This chapter should be cited as follows:
Couck I, Lewi L, Glob Libr Women's Med
ISSN: 1756-2228; DOI 10.3843/GLOWM.413733
The Continuous Textbook of Women’s Medicine Series – Obstetrics Module
Volume 4
Fetal development and maternal adaptation
Volume Editor: Professor Asma Khalil, The Royal College of Obstetricians and Gynaecologists, London, UK; Fetal Medicine Unit, Department of Obstetrics and Gynaecology, St George’s University Hospitals NHS Foundation Trust, London, UK

Chapter
Other Pathologies in Identical Twins
First published: February 2022
Study Assessment Option
By answering four multiple-choice questions (randomly selected) after studying this chapter, readers can qualify for Continuing Professional Development points plus a Study Completion Certificate from GLOWM.
See end of chapter for details.
TWIN ANEMIA POLYCYTHEMIA SEQUENCE
Twin anemia polycythemia sequence (TAPS) is a chronic transfusion imbalance that is characterized by a discordance in hemoglobin. The placenta in TAPS has a very typical appearance, with only few minuscule (less than 1 mm diameter) anastomoses (Figure 1). These facilitate the slow transfusion of red blood cells, without massive volume shifts. This results in an anemic donor twin and a polycythemic recipient twin.1,2 Both twin-to-twin transfusion syndrome (TTTS) and TAPS are thus caused by an imbalance in intertwin transfusion and are pathologies that are only seen in monochorionic twins. However, unlike in TTTS, the red blood cell imbalance in isolated TAPS is not accompanied by a severe discordance in amniotic fluid.3 TTTS can sometimes be accompanied by TAPS. However, these cases are considered as TTTS cases, as the laser coagulation of placental anastomoses is the treatment of choice for TTTS.4
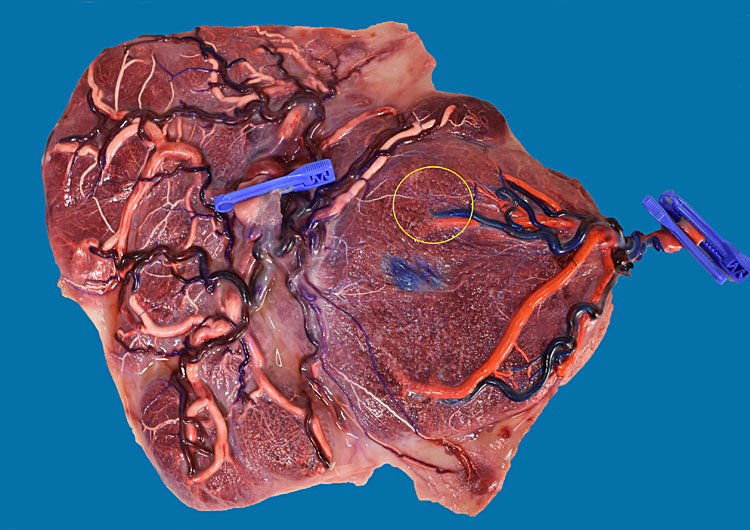
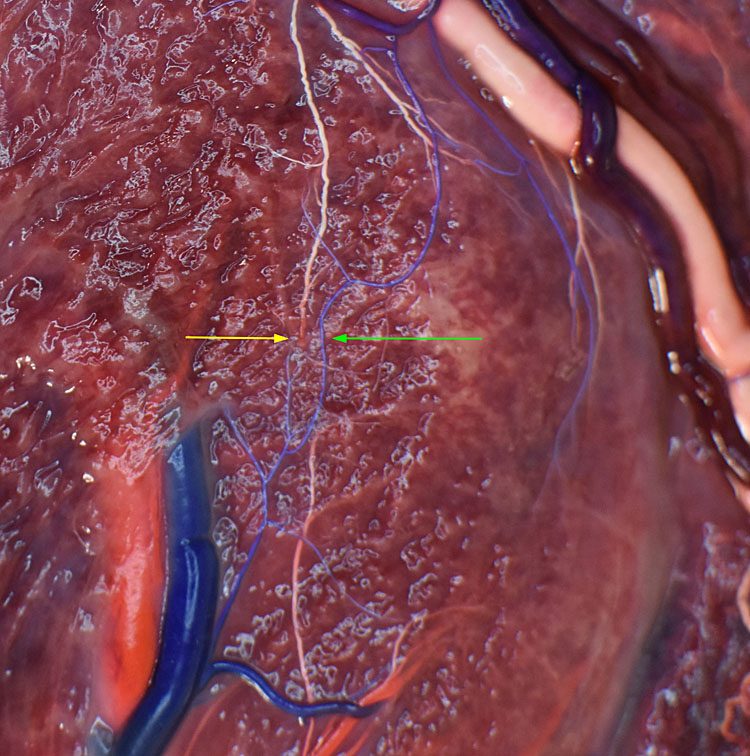
1
Typical placenta of an MCDA twin with TAPS. This case was picked up antenatally but was managed conservatively. The patients was delivered at 32 weeks and 1 day because of pre-eclampsia. The babies were born with a hemoglobin of 20.5 g/dl and 13.3 g/dl. The polycythemic twin required partial exchange transfusion on day 1. Figure 1 (lower) shows a detail of the tiny artery-to-artery (green arrow) and artery-to-vein (yellow arrow) anastomosis.
There are two types of TAPS. Spontaneous TAPS occurs in about 5% of monochorionic diamniotic (MCDA) twins and usually arises after 26 weeks in previously uncomplicated monochorionic twin pregnancies.5 The iatrogenic type or “postlaser” TAPS can occur after incomplete laser surgery for TTTS. In these cases, very small anastomoses are missed and left patent at the time of the surgery, which can eventually lead to TAPS later on. The prevalence of postlaser TAPS depends on the surgical technique that is used.6 With the classic technique of selective coagulation of placental anastomoses, the prevalence of TAPS is around 15%, while this is reduced to 3% when the a coagulation line is drawn across the entire vascular equator.7
Diagnosis and staging of TAPS
TAPS is a condition that can be detected antenatally. This is possible with careful ultrasound surveillance. Since the key feature in TAPS is a discordance in hemoglobin, TAPS can be diagnosed using the peak systolic velocity in the middle cerebral artery (MCA-PSV) of each twin. In the anemic donor, the MCA-PSV will be increased to >1.5 MoM, while the MCA-PSV of the polycythemic recipient will be <1.0 MoM.8 However, there is still uncertainty whether these diagnostic criteria are ideal for the detection of polycythemia. Recently, it has been suggested that maybe in intertwin difference in MCA-PSV is more important than the actual values.9 In any case, a difference in MCA-PSV of >0.5 MoM should be a warning sign and warrants careful monitoring.
There are other ultrasound features that can alert the clinician to be on the lookout for TAPS. First, the liver of the recipient twin can have a “starry sky” appearance, with portal venules that are accentuated in the parenchyma.10 Secondly, the placental share of the anemic twin can appear thicker, with an increased echogenicity, compared to the recipient’s share (Figure 2).8 While these features are not mandatory diagnostic criteria, they should ring a bell when present and should lead the investigator to carefully assess the MCA-PSV of both fetuses.
