This chapter should be cited as follows:
Ford H, Meagher S, et al., Glob Libr Women's Med
ISSN: 1756-2228; DOI 10.3843/GLOWM.419223
The Continuous Textbook of Women’s Medicine Series – Obstetrics Module
Volume 18
Ultrasound in obstetrics
Volume Editors:
Professor Caterina M (Katia) Bilardo, Amsterdam UMC, Amsterdam and University of Groningen, Groningen, The Netherlands
Dr Valentina Tsibizova, PREIS International School, Florence, Italy

Chapter
Ultrasound Screening for Chromosomal and Genetic Abnormalities
First published: July 2025
Study Assessment Option
By answering four multiple-choice questions (randomly selected) after studying this chapter, readers can qualify for Continuing Professional Development points plus a Study Completion Certificate from GLOWM.
See end of chapter for details.
INTRODUCTION
Ultrasound examination at 11–13 weeks of gestation is a widely accepted tool for screening for fetal abnormalities. In addition to early detection of fetal abnormalities, advantages of screening at this gestation include accurate assessment of gestational age, diagnosis of multiple pregnancy and confirmation of chorionicity, as well as being an important part of aneuploidy screening.1,2,3 A pictorial guide based on the International Society of Ultrasound in Obstetrics and Gynecology (ISUOG)’s recommendations for structures that should be included in the first-trimester assessment was published in 2018 and updated more recently in 2023.3 This assessment can be performed by a transabdominal ultrasound approach, however, given multiple factors such as fetal age, fetal lie, presence of fibroids or other uterine anomalies or increased maternal body habitus, a transvaginal assessment may increase the yield of detection significantly.1,3 Anatomical examination at 11–13 weeks does not replace the need for a structural assessment of the fetus in the midtrimester, but rather provides an opportunity to assess the fetus for major structural abnormalities and risk of chromosomal and genetic abnormalities earlier in the pregnancy. The traditional role of the morphology ultrasound to assess for structural and potential chromosomal or genetic abnormalities will be discussed in this chapter, including the role of assessment of second-trimester markers of aneuploidy and assessment of structures that are not visible by the end of the first trimester.
DETECTION OF FETAL ANOMALIES
Whilst the mid-trimester examination is considered the official fetal anomaly screening assessment, due to its ability to detect a greater number of abnormalities as more structures are developed and amenable to visualization, an ultrasound performed at 11–13 weeks, when conducted using a standardized protocol, can identify many severe fetal abnormalities at a gestational age when termination of pregnancy may be technically easier and/or more acceptable to patients in some settings. ISUOG’s recommendations for structures that should be examined in the first trimester3 are listed in Table 1. In a multicenter, retrospective cohort study of over 100 000 women in the UK published in 2019, Syngelaki et al.2 demonstrated that trained ultrasound operators following a standardized protocol could confirm all cases of acrania, alobar holoprosencephaly, tricuspid or pulmonary atresia, encephalocele (Figure 1), exomphalos, gastroschisis and body-stalk anomaly through an 11–13-week assessment. Additionally, they detected a significant number of cases of open neural tube defects, hypoplastic left heart syndrome (Figure 2), atrioventricular septal defect, lower urinary tract obstruction, lethal skeletal dysplasia (Figure 3) and major limb abnormalities,2 as shown in Table 2.
1
Fetal structures that can be assessed on first-trimester ultrasound examination. Adapted from ISUOG's Practice Guidelines on performance of 11–14-week ultrasound scan3 *Indicates minimum requirement for scan.
Head | Axial view of head* Calcification of cranium* Contour/shape of cranium (with no bony defects)* Two brain halves separated by interhemispheric falx* Choroid plexuses almost filling lateral ventricles in their posterior two-thirds (butterfly sign)* Thalami Brainstem Cerebral peduncles with the aqueduct of Sylvius Intracranial translucency/fourth ventricle Cisterna magna |
Neck | Sagittal view of head and neck* Confirm whether nuchal translucency thickness <95th percentile* Exclude jugular sacs in neck |
Face | Forehead Orbits Nasal bone Maxilla Upper lip Mandible Retronasal triangle |
Thorax | Shape of thoracic wall Symmetrical lung fields Diaphragmatic continuity |
Heart | Heart inside chest with regular rhythm* Axial view of heart at the level of four-chamber view*; establish cardiac axis to left (30–60°) and occupying one-third of the thoracic space. Four-chamber view to establish two distinct ventricles on grayscale and color in diastole Left ventricular outflow tract (grayscale or color Doppler) Three-vessel-and-trachea view (grayscale or color Doppler) Antegrade ductus venosus A-wave on pulsed-wave Doppler/absence of tricuspid regurgitation Establish situs |
Abdomen | Stomach visible* and normally positioned on left Intact abdominal wall* with umbilical cord insertion visible Bladder visible and not dilated* (< 7 mm in sagittal view) Two umbilical arteries bordering bladder Kidneys: bilateral presence |
Spine | Regular shape and continuity of spine |
Extremities | Four limbs (each with three segments)* Hands and feet in normal orientation |
Placenta and amniotic fluid | Ascertain normal appearance without cystic structures* Location in relation to cervix and previous uterine cesarean section scar Cord insertion into placenta Amniotic fluid volume grossly normal Amniotic membrane and chorion dissociated physiologically |
Biometry | Crown–rump length* Nuchal translucency* Biparietal diameter* |

1
First-trimester demonstration of encephalocele at the posterior of the skull (occiput).

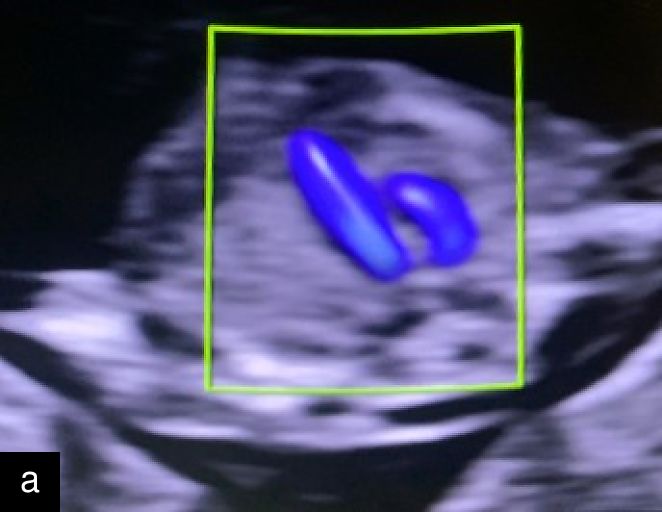
2
Hypoplastic left heart at 12 weeks. Arrow points to hypoplastic left ventricle (LV). RV, right ventricle.

