Genomics 23: 669, 1994 |
|
Zheng Q-X, Tease LA, Shupert WL et al: Characterization of cDNAs of the human pregnancy-specific b1 glycoprotein family: A new subfamily of the immunoglobulin gene superfamily. Biochemistry 29: 2845, 1990 |
|
Lee JN, Grudzinskas JG, Chard T: Circulating levels of pregnancy proteins in early and late pregnancy in relation to placental tissue concentration. Br J Obstet Gynaecol 86: 888, 1979 |
|
Wu SM, Arnold LL, Rone J et al: Effect of pregnancy-specific beta 1-glycoprotein on the development of preimplantation embryo. Exp Biol Med 220: 169, 1999 |
|
Chou JY, Plouzek CA: Pregnancy-specific b glycoprotein. Semin Reprod Endocrinol 10: 116, 1992 |
|
Sakuragi N, Ohkubo H, Yamamoto R et al: The serum pregnancy-specific b1 -glycoprotein to beta-human chorionic gonadotropin ratio as an index of prognosis in patients with choriocarcinoma. Br J Obstet Gynaecol 95: 614, 1988 |
|
Wald NJ, Watt HC, Norgaard-Pedersen B et al: SP1 in pregnancies with Down syndrome in the first trimester of pregnancy. Prenat Diagn 19: 517, 1999 |
|
Bischof P: Purification and characterization of pregnancy-associated plasma protein-A. Semin Reprod Endocrinol 10: 127, 1992 |
|
Bischof P: Pregnancy-associated plasma protein-A. Semin Reprod Endocrinol 10: 127, 1992 |
|
Bischof P, DuBera S, Schindler AM et al: Endometrial and plasma concentrations of pregnancy-associated plasma protein-A (PAPP-A). Br J Obstet Gynaecol 89: 701, 1982 |
|
Lawrence JB, Oxvig C, Overgaard MT et al: The insulin-like growth factor (IGF)-dependent IGF binding protein-4 protease secreted by human fibroblasts is pregnancy-associated plasma protein-A. Proc Natl Acad Sci USA 96: 3149, 1999 |
|
Byun D, Mohan S, Yoo M et al: Pregnancy-associated plasma protein-A accounts for the insulin-like growth factor (IGF)-binding protein-4(IGFBP-4) proteolytic activity in human pregnancy serum and enhances the mitogenic activity of IGF by degrading IGFBP-4 in vitro. J Clin Endocrinol Metab 86: 847, 2001 |
|
Westergaard JG, Hau J, Teisner B et al: Specific and reversible interaction between pregnancy-associated plasma protein A and heparin. Placenta 4: 13, 1983 |
|
Bishof P, Meisser A, Haenggeli L et al: Pregnancy-associated plasma protein-A (PAPP-A) inhibits thrombin-induced coagulation of plasma. Thromb Res 32: 45, 1983 |
|
Vuoral P, Hatva E, Lymboussaki A et al: Expression of vascular endothelial growth factor and placental factor in human placenta. Biol Reprod 56: 489, 1997 |
|
Athanassiades A, Lala PK: Role of placenta growth factor (PLGF) in human extravillous trophoblast proliferation, migration and invasiveness. Placenta 19: 465, 1998 |
|
Livingston JC, Chin R, Haddad B et al: Reductions of vascular endothelial growth factor and placental growth factor concentrations in severe preeclampsia. Am J Obstet Gynecol 183: 1554, 2000 |
|
Livingston JC, Haddad B, Gorski LA et al: Placenta growth factor is not an early marker for the development of severe preeclampsia. Am J Obstet Gynecol 184: 1218, 2001 |