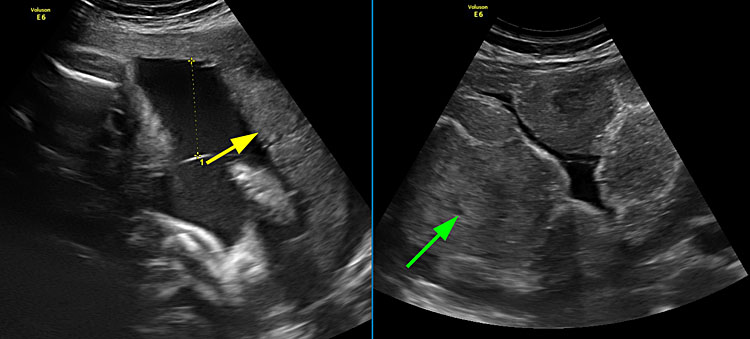
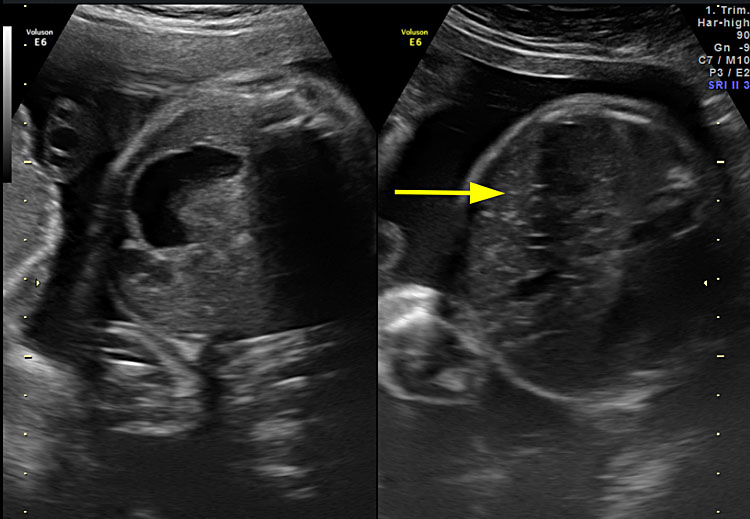
2
Ultrasound image of the same TAPS case as Figure 1. Figure 2 (upper) shows the placenta of the recipient on the left, which appears normal, and the thicker placental share of the donor on the right. Figure 2 (lower) shows the normal liver of the donor twin on the left and the starry sky appearance of the recipient’s liver on the right.
Unfortunately, not all TAPS cases are detected before birth. Therefore, postnatal criteria for TAPS were proposed to identify those cases that were missed antenatally. A first hint at TAPS in the postnatal period can be the skin color of the babies: a pale donor twin and a plethoric recipient. For the postnatal diagnosis, an intertwin difference in hemoglobin of >8 g/dl is required in the presence of only miniscule (< 1 mm) anastomoses after placental color dye injection.11 Placental injection studies should be the gold standard in these cases, to distinguish TAPS from acute intertwin transfusion through large anastomoses. When this is not possible, a reticulocyte count ratio (reticulocyte count of the donor/reticulocyte count of the recipient) of >1.7 may be helpful to differentiate between the chronic process of TAPS and acute transfusion.11 In contrast to an acute intertwin transfusion, there is typically a striking difference in color of the maternal side of each placental share in TAPS, with a pale placental share for the donor and a dark, plethoric share for the recipient.12
TAPS is staged according to the criteria defined by Slaghekke et al.8 These criteria differ in the antenatal and postnatal detection of TAPS. In the antenatal period, the stages of TAPS are defined by different ultrasound criteria, while postnatal staging is based on the difference in hemoglobin (Table 2).
1
Classification of sIUGR according to umbilical artery Doppler of the smaller twin with the respective placental characteristics and outcome with expectant management.21,23,26,32,67
Type 1 | Type 2 | Type 3 | |||||
Umbilical artery Doppler smaller twin | Positive EDF | Continuous absent or reversed EDF | Intermittent absent or reversed EDF | ||||
Fetal weight ratio | 1.4 | 1.6 | 1.6 | ||||
Placenta territory ratio | 1.8 | 2.6 | 4.4 | ||||
Large artery-to-artery anastomoses (>2 mm) | 70 | 18 | 98 | ||||
Survival (%) | Expectant | Expectant | Laser | Cord occlusion | Expectant | Laser | Cord occlusion |
- Overall | 96 | 59 | 53 | 46 | 85 | 64 | 47 |
- Smaller twin | 96 | 52 | 39 | 0 | 81 | 33 | 0 |
- Larger twin | 96 | 67 | 68 | 93 | 90 | 94 | 93 |
Gestational age at birth (weeks) | 36 | 28 | 32 | 36 | 31 | 32 | 36 |
* Defined as within the first 28 days of life
EDF, end-diastolic flow.
2
Staging of TAPS in the antenatal and postnatal period.8
Antenatal | Postnatal | |
Stage 1 | MCA-PSV >1.5 MoM in donor MCA-PSV <1.0 MoM in recipient No other signs of fetal compromise | Intertwin difference in hemoglobin >8 g/dl |
Stage 2 | MCA-PSV >1.5 MoM in donor MCA-PSV <0.8 MoM in recipient No other signs of fetal compromise | Intertwin difference in hemoglobin >11 g/dl |
Stage 3 | MCA-PSV >1.5 MoM in donor MCA-PSV <1.0 MoM in recipient Additional cardiac compromise of the donor, defined as critically abnormal flow | Intertwin difference in hemoglobin >14 g/dl |
Stage 4 | Hydrops of the donor | Intertwin difference in hemoglobin >17 g/dl |
Stage 5 | Intra-uterine fetal demise of one or both twins preceded by TAPS | Intertwin difference in hemoglobin >20 g/dl |
MCA-PSV, peak-systolic velocity in the middle cerebral artery; MoM, multiples of median.
Management of TAPS
The best management of antenatally detected TAPS is still undetermined and depends both on the gestational age and the severity of the disease.
Conservative management may be possible in TAPS cases without signs of fetal compromise (stages 1 and 2), as long as the disease remains stable. This means that these pregnancies should be monitored frequently in order to exclude deterioration. In the rare cases when TAPS presents very early (around 16 weeks), it is our experience that this may resolve spontaneously because the placenta still grows a lot at this stage, and we hypothesize that tiny anastomoses may obliterate spontaneously in the process of placental growth.
It is unclear when to deliver pregnancies with mild TAPS that appear to be stable. Tollenaar et al. suggest to deliver from 32 weeks onwards in TAPS stage 3 or more, or if stage 2 TAPS proves to be progressive.2
Of course, prior to 32 weeks, our aim should be to delay delivery, even in advanced stages. Intra-uterine transfusion of the donor can be used to gain time, but is only a temporary fix, as the red blood cells will eventually leak towards the recipient again, worsening the polycythemia. Therefore, partial exchange transfusion of the recipient can be performed at the same time. This procedure aims to decrease the viscosity of the recipient’s blood, reducing the risk of polycythemia-related complications.13,14 In most cases, multiple transfusions are needed, until an appropriate gestational age for delivery is reached.
The only causal therapy for TAPS is laser coagulation of the tiny intertwin anastomoses (Video 1). However, this is a lot more challenging than in TTTS, as there is no polyhydramnios and oligohydramnios. Especially in postlaser TAPS, where the previous laser surgery was incomplete, a second surgery may prove to be difficult. Nevertheless, laser surgery appears to be the most successful intervention to postpone delivery in TAPS. One non-randomized study showed that laser surgery resulted in a longer interval between diagnosis and delivery compared to intra-uterine transfusion (11 versus 5 weeks), which in turn benefits the perinatal outcome.15 Currently, a randomized controlled trial is ongoing to examine whether laser surgery can indeed prolong the pregnancy in case of TAPS, compared to standard care (https://www.trialregister.nl/trial/6879).
1
Clip of fetoscopy for TAPS. Three tiny anastomoses are identified and coagulated with laser.
In severe cases where (re-)laser is not possible and transfusion cannot buy enough time to reach a viable gestational, selective reduction can be considered as a management option.
The postnatal management of TAPS usually includes transfusion for the anemic twin and often partial exchange transfusion for the recipient twin.
Neurodevelopmental outcome
The number of studies on long-term outcome of TAPS survivors is limited, but there does seem to be an increased risk of impairment, comparable to TTTS survivors. Neurodevelopmental delay has been described in 9% of children and mild to moderate cognitive delay in 17%,16 as well as deafness and spastic paralysis.17 However, these studies report on the outcome of TAPS after incomplete laser surgery for TTTS. It is therefore unclear whether the adverse outcome is to be attributed to TTTS or to TAPS.