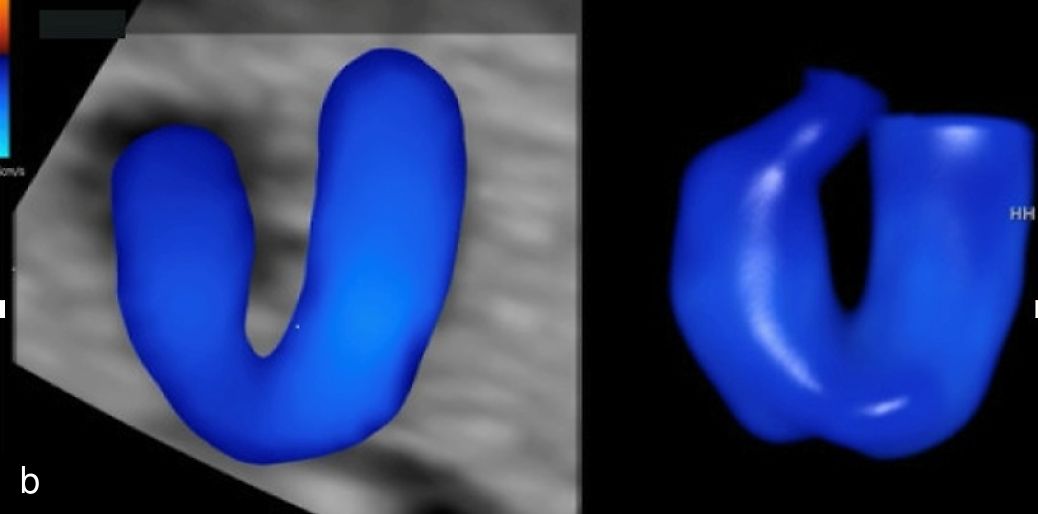
3
Skeletal dysplasia in the first trimester (3D render mode) with significantly shortened limb segments.
2
Numbers and proportions of fetal abnormalities detected by ultrasound in each trimester of pregnancy and postnatally in a study published by Syngelaki et al.2
Defect | n | Detection rate (%) | |||
First trimester | Second trimester | Third trimester | Postnatal | ||
Central nervous system | |||||
Acrania | 48 | 100 | – | – | – |
Alobar holoprosencephaly | 10 | 100 | – | – | – |
Encephalocele | 15 | 100 | – | – | – |
Open spina bifida | 59 | 59.3 | 40.7 | – | – |
Hypoplastic cerebellum/vermis | 15 | 13.3 | 86.7 | – | – |
Agenesis of the corpus callosum | 26 | 0 | 96.2 | 3.8 | – |
Microcephaly | 9 | 0 | 11.1 | 88.9 | – |
Craniosynostosis | 2 | 0 | 50.0 | 50.0 | – |
Blake’s pouch cyst | 4 | 0 | 100 | – | – |
Face | |||||
Anophthalmia/microphthalmia | 5 | 0 | 100 | – | – |
Cleft lip and palate | 52 | 34.6 | 65.4 | – | – |
Cleft palate only | 10 | 0 | 0 | 0 | 100 |
Cleft lip only | 28 | 0 | 85.7 | 0 | 14.3 |
Micrognathia | 7 | 14.3 | 85.7 | – | – |
Congenital diaphragmatic hernia | 24 | 29.2 | 58.3 | 8.3 | 4.2 |
Congenital pulmonary airway malformation | 43 | 0 | 90.7 | 9.3 | – |
Heart | |||||
Pulmonary atresia | 11 | 100 | – | – | – |
Hypoplastic left heart syndrome | 40 | 92.5 | 7.5 | – | – |
Atrioventricular septal defect | 11 | 90.9 | 9.1 | – | – |
Tetralogy of Fallot | 28 | 39.3 | 53.6 | 3.6 | 3.6 |
Transposition of the great arteries | 15 | 13.3 | 80.0 | 0 | 6.7 |
Ventricular septal defect | 136 | 0 | 71.3 | 22.8 | 5.9 |
Gastrointestinal tract | |||||
Right-sided stomach | 1 | 100 | – | – | – |
Esophageal atresia | 8 | 0 | 50.0 | 25.0 | 25.0 |
Duodenal atresia | 9 | 0 | 11.1 | 88.9 | – |
Imperforate anus | 3 | 0 | 0 | 0 | 100 |
Omphalocele | 44 | 100 | – | – | – |
Gastroschisis | 40 | 100 | – | – | – |
Genitourinary | |||||
Lower urinary tract obstruction | 52 | 71.2 | 21.2 | 7.7 | – |
Bilateral renal agenesis | 13 | 15.4 | 84.6 | – | – |
Bilateral polycystic kidneys | 14 | 7.2 | 71.4 | 21.4 | – |
Unilateral multicystic kidney | 58 | 0 | 87.9 | 12.1 | – |
Duplex kidney | 87 | 0 | 79.3 | 20.7 | – |
Horseshoe kidney | 5 | 0 | 80.0 | 20.0 | – |
Ovarian cyst | 27 | 0 | 0 | 100 | – |
Ambiguous genitalia | 5 | 0 | 80.0 | 0 | 20.0 |
Hypospadias | 26 | 0 | 3.8 | 0 | 96.2 |
Skeletal | |||||
Absent limb (hand/arm/leg/foot) | 24 | 75.0 | 25.0 | – | – |
Lethal skeletal dysplasia | 14 | 71.4 | 28.6 | – | – |
Talipes | 93 | 2.2 | 88.2 | 5.4 | 4.3 |
Some fetal anomalies cannot be definitively detected in the first trimester due to inadequate visualization or incomplete development of relevant structures.2,3,4 Examples include structures of the central nervous system (agenesis of the corpus callosum), chest and heart (pulmonary and aortic stenosis, ventricular septal defect, congenital airway pulmonary malformation), gastrointestinal tract (GIT) (esophageal or duodenal atresia, bowel obstruction) and genitourinary system abnormalities (multicystic kidney, horseshoe or duplex kidney, cloacal abnormality, ambiguous genitalia).2
Screening for aneuploidy has evolved from using a simple age-related risk cut-off to offer invasive diagnostic testing, to incorporating biochemical and ultrasound markers, and now includes cell-free DNA (cfDNA) screening technology, also known as non-invasive prenatal testing (NIPT). These screening options are discussed later in this chapter. The recommendation for an ultrasound examination to precede cfDNA testing at 8–11 weeks of gestation, primarily to exclude contraindications such as a non-viable pregnancy or ‘vanishing twin’, has improved ultrasound access in high-income countries and provided an opportunity for early assessment of the fetus from 10 weeks of gestation and subsequent population analysis at this stage. In an opinion piece by Rolnik et al.4 describing their own research in this area, the authors state that recent advances in ultrasound technology, such as high-frequency, high-resolution transvaginal probes and three-dimensional ultrasound modalities can facilitate a definitive diagnosis of some fetal defects, such as acrania and conjoined twins, at this early gestation, and additionally provide a high degree of suspicion for many other conditions, which can then be confirmed at the 11–14-week ultrasound assessment. Examples include nuchal edema with suspicion of major cardiac or other anomaly (Figure 4), major limb abnormalities such as sirenomelia and spinal defects.4 Early detection of major fetal anomalies prior to the 11-week stage in some cases may then guide the choice of screening or diagnostic modality, including moving straight to invasive testing rather than non-invasive screening options. Indeed, these authors concluded that, in their review of over 6000 ultrasound assessments performed prior to cfDNA testing, changes in management were observed in 1 in 10 pregnancies. These changes included delaying cfDNA testing (in cases of incorrect gestational dating), offering invasive testing instead of screening (in cases of fetal malformation or hydrops) and foregoing testing altogether (in cases of miscarriage or vanishing twin).4

4
Sagittal power Doppler ultrasound (with 'slowflow HD') image of 10-week fetus with increased nuchal translucency and omphalocele.
However, regardless of the type of aneuploidy screening performed, current recommendations still advocate for a mid-trimester assessment for all pregnant women. With respect to the mid-trimester evaluation of fetal anatomy, clear guidelines from ISUOG outline the structures that should be assessed5, as detailed in Table 3. These guidelines are useful for the development and implementation of clinical practice protocols in individual settings.
3
ISUOG suggested minimum and optional requirements for basic mid-trimester sonographic assessment of fetal anatomy.5
Head | Intact cranium Normal skull shape Cavum septi pellucidi (CSP) present and normal shape Midline falx Thalami Lateral cerebral ventricles normal in appearance and size (< 10 mm) Cerebellum normal in appearance and size Cisterna magna normal in appearance and size Nuchal fold normal in appearance and thickness (< 6 mm) |
Face | Orbits and lenses present Midsagittal profile Nasal bone Upper lip intact |
Neck | Absence of masses |
Chest/heart | Chest and lung normal in shape and size Fetal heart rate present Four-chamber view of heart (left side of chest; left-sided cardiac structures on left side of heart) Aortic and pulmonary outflow tracts normal in size and their relationship to each other Left ventricular outflow tract, three-vessel or three-vessel-and-trachea view normal No evidence of diaphragmatic hernia |
Abdomen | Stomach in normal position on left side Bowel not dilated or echogenic Gallbladder normally positioned on right Both kidneys present, no dilatation in the anteroposterior diameter Urinary bladder normal in appearance Cord insertion into anterior abdominal wall |
Skeletal | No spinal defects or masses (transverse and sagittal views) Arms and hands present; normal joint positioning Legs and feet present; normal joint positioning |
Placenta | Placental site and relationship to cervix |
Umbilical cord | Three-vessel cord Cord insertion into placenta |
Genitalia | Sex and appearance of genitalia |
Cervix | Cervical length |
ANEUPLOIDY SCREENING
Options for aneuploidy screening vary between countries, both in regard to differences between availability and accessibility of screening in low- vs middle- or high-income countries, as well as based on recommendations from national consensus groups. The detection rates of the different methods of fetal aneuploidy screening are presented in Table 4.
4
Detection rates of fetal trisomy 21 of different screening methods used over time, at a fixed false-positive rate of 5%. (Adapted from The 11–13+6 weeks scan: Fetal Medicine Foundation.6)
Method of screening | Detection rate (%) |
Maternal age | 30 |
Maternal age and second-trimester serum screening | 50–70 |
Maternal age and fetal nuchal translucency at 11–13+6 weeks | 70–80 |
Maternal age, nuchal translucency and maternal serum free beta-hCG and PAPP-A at 11–13+6 weeks | 85–90 |
Maternal age, nuchal translucency and fetal nasal bone at 11–13+6 weeks | 90 |
Maternal age, nuchal translucency, nasal bone, maternal serum free beta-hCG and PAPP-A at 11–13+6 weeks | 95 |
hCG, human chorionic gonadotropin; PAPP-A, pregnancy-associated plasma protein-A.
Age-based assessment of risk for trisomy 21
Before the development of the various non-invasive screening methods available today, age-based risk stratification was the only approach for prenatal screening for trisomy 21 (Down syndrome). Women above a certain age threshold were offered invasive testing based solely on their age-related risk. For example, in the UK, women aged ≥38 years were offered invasive testing, which resulted in a detection rate of approximately 30% at a screen-positive rate of about 5%.6
Markers of trisomy 21 in the second trimester
Mid-trimester ultrasound (at 19–24 weeks of gestation) was one of the first methods used in routine practice to assess for trisomy 21,7 with many studies reporting anomalies such as ventriculomegaly, absent or hypoplastic nasal bone, increased nuchal fold thickness, intracardiac echogenic foci, aberrant right subclavian artery, short femur or humerus, echogenic bowel, and hydronephrosis as markers of trisomy 21.8 This is because such ‘markers’ are more commonly seen in fetuses with trisomy 21 than in those with normal karyotype. However, the strengths of these associations are variable, with some of these markers remaining important in the absence of other findings, whilst others do not increase the risk of trisomy 21 when found in isolation. A large meta-analysis by Agathokleous et al.8 published in 2013 reviewed these markers and their association with trisomy 21, providing positive and negative likelihood ratios for each marker. These can be used to help determine those at increased risk, in combination with results from combined first-trimester screening, or in isolation with age-related risk when these results are not available. Of note, in the absence of any of the defined markers in this meta-analysis, there was a 7.7-fold reduction in the risk of trisomy 21.8 When using the markers in isolation, there is a 3- to 4-fold increase in risk in the presence of either ventriculomegaly, increased nuchal fold thickness or aberrant right subclavian artery, and a 6- to 7-fold increase with hypoplastic or absent nasal bone.8 However, when performing a detailed morphological ultrasound examination, these markers are best used along with their positive and negative likelihood ratios to determine the combined likelihood ratio and the risk for a patient rather than using the markers in isolation.8
Second-trimester serum screening for trisomy 21 (quadruple test)
In the 1980s, it was discovered that certain biochemical markers in maternal blood differed between pregnancies with trisomy 21 and those with normal karyotype. This led to the development of maternal serum screening. The screening panel measures four markers from 16 weeks of gestation: alpha-fetoprotein, unconjugated estriol, human chorionic gonadotropin and inhibin A. When these results are combined with maternal age, the test can identify approximately 70% of fetuses with trisomy 21 at a false-positive rate of approximately 5%.6
Nuchal translucency and combined first-trimester screening assessment for trisomy 21
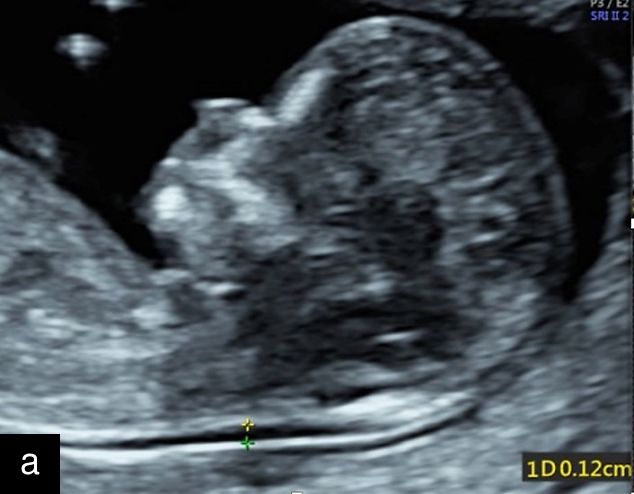
From the 1990s, the measurement of nuchal translucency was standardized and measurement protocols developed as a non-invasive way of risk assessment. Nuchal translucency thickness (Figure 5) can be measured between 11+0 and 13+6 weeks, which is equivalent to a crown–rump length of 45–84 mm.6 When combined with maternal age, this was shown to identify approximately 75% of affected fetuses with a false-positive rate of 5%.6 Research into additional maternal first-trimester serum biomarkers, specifically the free beta subunit of human chorionic gonadotropin (free β-hCG) and pregnancy-associated plasma protein A (PAPP-A), demonstrated that, when combined with maternal age and nuchal translucency, detection rates increased to approximately 90% at a fixed false-positive rate of 5%. The inclusion of additional first-trimester ultrasound markers, such as the presence or absence of the nasal bone, further improved detection rates to over 95%.6 A predetermined risk cut-off is often used to define 'high risk,' such as 1 : 300 or 1 : 150. However, individual patient factors, biochemical results and the nuchal translucency measurement should all be considered alongside the calculated risk value. For example, a risk of 1 : 200 at maternal age of 42 is actually lower than the age-related risk alone but still corresponds to a 0.5% probability. Conversely, a markedly increased nuchal translucency may elevate the risk of trisomy 21, but should also prompt detailed assessment of fetal anatomy, particularly when biochemical markers are within normal limits, as there may be an underlying structural anomaly causing the increased nuchal translucency. In such cases, if a fetal abnormality is detected, counseling regarding invasive diagnostic testing is a reasonable next step.