DISCORDANT GROWTH
While discordant growth can also occur in dichorionic twins, the risks and management differ greatly when discordant growth is present in a monochorionic pair. Because of the vascular intertwin anastomoses, the twins depend on each other for their well-being. If the growth-restricted twin should die, this can cause exsanguination of the co-twin, leading to brain damage or double fetal demise, as discussed further in this chapter (section on single demise). On the other hand, the twins have opposing interests: while the growth-restricted twin may benefit from preterm delivery, this puts the larger twin at risk of prematurity-related complications. In managing monochorionic pregnancies with discordant growth, the clinician should therefore constantly weigh the needs of both fetuses.
Definition and incidence of discordant growth
Discordant growth and selective intra-uterine growth restriction (sIUGR) are often used as synonyms. There is a lot of controversy on the exact definition of sIUGR. Some authors use a discordance in estimated fetal weight of more than 20 or 25%,18,19,20 others define sIUGR as growth of at least one twin below the 10th centile.21,22 In some studies, a combination of both is used.23 In an attempt to reach a consensus on the definition of sIUGR, a Delphi procedure resulted in the following definition to use for research purposes: a monochorionic twin pregnancy is classified as having sIUGR if one of the twins has an estimated fetal weight (EFW) <3rd centile or if at least two out of the following four parameters are present: EFW of one twin <10th centile, abdominal circumference of one twin <10th centile, EFW discordance ≥25%, and umbilical artery pulsatility index of the smaller twin >95th centile.24
Since monochorionic twins are identical, one could argue that they have the same genetic potential for growth and any discordance in fetal growth is therefore abnormal, irrespective of the exact measurements. In light of this, we prefer to use a discordance of more than 20% to define discordant growth as it is also easier to use in daily clinical practice. Discordance is calculated using the following formula: (EFW of the larger twin – EFW of the smaller twin)/EFW of the larger twin. The average discordance in birth weight in MCDA twins is around 10%.19 A discordance in EFW of at least 20% is found in about 15% of cases.25
Fetal growth in the MCDA twin is a result of placental sharing and intertwin transfusion. sIUGR cases that present early in pregnancy seem to have more unequally shared placentas and more often have Doppler abnormalities of the umbilical artery of the smaller twin. When discordant growth first manifests after 26 weeks, placentas tend to be less unequally shared. On the other hand, twin anemia polycythemia sequence is more frequent in these cases of late sIUGR.5 Of course, these late-onset cases are less complicated to manage, as delivery is possible if needed.
Classification of discordant growth
As mentioned above, it is possible to classify sIUGR based on the time when it first presents itself: early-onset when diagnosed before 26 weeks and late-onset when first detected after 26 weeks.5
Alternatively, sIUGR pregnancies can be classified based on the umbilical artery Doppler of the smaller twin developed by Gratacos et al. sIUGR type 1 comprises those cases with positive end-diastolic flow (EDF) in the umbilical artery of the smaller twin (Figure 3). Both type 2 and type 3 are characterized by absent or reversed EDF, but in type 2 this is continuous, while there is a pattern of intermittent absent or reversed EDF in type 3. This Doppler pattern in type 3 sIUGR is typically a cyclic variation that is best picked up if the Dopplers are measured at the placental cord insertion site. An important difference between type 2 and type 3 is the placental architecture: placentas are usually more unequally shared in type 3, with larger anastomoses that allow for more elaborate intertwin blood exchange (Figures 4 and 5, Video 2). This intertwin transfusion may partly “rescue” the smaller twin and allow for acceptable better fetal growth compared to type 2 cases, where there are only small anastomoses and the smaller twin behaves more as a singleton with a dysfunctional placenta.26
3
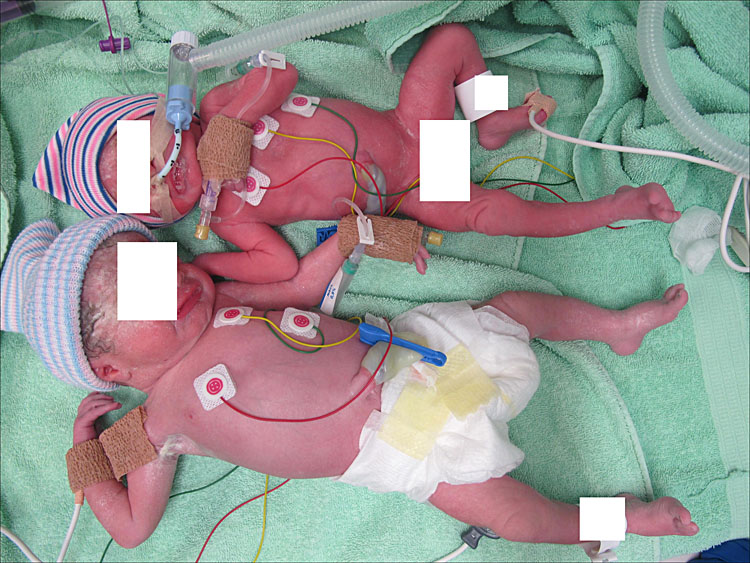
Figure 3a (upper) shows MCDA twins with sIUGR type 1, born at 24 weeks, weighing 1380 and 2160 g. Figure 3b (lower) shows the placenta. The white line indicates the approximate location of the vascular equator. The yellow arrows indicate 2 artery-to-artery anastomoses and the green arrows indicate 3 vein-to-vein anastomoses. Amniotic membranes have been removed.
4
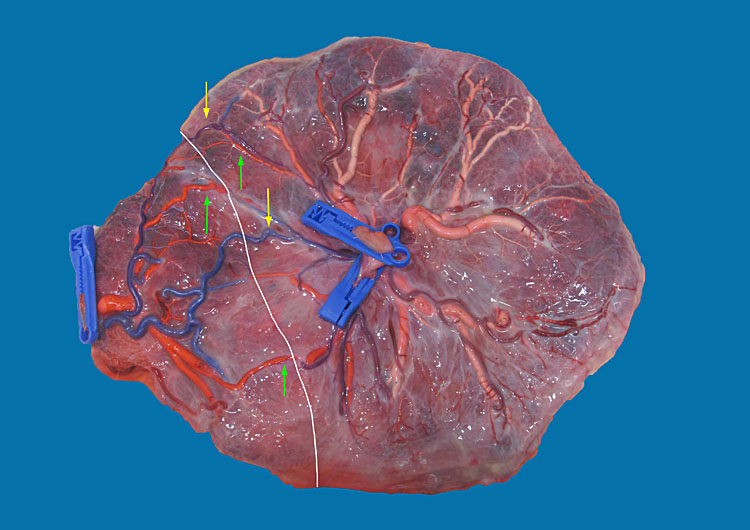
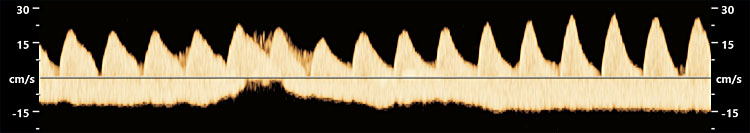
Figure 4 (upper) shows the placenta of MCDA twins with sIUGR type 2, delivered at 30 weeks with a birth weight of 1050 and 660 g. The white line indicates the approximate location of the vascular equator. There are only some very small artery-to-vein (green arrow) and vein-to-artery (yellow arrow) anastomoses. Amniotic membranes have been removed. Figure 4 (lower) shows the typical Doppler pattern in the umbilical artery of the smaller twin, with continuous absent and/or reversed end-diastolic flow.
5
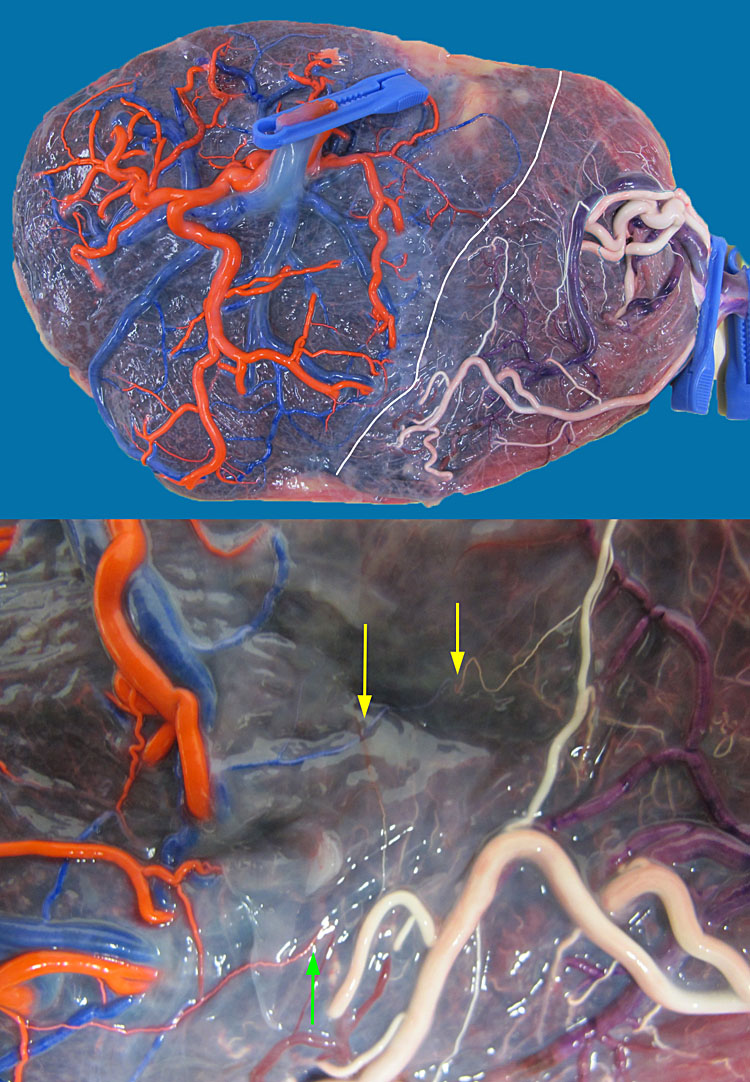
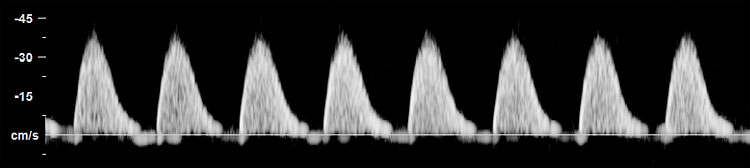
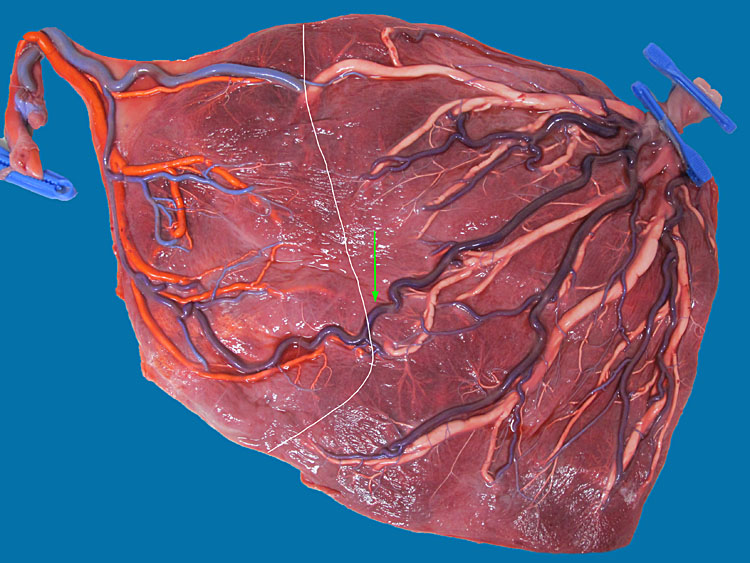
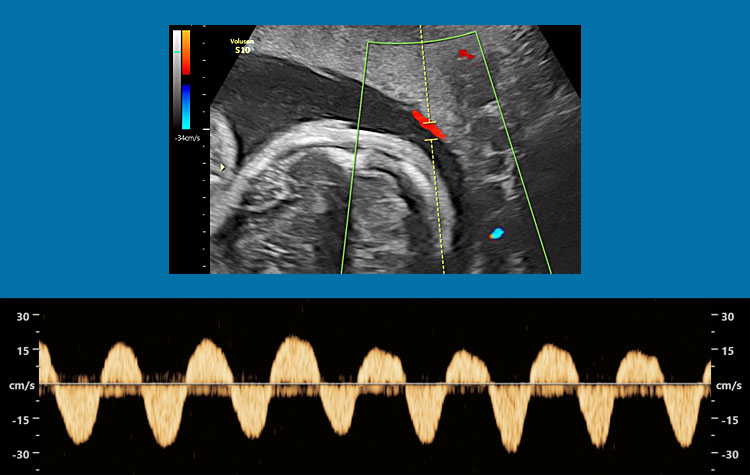
Figure 5(upper) shows the placenta of MCDA twins with sIUGR type 3, delivered at 33 weeks and 1 day, with a birth weight of 2061 and 1564 g. The white line indicates the approximate location of the vascular equator. The green arrow indicates a large artery-to artery connection. Note the tightly linked circulation that allows for elaborate intertwin blood exchange. Amniotic membranes have been removed. Figure 5 (middle) shows the intermittent absent end-diastolic flow in the umbilical artery of the smaller twin. Note how the velocity varies. Figure 5 (lower) shows bidirectional flow in a large artery-to-artery anastomosis.
2
Ultrasound clip demonstrating bidirectional flow in a large artery-to-artery anastomosis. The color Doppler pattern constantly changes from blue to red, indicating that blood flows in opposite directions.
Survival is best in type 1 cases and worst in type 2 cases (details in Table 2). An important difference between type 2 and type 3 sIUGR lies in the predictability of fetal demise. In type 2, cases typically deteriorate slowly with progressive worsening of Doppler measurements similar to singleton pregnancies, which allows time for intervening if necessary. In type 3 on the other hand, fetal demise is often unexpected and unpredictable. This is probably due to the difference in vascular anastomoses in these placentas: small anastomoses in type 2 versus larger artery-to-artery anastomoses in type 3, which allow for massive acute volume shifts.5,26,27
Management of discordant growth
The management of sIUGR strongly depends on the type and moment of onset. In type 1 sIUGR, the outcomes are excellent with expectant management and fetal intervention does not have any clear benefits.28 Expectant management is thus the best option for these pregnancies. Weekly follow-up with ultrasound and after viability in combination with biophysical profile scoring is recommended to detect deterioration.29 Of course, in case of subsequent development of TTTS or TAPS, the patient should receive the appropriate treatment. Elective delivery should be scheduled somewhere between 34 and 36 weeks.30 If cesarean section is planned, corticosteroids for lung maturity should be administered beforehand.
For type 2 and 3 sIUGR, the management is more difficult. As mentioned before, demise can be unpredictable, especially in type 3 cases. However, there are a few ultrasound signs, which can serve as “red flags”. If present, they should prompt the clinician to carefully reconsider all management options. These “red flags” are the following: important oligohydramnios with deepest vertical pool less than 2 cm, growth discordance of more than 35%, growth stop of the smaller twin, persistent abnormal Doppler findings in the ductus venosus, persistent reversed end-diastolic flow in the umbilical artery and (after viability) abnormal fetal heart rate tracing.26,27,31
In case deterioration of the fetal condition occurs when both have a good chance of intact survival, of course the most logical approach is to deliver the babies. The difficulty lies in what to do when this occurs before this milestone is reached. The main options in this case are laser coagulation of placental anastomoses or selective reduction. Laser coagulation aims to give both fetuses a chance of survival and protects the larger twin from exsanguination in case of spontaneous demise of the growth-restricted twin. With selective reduction, the smaller twin is sacrificed in order to protect the larger twin. In the latter case, management afterwards becomes similar to a singleton pregnancy.
As the figures in Table 2 indicate, observational studies do not indicate that laser surgery improves survival. Laser may even decrease the survival rate of the smaller twin especially in type 3, as this fetus now must survive on only a small placental share.21,32 It is important to keep in mind as well that laser surgery may not always be possible, as there is not the typical oligo-polyhydramnios sequence as in TTTS, making surgery more challenging.