|
|
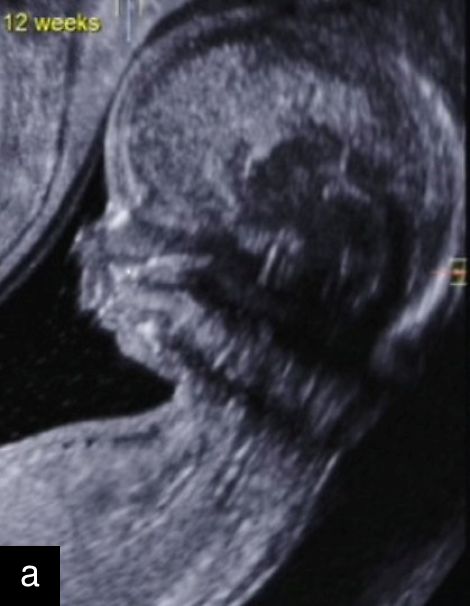
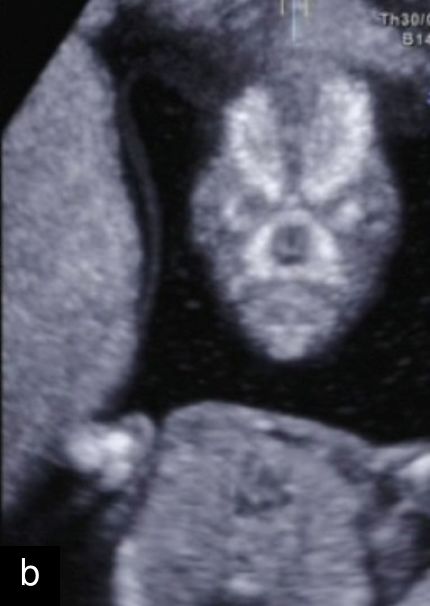
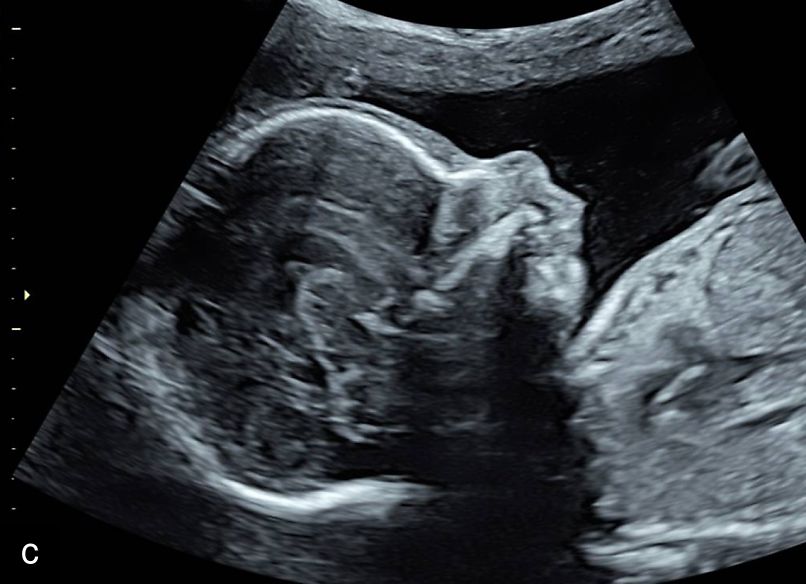
5
Sagittal ultrasound images, showing normal nuchal translucency (NT) at 12 weeks (a) and increased NT at 13 weeks (b).
Cell-free DNA screening
Trisomy 21 is not the only common aneuploidy amenable to screening. Trisomy 18 (Edward syndrome) can be detected with the second-trimester maternal serum screen, and risks for trisomies 13 (Patau syndrome) and 18 can be calculated for those undergoing a first-trimester combined screening assessment. A large meta-analysis published in 2014 by Metcalfe et al.9 found detection rates of 83.1% for trisomy 13 (with a false-positive rate of 4.4%) and 91.9% for trisomy 18 (with a false-positive rate of 5.4%) using the combined screening assessment. These common aneuploidies were among the first to be evaluated for detection through fetal cfDNA analysis. What was initially assumed to be fetal DNA, and therefore thought to be a potential diagnostic test, was then found to be trophoblastic DNA fragments from the placenta, and forms the basis of cfDNA testing as currently performed.
Given the improved detection rate, in higher-income countries there has been an increased uptake in cfDNA as a first-line aneuploidy screening tool. However, the significant cost of this test and the lack of consistent cost-effectiveness data on universal cfDNA screening compared to other screening modalities, has prevented universal uptake, even amongst higher-resource settings. At the time of writing, Belgium and the Netherlands are using cfDNA as their first-line test, and there have been calls in the UK to consider a risk-based (contingent) model to use cfDNA as a second-line screening test.10 In this scenario, if screening is offered, combined first-trimester screening is first undertaken and, if the risk of aneuploidy is between 1 : 150 and 1 : 1000 (so-called ‘intermediate risk’), cfDNA is offered as a second-line test.11,12 For those with a risk <1 : 1000, no further testing is undertaken, and those with a risk of greater than or equal to 1 : 150 are offered invasive testing or cfDNA testing.12 If structural abnormalities are detected, then invasive testing rather than cfDNA screening is recommended.10,11,12 Many other countries leave the choice of screening up to the individual practitioner, which requires an important discussion regarding pretest probability, appropriate counseling in cases of abnormal results, and a possible recommendation for invasive testing based on the result.
cfDNA screening is a constantly evolving space. Whilst outside the scope of this chapter, cfDNA is increasingly being used to screen for sex chromosome abnormalities, rare autosomal trisomies, deletions and duplications, and microdeletion syndromes such as 22q.11 (formerly known as DiGeorge) syndrome. However, cfDNA testing for these rarer conditions has not been properly clinically validated, performance is lower than that for the common trisomies, and counseling is often challenging due to the variable and commonly unpredictable phenotype, all of which dramatically increase the need for practitioner education and pretest counseling on the limitations of the test. Positive predictive values (PPV) for sex chromosome abnormalities overall may be in the order of 50%13 (with PPV depending on the sex chromosome abnormality in question)13 and rare autosomal trisomies only approximately 4%,14 all of which should be clearly outlined to the patient prior to undertaking these tests. The decision to undergo this screening should be made with the knowledge that it may lead to higher rates of false-positive results that cannot be confirmed antenatally without the need for invasive testing.
FETAL ANOMALIES AND CHROMOSOMAL ABNORMALITY
Fetal anomalies highly suggestive of chromosomal abnormality
When multiple fetal abnormalities are seen, the suspicion of an underlying chromosomal or syndromic cause should be raised. However, there are some abnormalities that, even when seen in isolation, should raise the suspicion for an underlying chromosomal abnormality. Fetal abnormalities and markers commonly seen with the most prevalent chromosomal or genetic syndromes are listed in Table 5, and examples of fetal structural abnormalities are shown in Figures 6–12.
5
Common fetal abnormalities and markers seen with trisomy 13, trisomy 18, trisomy 21, Turner syndrome (monosomy X) and Di George syndrome (22q11 microdeletion).