Cord occlusion on the other hand, will for sure result in demise of the smaller twin, thereby reducing overall survival. The advantage may be that it obviates the need to deliver the larger twin early and pregnancy may be prolonged to 36 weeks.23 It seems reasonable that this approach may therefore limit the risk of prematurity-related complications for the larger twin, although this remains to be proven. Currently, there is no randomized research in this domain.
Of course, the parents’ wishes are an important factor in deciding the management of these pregnancies. Therefore, honest counseling with an overview of the pros and cons of all scenarios is mandatory.
In the follow-up of type 2 and 3 sIUGR, we advise inpatient monitoring from 28 weeks onwards with cardiotocogram three times a day, ultrasound twice a week and elective delivery planned around 32 to 33 weeks. Corticosteroids are administered upon admission and a repeat course is given prior to elective cesarean section.
MONOAMNIOTIC TWINS
Monoamniotic (MA) twins not only share the placenta, but also the amniotic cavity. This type of placentation arises when the inner cell mass splits late, probably between day 7 and 9 after fertilization.33,34This occurs in 1 in 7500 pregnancies overall or 5% of monochorionic twin pregnancies.35 Interestingly, the ratio of female to male fetuses is increased to about 70% in monoamniotic conceptions. The reason behind this is not clear yet: it may be related to the process of X-inactivation, which occurs around the time of the monoamniotic split, or it may be the case that female embryos are more likely to survive the split.36,37 Twins that are initially monochorionic diamniotic can become monoamniotic later on, after spontaneous (rare) or iatrogenic rupture of the intertwin septum. Iatrogenic rupture can occur after amniocentesis or fetal surgery.38 In rare cases, the intertwin septum can be present, but incomplete, resulting in partial monoamniotic twins.39
Diagnosis
The most typical ultrasound feature of MA twins is of course the absence of an intertwin membrane. This should be diagnosed in the first trimester. However, prior to 8 weeks one cannot determine the amnionicity, as the amnion only becomes visible at around 7.5 to 8 weeks. From 8 weeks onward, it should be possible to make a correct assessment.40 It is important to distinguish between monoamniotic twins and severe TTTS in more advanced pregnancies, as the stuck twin can be entirely embedded in its intertwin membrane, mimicking an absent intertwin membrane.34 However, in TTTS the donor is stuck, whereas in a monoamniotic pregnancies, both twins move freely within the common sac. The number of yolk sacs (either 1 or 2) cannot be used reliably to determine amnionicity.41
Spontaneous MA twins typically have one placenta with cords that insert close together, connected by large intertwin anastomoses, with an artery-to-artery anastomosis in almost all cases (Figure 6).42,43,44 These proximate insertions can be visualized on ultrasound and even large anastomoses may sometimes be visible. However, these signs are not useful to differentiate between monoamniotic and MCDA twins, as they can be present in both. Since the cords of monoamniotic twins are in the same amniotic cavity, they are usually entangled, although this is not a mandatory feature for ultrasound diagnosis (Video 3).40 When present on the other hand, cord entanglement does confirm monoamnionicity.34 As a result of this entanglement, it is usually not feasible to follow the umbilical cord all the way from the abdominal wall insertion to the placenta. Therefore, it is virtually impossible to label the twins as in other twin pregnancies, unless there is another sonographic feature, which sets them apart (such as a single umbilical artery or an anomaly in one twin).
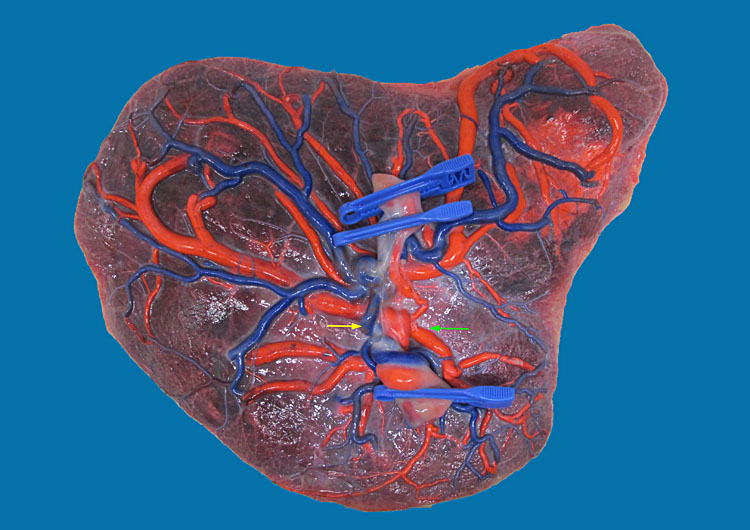
6
Placenta of a monoamniotic twin pair, delivered at 31 weeks and 5 days after spontaneous rupture of membranes. Birth weights were 1720 and 1370 g. There are larger artery-to-artery (yellow arrow) and vein-to-vein anastomoses (green arrow), and the vessels of both twins filled up completely after injection of one umbilical cord, demonstrating the tightly linked circulation.
3
Ultrasound clip of a monoamniotic pregnancy at 12 weeks. There is only an amnion layer around both fetuses and not in between. The fetuses are in the same amniotic cavity and their cords are entangled.
Follow-up and delivery
Monoamniotic twins have an increased risk of in utero demise, often of both twins. In a large unselected series of monoamniotic twins, intra-uterine demise occurred in 22% of pregnancies, and in two thirds of these cases, both twins died. In this series however, congenital anomalies were not excluded.45 A meta-analysis showed that the overall incidence of fetal loss in monoamniotic twins is only 6%, but here only pregnancies that progressed after 24 weeks were included and pregnancies with congenital anomalies were excluded. An important finding however, was that half of all fetal demises were unpredictable and this was even the case for all of the fetal demises after 30 weeks.46 Demise in monoamniotic pregnancies is often labeled as a “cord accident”, but in reality it is probably a combination of cord compression and acute intertwin transfusion imbalance across the large anastomoses.47 In our own patient population, there is 85% survival in monoamniotic twins when the first trimester scan does not reveal any major anomalies (80% double survival, 5% single demise, and 15% double demise), in contrast with 90% survival in MCDA twins with a normal first trimester scan (85% double survival, 7.5% single demise, and 7.5% double demise) (unpublished data).48
It seems evident that these high-risk pregnancies should be monitored by centers with expertise in complicated monochorionic pregnancies. Van Mieghem et al. demonstrated between 32 and 33 weeks is the optimal time for delivery of these pregnancies, when balancing the risks of the intra-uterine environment with those of preterm birth.45 Cesarean section is the preferred mode of delivery, because of the risk of cord entanglement. However, there is no consensus on how the follow-up of these pregnancies should be organized.
A large series demonstrated there is no difference in the risk of fetal loss between inpatient and outpatient follow-up.45 In contrast, a recent meta-analysis suggested that inpatient follow-up may be superior.46 In reality, the protocol for follow-up of these pregnancies is probably more important than the location where the patient is monitored.49 At present, there is no reason to assume intensive follow-up as an outpatient is inferior to inpatient monitoring. Unfortunately, we do not know what this ideal protocol for follow-up entails. Van Mieghem et al. performed ultrasound once or twice a week in their outpatient group, as well as cardiotocogram four times a week. In our own center, we usually propose inpatient management from 28 weeks onwards, with ultrasound scan twice a week and cardiotocogram three times a day. Corticosteroids for lung maturation are administered upon admission at 28 weeks and repeated once prior to elective delivery around 33 weeks.34 Other centers may opt to start intensive follow-up earlier, around 26 weeks, depending on local practices. Prior to this time, monoamniotic twins are followed with ultrasound every 2 weeks, as is the case for MCDA twins.
Other complications in monoamniotic pregnancies
Congenital anomalies appear to be more frequent in monoamniotic twins, with a reported incidence of 23% of pregnancies and 14% of fetuses. Cardiac defects were responsible for more than one third of cases in this series.45 Another study reported an increased incidence of congenital heart disease and cerebral abnormalities of 4% and 5%, respectively.50 The anomaly is often present in only one of the twins, complicating management further.40 In case of an anomaly that is lethal in pregnancy, expectant management is not an option as there is the risk of co-twin demise. There are two factors that contribute to this risk. First, large intertwin anastomoses may predispose to exsanguination of the co-twin in case of fetal demise. Secondly, the cord of the dead fetus can wind around the cord of the living twin. As this dead tissue loses its elasticity, it can eventually block blood flow in the living cord, leading to demise of the co-twin, even weeks or months after the initial demise. Therefore, active management of these pregnancies is preferred, with selective feticide of the affected twin by cord coagulation and additional cord transection. This procedure is usually only feasible from 18 weeks onward, as this technique requires the use of a bipolar forceps and the corresponding 10 French trocar (Video 4). For urgent interventions before 16 weeks, laser cord coagulation using a 1 mm embryoscope is possible. Cutting the cord of the demised twin is probably effective in reducing the risk for the co-twin compared to selective feticide alone, as was suggested by several groups, although there are no randomized trials.51 Survival of the healthy twin after cord occlusion and transection is reported to be around 75%, with a gestational age at birth of 34 to 35 weeks on average.51,52 There is only one study comparing the outcome between monoamniotic twins treated with cord occlusion and transection and MCDA twins treated with conventional cord occlusion. In this series, the outcomes did not differ between both groups, but sample size was small in the monoamniotic group. They did observe a trend towards higher rates of preterm prelabour rupture of membranes (PPROM) (35% versus 21%) and lower gestational age at birth (35 weeks versus 37 weeks) in the monoamniotic group. In any case, selective reduction in a monoamniotic setting does always require entry in the amniotic sac of the healthy twin, in contrast with MCDA twins where the point of entry is the sac of the target twin. Therefore, iatrogenic PPROM in monoamniotic twins will result in an oligohydramnios of the survivor, while this is usually not the case in MCDA twins.52 Since this is an invasive strategy in an already high-risk pregnancy, the parents should also be counseled about their options regarding termination of pregnancy.
4
Fetoscopic clip of a cord transection by laser in a monoamniotic setting after single demise.
Monoamniotic pregnancies are less likely to develop TTTS and TAPS. The reported incidence of TTTS in monoamniotic twins is around 3%,45,53 while this is around 12% in MCDA twins.25 TAPS in monoamniotic twins is an extremely rare phenomenon, with only sporadic case reports.54 Twin reversed arterial perfusion or TRAP sequence on the other hand, is more common in monoamniotic twins compared to MCDA twins.55,56 This is probably due to the difference in placental architecture, with the presence of large artery-to-artery anastomoses that are protective against TTTS and TAPS, but predispose to TRAP if there is an early embryonic demise of one of a monoamniotic pair.34,42,57 Laser surgery in monoamniotic twins is challenging but possible, and may be considered in case of TTTS or TAPS.51 Selective reduction or termination of pregnancy may be offered as an alternative when laser is not possible or in case of additional malformations. For TRAP, intrafetal coagulation to arrest the reversed perfusion is not indicated as the cord of the acardiac twin needs to be coagulated and transected. In case of spontaneous arrest of flow in the acardiac twin, cord transection can still be considered to minimize the risk of remote intrauterine demise for the co-twin.
Recently, it was demonstrated that a weight discordance of more than 20% in monoamniotic twins increases the risk of intrauterine demise. Due to the elaborate intertwin transfusion and proximate cord insertions, sIUGR is less common in a monoamniotic setting (3–7%).45,58 Weight discordance alone does not seem to warrant intervention.58
Conjoined twins are a special type of monoamniotic twins, where the fetuses not only share the amniotic cavity, but also body parts. They arise when the monochorionic split occurs later than day 9 postfertilization.33,34 This condition should be diagnosed in the first trimester and due to the poor outcome most parents opt for termination of pregnancy as many of these cases will result in spontaneous miscarriage, stillbirth, or early neonatal demise.59
SINGLE DEMISE IN MONOCHORIONIC TWINS
When one twin dies in a monochorionic setting, there is a risk of exsanguination of the co-twin through large vascular anastomoses, leading to double fetal loss. In our own series, the risk of spontaneous double fetal loss after the first trimester was higher compared to spontaneous single fetal loss: 3% versus 1%.48 Also, Sebire et al. – before fetal therapy was the standard of care – reported that most cases of fetal demise were double losses.60 But even when the co-twin survives initially, this fetus remains at risk: a meta-analysis showed that in case of single demise in a monochorionic pair where one initially survives, there is still a 15% risk of subsequent double demise. In case of survival of the co-twin, the temporary hypotension around the time of demise of the first fetus may lead to impaired cerebral perfusion, resulting in a 25% risk of neurodevelopmental impairment.61 This study also demonstrated that these risks were significantly higher compared to cases with single demise in a dichorionic setting.
When single demise is noted in the first trimester, it is important to exclude evolution towards TRAP sequence. This is a condition where the healthy twin acts as a pump twin and perfuses the demised body of the co-twin (or acardiac twin). This is only possible by means of reversed perfusion in the umbilical cord of the acardiac twin, through an artery-to-artery and vein-to-vein anastomosis. Therefore, when single demise is diagnosed, the sonographer should always carefully assess the presence of absence of reversed perfusion in the cord of the demised twin. Even when this cannot be demonstrated at the time of diagnosis, a follow-up scan after 1–2 weeks is indicated because the reversed perfusion may only be demonstrated intermittently. Also, when the body of the demised twin after single demise appears to increase in size, this should prompt the clinician to carefully reexamine for reversed flow.
Management
As this hypotensive crisis occurs at the time of demise of the first twin, the damage is already done by the time single demise is diagnosed on ultrasound. Therefore, emergency delivery of the second twin will not prevent neurological damage. When single demise is diagnosed, the co-twin needs to be examined for signs of anemia and fetal distress. In case of anemia, intra-uterine transfusion can be considered. The purpose should be to delay delivery as much as possible, in order to gain time to assess the neurological development.30 It is preferable to start with sonographic follow-up. Cerebral lesions may only become apparent several weeks after the noxious event has occurred. As such, ultrasound guidelines advise detailed evaluation of the fetal brain 4–6 weeks after demise.30 However, even when detailed neurosonography is negative, a magnetic resonance imaging (MRI) scan can add important information that might change counseling. Several authors have demonstrated the usefulness of prenatal sonography and fetal MRI for the evaluation of brain development after single demise.62,63 There is not much evidence on when MRI should be performed. One study suggests planning the MRI after 24 weeks and at least 2 weeks after co-twin demise.64 However, postponing the MRI scan until after 30 weeks is preferable in order to maximize the benefits from this exam.65
A special situation is single demise in monoamniotic twins, either spontaneous monoamniotic twins or iatrogenic after septostomy (for example, after laser for TTTS). As discussed previously, the cord of the dead fetus can wind around the cord of the living twin, blocking blood flow in the living cord and leading to demise of the co-twin, even weeks or months after the initial demise. Therefore, elective cord transection of the dead cord can be an option in an attempt to maximize the chances of survival for the living twin, similarly to what is sometimes done in twin reversed arterial perfusion with spontaneous arrest of flow in a monoamniotic setting.51 However, this may be complicated as the maceration of the demised twin causes the amniotic fluid to become turbid, limiting visualization at the time of fetoscopy.66
PRACTICE RECOMMENDATIONS
- The MCA-PSV should be measured every 2 weeks as part of the routine ultrasound examination in monochorionic twins, in order to timely detect TAPS.
- In MCDA twins, the umbilical artery Doppler should be measured at placental cord insertion site, in order to detect absent or reversed EDF and cyclic variations. In monoamniotic twins, umbilical artery Doppler should be measured at the level of the fetal bladder as it is otherwise impossible to correctly determine which cord insertion belongs to each fetus.
- Chorionicity and amnionicity should be determined between 8 and 13 weeks. If chorionicity is unsure, the patient should be referred to a tertiary care center by the end of the first trimester. If monoamniotic twins are suspected prior to 8 weeks, the pregnancy should be reevaluated between 8 and 13 weeks. Most of these pregnancies will turn out to be MCDA twins.