Chromosomal abnormality | Structural abnormalities | Additional markers |
Trisomy 13 | First trimester: complex cardiac abnormality (see second-trimester findings), omphalocele,15,16 holoprosencephaly,15,16 megacystis,15,16 complex cardiac abnormalities,15 cleft lip/palate,15 limb abnormalities (postaxial polydactyly15 [Figure 6], clenched or overlapping digits)15 Second trimester (additional to above): brachycephaly,15 microcephaly,15 posterior fossa abnormalitY/Dandy–Walker malformation,15 ventriculomegaly,15 neural tube defect,15 micrognathia,15 cardiac abnormalitY (VSD,15,17 ASD,15,17 DORV,15,17 HLHS,15,17 anomalous pulmonary venous return,15 isomerism)17, CDH15 [Figure 7], renal cystic dysplasia,15 multicystic kidney,15 enlarged and/or echogenic kidney,15 hydronephrosis,15 rocker-bottom feet15 *> 90% detection rate using the findings of increased NT, fetal tachycardia, holoproscencephaly, omphalocele and megacystis16 | Increased nuchal translucency,15,16 fetal tachycardia,15,16 absent nasal bone,15 small for dates/FGR15 |
Trisomy 18 | First trimester: complex cardiac abnormality (see second-trimester findings), omphalocele,18,19 cleft lip/palate,18 limb abnormality19,20 (clenched hands, abnormal finger positioning or overlapping fingers, absence of hypoplasia of forearm bones), complex cardiac abnormality19 Second trimester (additional to above): strawberry-shaped skull,18,19 ventriculomegaly,18 posterior fossa cyst,18 choroid plexus cyst,18,19 cerebellar hypoplasia,19 facial cleft,18 micrognathia,18,19 esophageal atresia,18,19 diaphragmatic hernia,18 cardiac abnormality (AVSD,17,19 VSD,17,19 TOF,17,19 double outlet left ventricle,17 TGA)17,18, omphalocele,18 renal abnormality18 including megacystis,19 neural tube defect,19 talipes,18,19 rocker-bottom feet20 | Increased nuchal translucency,18,19 small for dates/FGR,18,19 relative bradycardia18 |
Trisomy 21 | First trimester: complex cardiac abnormality (AVSD)17,21 Second trimester (additional to above): other cardiac abnormality (ASD,17 VSD17,21), evidence of hydrops21 (ascites, pericardial or pleural effusion), ventriculomegaly8 | Increased nuchal translucency,21 increased nuchal fold,8 echogenic bowel,8 renal pyelectasis,8 short femur,8 short humerus,8 aberrant right subclavian artery,8 absent or hypoplastic nasal bone8 |
Turner syndrome | First trimester: cystic hygroma,17 HLHS17 Second trimester (additional to above): coarctation of the aorta,17 aortic stenosis,17 horseshoe kidney17 | |
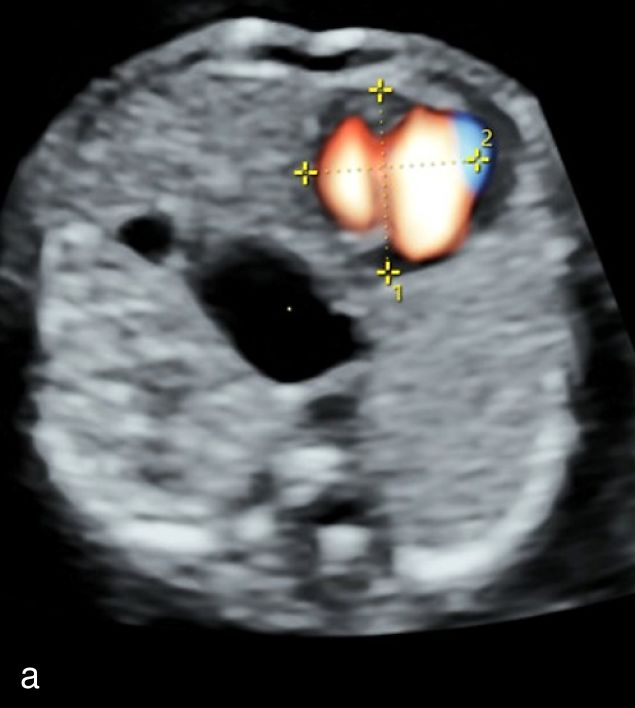
Di George syndrome | First trimester: complex cardiac abnormality (see second-trimester findings), cleft palate17 Second trimester (additional to above): cardiac abnormality (right-sided aortic arch17,22 [Figure 8], double aortic arch,22 TOF,17 DORV,17 truncus arteriosis,17 TGA),17 microcephaly,17 micrognathia,17 thymus aplasia,17 hydronephrosis17 |
ASD, atrial septal defect; AVSD, atrioventricular septal defect; CDH, congenital diaphragmatic hernia; DORV, double outlet right ventricle; FGR, fetal growth restriction. HLHS, hypoplastic left heart syndrome; TGA, transposition of the great arteries; TOF, tetralogy of Fallot; VSD, ventricular septal defect.

6
Post-axial polydactyly of the foot in the first trimester (3D render mode).
|
|
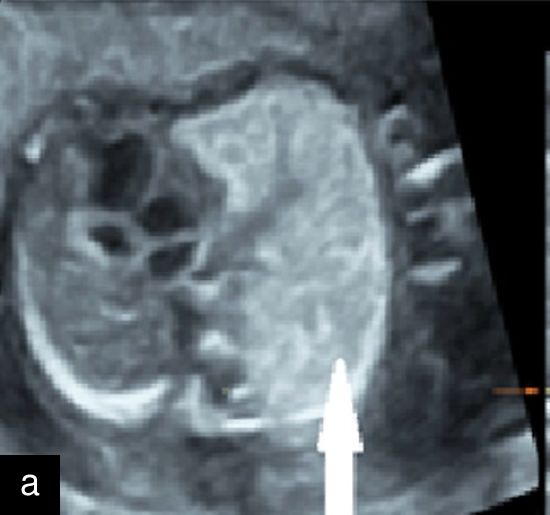
7
(a) Congenital diaphragmatic hernia at 13 weeks with a four-chamber view of the heart (in color) with mediastinal shift and stomach visible in left hemithorax. (b) Congenital diaphragmatic hernia at 20 weeks with a four-chamber view of the heart with the small left ventricle and mediastinal shift, and stomach visible in left hemithorax.
|
|
8
Ultrasound imaging of right-sided aortic arch at 12+3 weeks’ gestation using color Doppler (a) and spatiotemporal image correlation (b) modes.
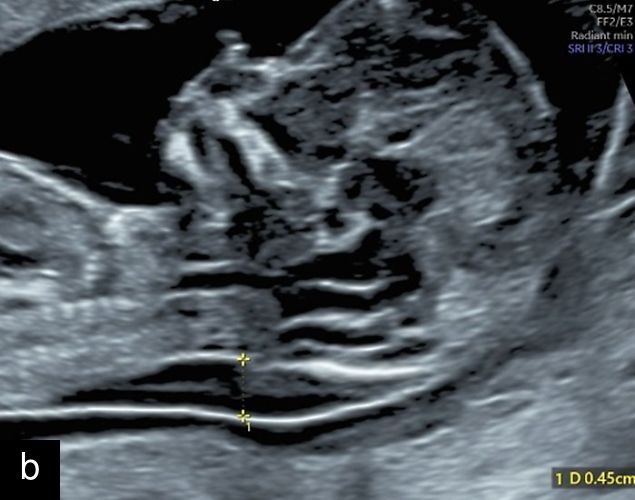
Micrognathia
Micrognathia refers to the arrested development of the fetal mandible, resulting in a small chin17 (Figure 9). Agnathia is the most severe form of mandible maldevelopment, with complete agenesis of the mandible,17 and retrognathia refers to a chin that is posteriorly positioned when compared in position to the maxilla,17,23,24 and the term is commonly used synonymously with micrognathia.23 The prevalence of micrognathia is approximately 1 : 150023 and is associated with over 300 genetic abnormalities, including trisomy 13, trisomy 18, Turner syndrome and DiGeorge Syndrome.23 It may also be seen with primary mandibular disorders, such as Pierre Robin sequence (particularly if seen with cleft palate),17,23 skeletal dysplasias23 and other syndromes such as Meckel–Gruber syndrome,23 Noonan’s syndrome23 and Smith–Lemli–Opitz syndrome.23 Aside from the association with chromosomal and syndromic causes, micrognathia is an important finding due to its potential for postnatal complications including airway obstruction at birth and feeding difficulties.23
|
|
|
9
Micrognathia at 12 weeks on sagittal (a) and coronal (b) views and at 20 weeks on sagittal view (c).