- When single demise is diagnosed in a monochorionic setting, the aim should be to delay delivery as long as possible in order to allow dedicated follow-up of the brain development of the surviving twin. The survivor should be examined for signs of fetal distress and anemia at diagnosis. Intra-uterine transfusion can help the surviving twin in case of anemia, but will not prevent brain damage.
- If single demise is noted in early pregnancy in monochorionic twins, the pregnancy should be followed carefully in order to exclude evolution to TRAP sequence.
- After single demise or selective reduction in a monoamniotic setting, cord transection of the cord of the demised twin is recommended to avoid demise of the co-twin later on in pregnancy.
CONFLICTS OF INTEREST
The author(s) of this chapter declare that they have no interests that conflict with the contents of the chapter.
Feedback
Publishers’ note: We are constantly trying to update and enhance chapters in this Series. So if you have any constructive comments about this chapter please provide them to us by selecting the "Your Feedback" link in the left-hand column.
REFERENCES
Lopriore E, Middeldorp JM, Oepkes D, et al. Twin anemia-polycythemia sequence in two monochorionic twin pairs without oligo-polyhydramnios sequence. Placenta 2007;28(1):47–51. | |
Tollenaar LS, Slaghekke F, Middeldorp JM, et al. Twin Anemia Polycythemia Sequence: Current Views on Pathogenesis, Diagnostic Criteria, Perinatal Management, and Outcome. Twin Res Hum Genet 2016;19(3):222–33. | |
Couck I, Lewi L. The Placenta in Twin-to-Twin Transfusion Syndrome and Twin Anemia Polycythemia Sequence. Twin Res Hum Genet 2016;19(3):184–90. | |
Senat MV, Deprest J, Boulvain M, et al. Endoscopic laser surgery versus serial amnioreduction for severe twin-to-twin transfusion syndrome. The New England Journal of Medicine 2004;351(2):136–44. | |
Lewi L, Gucciardo L, Huber A, et al. Clinical outcome and placental characteristics of monochorionic diamniotic twin pairs with early- and late-onset discordant growth. Am J Obstet Gynecol 2008;199(5):511 e1–7. | |
Slaghekke F, Oepkes D. Solomon Technique Versus Selective Coagulation for Twin-Twin Transfusion Syndrome. Twin Res Hum Genet 2016;19(3):217–21. | |
Slaghekke F, Lopriore E, Lewi L, et al. Fetoscopic laser coagulation of the vascular equator versus selective coagulation for twin-to-twin transfusion syndrome: an open-label randomised controlled trial. The Lancet 2014;383(9935):2144–51. | |
Slaghekke F, Kist WJ, Oepkes D, et al. Twin anemia-polycythemia sequence: diagnostic criteria, classification, perinatal management and outcome. Fetal Diagn Ther 2010;27(4):181–90. | |
Fishel-Bartal M, Weisz B, Mazaki-Tovi S, et al. Can middle cerebral artery peak systolic velocity predict polycythemia in monochorionic-diamniotic twins? Evidence from a prospective cohort study. Ultrasound Obstet Gynecol 2016;48(4):470–5. | |
Soundararajan LP, Howe DT. Starry sky liver in twin anemia-polycythemia sequence. Ultrasound Obstet Gynecol 2014;43(5):597–9. | |
Lopriore E, Slaghekke F, Oepkes D, et al. Hematological characteristics in neonates with twin anemia-polycythemia sequence (TAPS). Prenat Diagn 2010;30(3):251–5. | |
Tollenaar LS, Zhao DP, Middeldorp JM, et al. Color Difference in Placentas with Twin Anemia-Polycythemia Sequence: An Additional Diagnostic Criterion? Fetal Diagn Ther 2016;40(2):123–7. | |
Slaghekke F, van den Wijngaard JP, Akkermans J, et al. Intrauterine transfusion combined with partial exchange transfusion for twin anemia polycythemia sequence: modeling a novel technique. Placenta 2015;36(5):599–602. | |
Genova L, Slaghekke F, Klumper FJ, et al. Management of twin anemia-polycythemia sequence using intrauterine blood transfusion for the donor and partial exchange transfusion for the recipient. Fetal Diagn Ther 2013;34(2):121–6. | |
Slaghekke F, Favre R, Peeters SH, et al. Laser surgery as a management option for twin anemia-polycythemia sequence. Ultrasound Obstet Gynecol 2014;44(3):304–10. | |
Slaghekke F, van Klink JM, Koopman HM, et al. Neurodevelopmental outcome in twin anemia-polycythemia sequence after laser surgery for twin-twin transfusion syndrome. Ultrasound Obstet Gynecol 2014;44(3):316–21. | |
Taniguchi K, Sumie M, Sugibayashi R, et al. Twin anemia-polycythemia sequence after laser surgery for twin-twin transfusion syndrome and maternal morbidity. Fetal Diagn Ther 2015;37(2):148–53. | |
Ortibus E, Lopriore E, Deprest J, et al. The pregnancy and long-term neurodevelopmental outcome of monochorionic diamniotic twin gestations: a multicenter prospective cohort study from the first trimester onward. Am J Obstet Gynecol 2009;200(5):494 e1–8. | |
Van Mieghem T, Deprest J, Klaritsch P, et al. Ultrasound prediction of intertwin birth weight discordance in monochorionic diamniotic twin pregnancies. Prenat Diagn 2009;29(3):240–4. | |
Chalouhi GE, Marangoni MA, Quibel T, et al. Active management of selective intrauterine growth restriction with abnormal Doppler in monochorionic diamniotic twin pregnancies diagnosed in the second trimester of pregnancy. Prenat Diagn 2013;33(2):109–15. | |
Gratacos E, Antolin E, Lewi L, et al. Monochorionic twins with selective intrauterine growth restriction and intermittent absent or reversed end-diastolic flow (Type III): feasibility and perinatal outcome of fetoscopic placental laser coagulation. Ultrasound Obstet Gynecol 2008;31(6):669–75. | |
Valsky DV, Eixarch E, Martinez JM, et al. Selective intrauterine growth restriction in monochorionic twins: pathophysiology, diagnostic approach and management dilemmas. Seminars in Fetal & Neonatal Medicine 2010;15(6):342–8. | |
Parra-Cordero M, Bennasar M, Martinez JM, et al. Cord Occlusion in Monochorionic Twins with Early Selective Intrauterine Growth Restriction and Abnormal Umbilical Artery Doppler: A Consecutive Series of 90 Cases. Fetal Diagn Ther 2015. | |
Khalil A, Beune I, Hecher K, et al. Consensus definition and essential reporting parameters of selective fetal growth restriction in twin pregnancy: a Delphi procedure. Ultrasound Obstet Gynecol 2019;53(1):47–54. | |
Couck I, Mourad Tawfic N, Deprest J, et al. Does the Site of The Cord Insertion increase the risk of Adverse Outcome, Twin-To-Twin Transfusion Syndrome and Discordant Growth in monochorionic twin pregnancies? Ultrasound Obstet Gynecol 2017. | |
Gratacos E, Lewi L, Munoz B, et al. A classification system for selective intrauterine growth restriction in monochorionic pregnancies according to umbilical artery Doppler flow in the smaller twin. Ultrasound Obstet Gynecol 2007;30(1):28–34. | |
Ishii K, Murakoshi T, Hayashi S, et al. Ultrasound predictors of mortality in monochorionic twins with selective intrauterine growth restriction. Ultrasound Obstet Gynecol 2011;37(1):22–6. | |
Townsend R, D'Antonio F, Sileo FG, et al. Perinatal outcome of monochorionic twin pregnancy complicated by selective fetal growth restriction according to management: systematic review and meta-analysis. Ultrasound Obstet Gynecol 2019;53(1):36–46. | |
Rustico MA, Consonni D, Lanna M, et al. Selective intrauterine growth restriction in monochorionic twins: changing patterns in umbilical artery Doppler flow and outcomes. Ultrasound Obstet Gynecol 2017;49(3):387–93. | |