Micrognathia can be determined subjectively but, more recently, diagnostic indices such as the inferior facial angle (comparing the frontal bone to a line drawn at the angle of the mentum), frontonasomental angle (comparing the frontal bone to a line drawn at the tip of the nose and mentum) and jaw index (comparing the axial mandibular diameter to the anteroposterior distance of the mandible) have been developed.23,24 Micrognathia may be less apparent with continued growth and development, particularly if diagnosed in the first or early second trimester, and therefore evaluation with these diagnostic indices may be particularly useful in these cases, along with careful follow-up.23
Omphalocele
Herniation of bowel at the umbilical cord insertion site is a physiological process, with the bowel contents expected to return to the abdomen by 13 weeks of gestation.25 Omphalocele (also known as exomphalos) is the failure of the contents to be relocated by this gestation; it may also contain liver along with bowel loops17,26 but rarely stomach,26 and its prevalence is approximately 1 in 5000.27 It can be distinguished from gastroschisis by the sac seen encompassing the bowel contents through the midline herniation at the level of the umbilicus, as opposed to free loops of bowel through a paraumbilical defect.17 Up to 60% of omphalocele may be explained by an underlying chromosomal abnormality or syndrome,27 including trisomy 18, trisomy 13, Beckwith–Wiedemann syndrome and pentalogy of Cantrell.17 This is in stark contrast to gastroschisis, in which associated chromosomal abnormality is rare.17 Aside from the association with underlying chromosomal abnormality, omphalocele is an important diagnosis to make when planning for delivery, particularly with respect to being in a location in which pediatric surgical services are available to plan for primary or delayed closure.26
Rib abnormalities
An increased number of ribs is rarely a normal variant, and is usually associated with trisomy 21 or VACTERL sequence (vertebral anomalies, anal atresia, cardiac defects, tracheoesophageal fistula, renal anomalies and limb defects).17 A reduced number of ribs may also be seen with trisomy 21, or skeletal dysplasias such as cleidocranial dysplasia and campomelic dysplasia.17 Short ribs are a common finding in many skeletal dysplasias, particularly those that are considered lethal due to the reduced thoracic circumference and associated pulmonary hypoplasia.17 These abnormalities may be seen in both the first and second trimesters; particularly in the case of skeletal dysplasias considered to be lethal, these abnormalities may be evident in the first trimester.
Fetal anomalies unlikely linked to chromosomal abnormality
When seen in isolation, the following fetal anomalies have a low risk of associated chromosomal abnormalities or genetic syndromes. As with all abnormalities that are seen, a careful fetal ultrasound examination should be performed to exclude additional abnormalities.
Muscular VSD
The classification of VSD depends on where within the septum the defect is seen, and may include the membranous septum (perimembranous VSD), muscular septum (muscular VSD, Figure 10) and atrioventricular valve inlet region (inlet VSD).28 Muscular VSDs are seen in up to 5% of term fetuses.29 The muscular portion of the septum can be divided into the mid-muscular region and apical region, with defects in the mid-muscular region approximately five times more common than those in the apical region.28 Isolated muscular VSDs are unlikely to be seen in the first trimester, although membranous VSDs and those associated with complex cardiac anomalies can be seen. While VSD accounts for approximately 30% of cardiac abnormalities in fetuses affected by trisomy 21, when muscular VSD is seen in isolation, there is unlikely to be an additional increased risk above baseline for trisomy 21 or chromosomal abnormalities in general.28,29 Whilst this is the most commonly seen congenital cardiac abnormality, when in isolation, spontaneous closure rates have been quoted as up to 90%.29

10
Ventricular septal defect with associated tricuspid atresia demonstrated with spatiotemporal image correlation (STIC) mode. MV, mitral valve; TV, tricuspid valve.
Congenital pulmonary airway malformation (CPAM)
CPAM (Figure 11) and bronchopulmonary sequestration are congenital lung malformations that occur in approximately 1 in 8000 births.30 CPAMs are cystic or solid lung masses, which may take on a macrocystic or microcystic appearance in the fetal lung, and are formed by an abnormal proliferation of immature respiratory bronchioles, for which the blood supply arises from the pulmonary vessels.30 This is in comparison to bronchopulmonary sequestration, in which the blood supply arises from the systemic circulation. If seen in isolation, these echogenic lung lesions are not commonly associated with chromosomal abnormalites.17 Fetuses with very large CPAM are at risk of hydrops, however, outcomes are generally good for children born with isolated CPAM, with reported survival rates over 95%.30 CPAM is a diagnosis predominantly made in the second trimester, although a very large CPAM may be seen in the first trimester if it causes mediastinal shift, and along with congenital diaphragmatic hernia, should be included in the differential diagnosis.
|
|
11
Axial (a) and sagittal (b) views of congenital pulmonary airway malformation (arrow) in the left lung with mediastinal shift at 20 weeks.
Gastroschisis
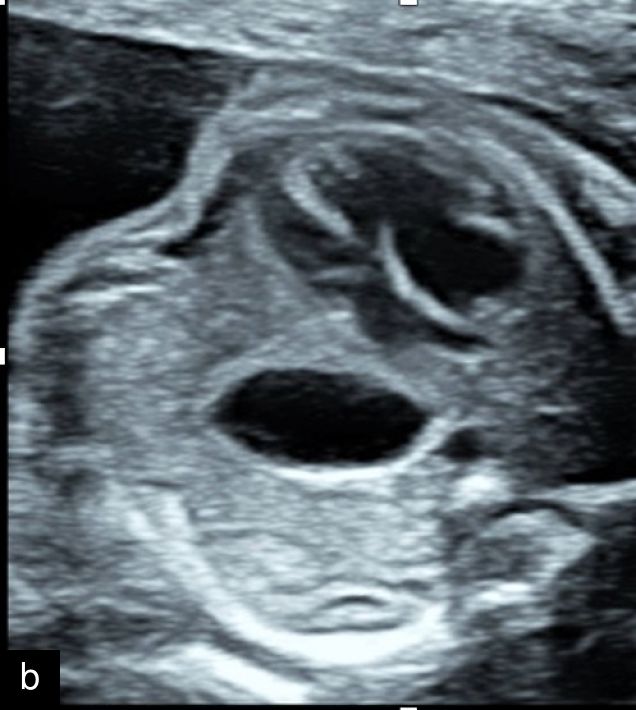
Gastroschisis (Figure 12) is an anterior abdominal wall defect, typically to the right side of the umbilical cord insertion site, which allows free loops of bowel to herniate outside the abdominal cavity and float freely in the amniotic fluid.31 Rarely, the stomach, bladder and internal genitalia can be exteriorized, and it can be differentiated from an omphalocele due to the lack of surrounding tissue membrane and a normal umbilical cord insertion site being identified.31 While risk factors such as young maternal age and recreational substance use have been attributed, it is not usually associated with chromosomal abnormalities when seen in isolation.31 However, the fetus should be carefully examined for both additional abnormalities, which increase suspicion for an underlying chromosomal or syndromic cause, and to exclude a rupture of an omphalocele membrane by carefully examining the cord insertion site.31 Fetal growth restriction is commonly associated with gastroschisis, however,31 and there is an increased risk of fetal distress, intrauterine fetal death and postnatal morbidity and mortality related to bowel complications.31 Diagnosis should prompt immediate referral to a center that can provide neonatal care and pediatric surgical services.31

12
Gastroschisis at 23 weeks, with free loops of bowel seen within the amniotic fluid.
CONCLUSION
Screening for fetal anomalies with ultrasound requires careful examination of the fetus, which is aided greatly by consensus and evidence-based standardized protocols to ensure that the fetus is adequately assessed at each gestation. It should always be remembered that ultrasound is an assessment of fetal structure, not of fetal genetics, and the diagnosis of fetal anomalies on ultrasound should always lead a clinician to consider the need for genetic testing. Even in the absence of fetal anomalies on ultrasound, screening for common chromosomal abnormalities such as trisomies 21, 18 and 13 should be considered. Whilst there are some ultrasound anomalies that, when seen in isolation, cause little concern for an underlying chromosomal or syndromic abnormality, such as muscular VSD or CPAM, other ultrasound anomalies, such as micrognathia, should always raise suspicion of an associated genetic abnormality, even when seen in apparent isolation.
PRACTICE RECOMMENDATIONS
- Perform a comprehensive ultrasound scan at 11–13 weeks of gestation to assess fetal anatomy, confirm gestational age, identify multiple pregnancies and chorionicity, and initiate early aneuploidy screening.
- When transabdominal imaging is suboptimal due to fetal position, maternal habitus or presence of fibroids, use a transvaginal approach to improve visualization and diagnostic yield.
- Follow standardized protocols for first-trimester anatomy scans. Adhere to ISUOG guidelines for anatomical structures to be assessed during first-trimester screening to enhance early detection of major anomalies.
- Reinforce that the 11–13-week scan does not replace the midtrimester scan (typically at 19–24 weeks), which remains essential for comprehensive evaluation of fetal structures.
- Incorporate combined first-trimester screening where available. Use maternal age, nuchal translucency thickness and biochemical markers (free β-hCG and PAPP-A) for more accurate trisomy 21 risk stratification and improve detection rates up to 90–95%.
- Use cell-free DNA (cfDNA) screening as part of a contingent model, offering it as a second-line test in intermediate-risk cases (e.g. 1 : 150–1 : 1000 based on combined screening), to balance sensitivity and cost-effectiveness.
- Always precede cfDNA testing with ultrasound. Conduct an early ultrasound (8–11 weeks) prior to cfDNA testing to confirm viability and rule out contraindications such as a vanishing twin or inaccurate gestational age.
- Refer for invasive testing when structural abnormalities are found. If major fetal structural anomalies are identified at any stage, prioritize referral for invasive diagnostic testing over cfDNA due to limitations in screening accuracy.
- Counsel patients about the reduced predictive value of cfDNA for rare chromosomal abnormalities (e.g. sex chromosome aneuploidies, microdeletions) and the possibility of false positives.
- In the context of isolated abnormalities, such as muscular VSD or CPAM, do not automatically assume chromosomal abnormality but perform a thorough anatomical survey to rule out additional findings.
- Use positive and negative likelihood ratios to interpret second-trimester soft markers for trisomy 21 (e.g. nuchal fold, nasal bone absence) instead of relying on individual markers in isolation.
- Plan delivery appropriately for surgical conditions. For fetal anomalies such as omphalocele, ensure delivery in a tertiary center with access to pediatric surgery to facilitate optimal neonatal management and outcomes.
CONFLICTS OF INTEREST
The author(s) of this chapter declare that they have no interests that conflict with the contents of the chapter.
Feedback
Publishers’ note: We are constantly trying to update and enhance chapters in this Series. So if you have any constructive comments about this chapter please provide them to us by selecting the "Your Feedback" link in the left-hand column.
REFERENCES
Springhall EA, Rolnik DL, Reddy M, Ganesan S, Maxfield M, Ramkrishna J, Meagher S, Teoh M, da Silva Costa F. How to perform a sonographic morphological assessment of the fetus at 11–14 weeks of gestation. Australas J Ultrasound Med. 2018 Aug 22;21(3):125–137. doi: 10.1002/ajum.12109. | |
Syngelaki A, Hammami A, Bower S, Zidere V, Akolekar R, Nicolaides KH. Diagnosis of fetal non-chromosomal abnormalities on routine ultrasound examination at 11–13 weeks' gestation. Ultrasound Obstet Gynecol. 2019;54(4):468–476. doi: https://doi.org/10.1002/uog.20844 | |
International Society of Ultrasound in Obstetrics and Gynecology; Bilardo CM, Chaoui R, Hyett JA, Kagan KO, Karim JN, Papageorghiou AT, Poon LC, Salomon LJ, Syngelaki A, Nicolaides KH. ISUOG Practice Guidelines (updated): performance of 11–14-week ultrasound scan. Ultrasound Obstet Gynecol. 2023 Jan;61(1):127–143. doi: 10.1002/uog.26106. | |
Rolnik DL, Wertaschnigg D, Benoit B, Meagher S. Sonographic detection of fetal abnormalities before 11 weeks of gestation. Ultrasound Obstet Gynecol. 2020;55(5):565–574. doi: https://doi.org/10.1002/uog.21921 | |
Salomon LJ, Alfirevic Z, Berghella V, Bilardo CM, Chalouhi GE, Da Silva Costa F, Hernandez-Andrade E, Malinger G, Munoz H, Paladini D, Prefumo F, Sotiriadis A, Toi A, Lee W. ISUOG Practice Guidelines (updated): performance of the routine mid-trimester fetal ultrasound scan. Ultrasound Obstet Gynecol. 2022 Jun;59(6):840–856. doi: 10.1002/uog.24888. Epub 2022 May 20. Erratum in: Ultrasound Obstet Gynecol. 2022 Oct;60(4):591. doi: 10.1002/uog.26067. | |
Nicolaides KH. The 11–13+6 weeks scan: Fetal Medicine Foundation, London, 2004. | |
Vintzileos AM, Egan JF. Adjusting the risk for trisomy 21 on the basis of second-trimester ultrasonography. Am J Obstet Gynecol. 1995 Mar;172(3):837–44. doi: 10.1016/0002-9378(95)90008-x. PMID: 7892872. | |
Agathokleous M, Chaveeva P, Poon LC, Kosinski P, Nicolaides KH. Meta-analysis of second-trimester markers for trisomy 21. Ultrasound Obstet Gynecol. 2013 Mar;41(3):247–61. doi: 10.1002/uog.12364. Epub 2013 Jan 24. PMID: 23208748. | |