Khalil A, Rodgers M, Baschat A, et al. ISUOG Practice Guidelines: role of ultrasound in twin pregnancy. Ultrasound Obstet Gynecol 2016;47(2):247–63. | |
Monaghan C, Kalafat E, Binder J, et al. Prediction of adverse pregnancy outcome in monochorionic diamniotic twin pregnancy complicated by selective fetal growth restriction. Ultrasound Obstet Gynecol 2019;53(2):200–7. | |
Peeva G, Bower S, Orosz L, et al. Endoscopic Placental Laser Coagulation in Monochorionic Diamniotic Twins with Type II Selective Fetal Growth Restriction. Fetal Diagn Ther 2015;38(2):86–93. | |
Hall JG. Twinning. Lancet (London, England) 2003;362(9385):735–43. | |
Post A, Heyborne K. Managing Monoamniotic Twin Pregnancies. Clinical Obstetrics and Gynecology 2015;58(3):643–53. | |
Quigley JK. Monoamniotic Twin Pregnancy: A Case Record with Review of the Literature. American Journal of Obstetrics & Gynecology 1935;29(3):354–62. | |
Derom C, Vlietinck R, Derom R, et al. Population-based study of sex proportion in monoamniotic twins. The New England Journal of Medicine 1988;319(2):119–20. | |
Chitnis S, Derom C, Vlietinck R, et al. X chromosome-inactivation patterns confirm the late timing of monoamniotic-MZ twinning. American Journal of Human Genetics 1999;65(2):570–1. | |
Cruz-Martinez R, Van Mieghem T, Lewi L, et al. Incidence and clinical implications of early inadvertent septostomy after laser therapy for twin-twin transfusion syndrome. Ultrasound Obstet Gynecol 2011;37(4):458–62. | |
Galjaard S, Moerman P, Corveleyn A, et al. Partial monochorionic and monoamniotic twin pregnancies: a report of two cases. Ultrasound Obstet Gynecol 2014;44(6):722–4. | |
Sebire NJ, Souka A, Skentou H, et al. First trimester diagnosis of monoamniotic twin pregnancies. Ultrasound Obstet Gynecol 2000;16(3):223–5. | |
Corbett SL, Shmorgun D. Yolk sac number does not predict reliably amnionicity in monochorionic twin pregnancies: a case of a monochorionic monoamniotic twin pregnancy with two distinct yolk sacs on early first-trimester ultrasound. Ultrasound Obstet Gynecol 2012;39(5):607–8. | |
Bajoria R. Abundant vascular anastomoses in monoamniotic versus diamniotic monochorionic placentas. Am J Obstet Gynecol 1998;179(3 Pt 1):788–93. | |
Paek B, Dorn M, Walker M. Atypical twin-to-twin transfusion syndrome: prevalence in a population undergoing fetoscopic laser ablation of communicating placental vessels. Am J Obstet Gynecol 2016;215(1):115.e1–5. | |
Hack KE, Nikkels PG, Koopman-Esseboom C, et al. Placental characteristics of monochorionic diamniotic twin pregnancies in relation to perinatal outcome. Placenta 2008;29(11):976–81. | |
Van Mieghem T, De Heus R, Lewi L, et al. Prenatal management of monoamniotic twin pregnancies. Obstetrics and Gynecology 2014;124(3):498–506. | |
D'Antonio F, Odibo A, Berghella V, et al. Perinatal mortality, timing of delivery and prenatal management of monoamniotic twin pregnancy: systematic review and meta-analysis. Ultrasound Obstet Gynecol 2019;53(2):166–74. | |
Lewi L. Cord entanglement in monoamniotic twins: does it really matter? Ultrasound Obstet Gynecol 2010;35(2):139–41. | |
Lewi L, Jani J, Blickstein I, et al. The outcome of monochorionic diamniotic twin gestations in the era of invasive fetal therapy: a prospective cohort study. Am J Obstet Gynecol 2008;199(5):514 e1–8. | |
Mieghem TV, Shub A. Management of monoamniotic twins: the question is not 'where?', but 'how?'. Ultrasound Obstet Gynecol 2019;53(2):151–2. | |
Hack KE, Derks JB, Schaap AH, et al. Perinatal outcome of monoamniotic twin pregnancies. Obstetrics and Gynecology 2009;113(2 Pt 1):353–60. | |
Peeters SH, Devlieger R, Middeldorp JM, et al. Fetal surgery in complicated monoamniotic pregnancies: case series and systematic review of the literature. Prenat Diagn 2014;34(6):586–91. | |
Valsky DV, Martinez-Serrano MJ, Sanz M, et al. Cord occlusion followed by laser cord transection in monochorionic monoamniotic discordant twins. Ultrasound Obstet Gynecol 2011;37(6):684–8. | |
Heyborne KD, Porreco RP, Garite TJ, et al. Improved perinatal survival of monoamniotic twins with intensive inpatient monitoring. Am J Obstet Gynecol 2005;192(1):96–101. | |
Diehl W, Glosemeyer P, Tavares De Sousa M, et al. Twin anemia-polycythemia sequence in a case of monoamniotic twins. Ultrasound Obstet Gynecol 2013;42(1):108–11. | |
Dias T, Mahsud-Dornan S, Bhide A, et al. Cord entanglement and perinatal outcome in monoamniotic twin pregnancies. Ultrasound Obstet Gynecol 2010;35(2):201–4. | |
Dias T, Contro E, Thilaganathan B, et al. Pregnancy outcome of monochorionic twins: does amnionicity matter? Twin Res Hum Genet 2011;14(6):586–92. | |
Hack KE, van Gemert MJ, Lopriore E, et al. Placental characteristics of monoamniotic twin pregnancies in relation to perinatal outcome. Placenta 2009;30(1):62–5. | |
Saccone G, Khalil A, Thilaganathan B, et al. Weight discordance and perinatal mortality in monoamniotic twin pregnancies: analysis of the MONOMONO, NorSTAMP and STORK multiple pregnancy cohorts. Ultrasound Obstet Gynecol 2019. | |
Tannuri AC, Batatinha JA, Velhote MC, et al. Conjoined twins: twenty years' experience at a reference center in Brazil. Clinics (Sao Paulo, Brazil). 2013;68(3):371–7. | |
Sebire NJ, Snijders RJ, Hughes K, et al. The hidden mortality of monochorionic twin pregnancies. British Journal of Obstetrics and Gynaecology 1997;104(10):1203–7. | |
Hillman SC, Morris RK, Kilby MD. Co-twin prognosis after single fetal death: a systematic review and meta-analysis. Obstetrics and Gynecology 2011;118(4):928–40. | |
Griffiths PD, Sharrack S, Chan KL, et al. Fetal brain injury in survivors of twin pregnancies complicated by demise of one twin as assessed by in utero MR imaging. Prenat Diagn 2015;35(6):583–91. | |
Simonazzi G, Segata M, Ghi T, et al. Accurate neurosonographic prediction of brain injury in the surviving fetus after the death of a monochorionic cotwin. Ultrasound Obstet Gynecol 2006;27(5):517–21. | |
Robinson A, Teoh M, Edwards A, et al. Fetal brain injury in complicated monochorionic pregnancies: diagnostic yield of prenatal MRI following surveillance ultrasound and influence on prognostic counselling. Prenat Diagn 2017;37(6):611–27. | |
Garel C. The role of MRI in the evaluation of the fetal brain with an emphasis on biometry, gyration and parenchyma. Pediatric Radiology 2004;34(9):694–9. | |
Greimel P, Csapo B, Haeusler M, et al. A Modified Technique for Cord Transection in Monochorionic Monoamniotic Twin Pregnancies. Fetal Diagn Ther 2018;44(3):236–40. | |
Ishii K, Murakoshi T, Takahashi Y, et al. Perinatal outcome of monochorionic twins with selective intrauterine growth restriction and different types of umbilical artery Doppler under expectant management. Fetal Diagn Ther 2009;26(3):157–61. |
Online Study Assessment Option
All readers who are qualified doctors or allied medical professionals can automatically receive 2 Continuing Professional Development points plus a Study Completion Certificate from GLOWM for successfully answering four multiple-choice questions (randomly selected) based on the study of this chapter. Medical students can receive the Study Completion Certificate only.
(To find out more about the Continuing Professional Development awards program CLICK HERE)