Metcalfe A, Hippman C, Pastuck M, Johnson JA. Beyond Trisomy 21: Additional Chromosomal Anomalies Detected through Routine Aneuploidy Screening. J Clin Med. 2014 Apr 8;3(2):388–415. doi: 10.3390/jcm3020388. PMID: 26237381; PMCID: PMC4449689. | |
RCOG. Non-invasive Prenatal Testing for Chromosomal Abnormality using Maternal Plasma DNA (Scientific Impact Paper No. 15). 2014. | |
Bowden B, de Souza S, Puchades A, Williams K, Morgan S, Anderson S, Tucker D, Hillier S. Implementation of non-invasive prenatal testing within a national UK antenatal screening programme: Impact on women's choices. Prenat Diagn. 2022 May;42(5):549–556. doi: 10.1002/pd.6131. Epub 2022 Mar 24. PMID: 35278232. | |
Chitty LS, Wright D, Hill M, Verhoef TI, Daley R, Lewis C, Mason S, McKay F, Jenkins L, Howarth A, Cameron L, McEwan A, Fisher J, Kroese M, Morris S. Uptake, outcomes, and costs of implementing non-invasive prenatal testing for Down's syndrome into NHS maternity care: prospective cohort study in eight diverse maternity units. BMJ. 2016 Jul 4;354:i3426. doi: 10.1136/bmj.i3426. PMID: 27378786; PMCID: PMC4933930. | |
Bussolaro S, Raymond YC, Acreman ML, Guido M, Da Silva Costa F, Rolnik DL, Fantasia I. The accuracy of prenatal cell-free DNA screening for sex chromosome abnormalities: A systematic review and meta-analysis. Am J Obstet Gynecol MFM. 2023 Mar;5(3):100844. doi: 10.1016/j.ajogmf.2022.100844. Epub 2022 Dec 24. PMID: 36572107. | |
Raymond YC, Fernando S, Menezes M, Meagher S, Mol BW, McLennan A, Scott F, Mizia K, Carey K, Fleming G, Rolnik DL. Cell-free DNA screening for rare autosomal trisomies and segmental chromosome imbalances. Prenat Diagn. 2022 Oct;42(11):1349–1357. doi: 10.1002/pd.6233. Epub 2022 Sep 22. PMID: 36068932; PMCID: PMC9826090. | |
Chen CP. Prenatal sonographic features of fetuses in trisomy 13 pregnancies (III). Taiwan J Obstet Gynecol. 2009 Dec;48(4):342–9. doi: 10.1016/S1028-4559(09)60322-3. PMID: 20045754. | |
Papageorghiou AT, Avgidou K, Spencer K, Nix B, Nicolaides KH. Sonographic screening for trisomy 13 at 11 to 13(+6) weeks of gestation. Am J Obstet Gynecol. 2006 Feb;194(2):397–401. doi: 10.1016/j.ajog.2005.08.010. PMID: 16458636. | |
Sivanathan J, Thilaganathan B. Book: Genetics for obstetricians and gynaecologists: Chapter: Genetic markers on ultrasound scan. Best Pract Res Clin Obstet Gynaecol. 2017 Jul;42:64–85. doi: 10.1016/j.bpobgyn.2017.03.005. Epub 2017 Mar 21. PMID: 28456373. | |
Sherod C, Sebire NJ, Soares W, Snijders RJ, Nicolaides KH. Prenatal diagnosis of trisomy 18 at the 10–14-week ultrasound scan. Ultrasound Obstet Gynecol. 1997 Dec;10(6):387–90. doi: 10.1046/j.1469-0705.1997.10060387.x. PMID: 9476321. | |
Viora E, Zamboni C, Mortara G, Stillavato S, Bastonero S, Errante G, Sciarrone A, Campogrande M. Trisomy 18: Fetal ultrasound findings at different gestational ages. Am J Med Genet A. 2007 Mar 15;143A(6):553–7. doi: 10.1002/ajmg.a.31615. PMID: 17318852. | |
Rosa RF, Rosa RC, Lorenzen MB, Zen PR, Oliveira CA, Graziadio C, Paskulin GA. Limb abnormalities on trisomy 18: evidence for early diagnosis. J Pediatr (Rio J). 2012 Sep-Oct;88(5):401–5. English, Portuguese. doi: 10.2223/JPED.2212. Epub 2012 Sep 22. PMID: 23002079. | |
Offerdal K, Blaas HG, Eik-Nes SH. Prenatal detection of trisomy 21 by second-trimester ultrasound examination and maternal age in a non-selected population of 49 314 births in Norway. Ultrasound Obstet Gynecol. 2008 Sep;32(4):493–500. doi: 10.1002/uog.5373. PMID: 18688793. | |
McElhinney DB, Clark BJ 3rd, Weinberg PM, Kenton ML, McDonald-McGinn D, Driscoll DA, Zackai EH, Goldmuntz E. Association of chromosome 22q11 deletion with isolated anomalies of aortic arch laterality and branching. J Am Coll Cardiol. 2001 Jun 15;37(8):2114–9. doi: 10.1016/s0735-1097(01)01286-4. PMID: 11419896. | |
Singhania S, Kashikar S, Dhawan V, Parihar P. Prenatal diagnosis of micrognathia and postnatal identification of cleft soft palate: A case report. Radiol Case Rep. 2025 Mar 15;20(6):2637–2641. doi: 10.1016/j.radcr.2025.02.028. PMID: 40151285; PMCID: PMC11937608. | |
Paladini D. Fetal micrognathia: almost always an ominous finding. Ultrasound Obstet Gynecol. 2010 Apr;35(4):377–84. doi: 10.1002/uog.7639. PMID: 20373481. | |
AIUM Practice Parameter for the Performance of Detailed Diagnostic Obstetric Ultrasound Examinations Between 12 Weeks 0 Days and 13 Weeks 6 Days. J Ultrasound Med. 2021 May;40(5):E1-E16. doi: 10.1002/jum.15477. Epub 2020 Aug 27. PMID: 32852128. | |
Poaty H, Pelluard F, Diallo MS, Ondima IPL, André G, Silou-Massamba JF. Omphalocele: a review of common genetic etiologies. Egypt J Med Hum Gen. 2019;20(1). doi: 10.1186/s43042-019-0040-3. | |
Chen CP. Chromosomal abnormalities associated with omphalocele. Taiwan J Obstet Gynecol. 2007 Mar;46(1):1–8. doi: 10.1016/S1028-4559(08)60099-6. PMID: 17389182. | |
Huang SY, Chao AS, Kao CC, Lin CH, Hsieh CC. The Outcome of Prenatally Diagnosed Isolated Fetal Ventricular Septal Defect. J Med Ultrasound. 2017 Apr-Jun;25(2):71–75. doi: 10.1016/j.jmu.2017.05.005. Epub 2017 Jun 10. PMID: 30065463; PMCID: PMC6029313. | |
Miyake T. A review of isolated muscular ventricular septal defect. World J Pediatr. 2020;16(2):120–128. doi: 10.1007/s12519-019-00289-5. | |
Reyna JC, Zagory JA, Yallapragada S, Santiago-Munoz P, Schindel DT. Impact of Additional Anomalies on Postnatal Outcomes in Congenital Lung Malformations. J Surg Res. 2020 Dec;256:611–617. doi: 10.1016/j.jss.2020.07.039. Epub 2020 Aug 15. PMID: 32810660. | |
Lepigeon K, Van Mieghem T, Vasseur Maurer S, Giannoni E, Baud D. Gastroschisis–what should be told to parents? Prenat Diagn. 2014 Apr;34(4):316–26. doi: 10.1002/pd.4305. Epub 2014 Jan 20. PMID: 24375446. |
Online Study Assessment Option
All readers who are qualified doctors or allied medical professionals can automatically receive 2 Continuing Professional Development points plus a Study Completion Certificate from GLOWM for successfully answering four multiple-choice questions (randomly selected) based on the study of this chapter. Medical students can receive the Study Completion Certificate only.
(To find out more about the Continuing Professional Development awards program CLICK HERE)