This chapter should be cited as follows:
Lapinsky SE, Glob. libr. women's med.,
ISSN: 1756-2228; DOI 10.3843/GLOWM.409343
The Continuous Textbook of Women’s Medicine Series – Obstetrics Module
Volume 9
Principles and practice of obstetric high-dependency and critical care
Volume Editor: Professor Stephen Lapinsky, University of Toronto, Canada

Chapter
Airway and Ventilator Support
First published: February 2021
Study Assessment Option
By completing 4 multiple-choice questions (randomly selected) after studying this chapter readers can qualify for Continuing Professional Development awards from FIGO plus a Study Completion Certificate from GLOWM
See end of chapter for details
INTRODUCTION
The need for critical care unit admission occurs in 0.7–13.5 women per 1000 deliveries, and of these about 41% require mechanical ventilator support for respiratory failure.1 Although respiratory failure is not a common complication of pregnancy, airway management and respiratory failure are a significant cause of maternal deaths.2 Short-term ventilator support, related to general anesthesia for obstetric or non-obstetric surgical procedures, is required far more commonly than prolonged ICU ventilation. This chapter addresses respiratory support from a critical care perspective, but many concepts may be relevant to the anesthesia environment as well.
PHYSIOLOGY
Several physiological changes occur during pregnancy, affecting the assessment and management of the patient with respiratory failure. Hormonal effects produce capillary congestion and mucus gland hyperplasia, causing airway hyperemia and edema. The enlarging uterus and hormonal effects producing ligamentous laxity affect configuration of the chest wall. The changes to the thoracic cage affect lung volumes, producing a decrease in functional residual capacity (FRC) by 10% to 25% by term,3 with these effects being measurable by 16–24 weeks' gestation and progressing towards term. Vital capacity is not changed, and total lung capacity decreases only a small amount. Lung compliance is not altered, but chest wall and, therefore, total respiratory system compliance are reduced in the third trimester.4 Minute ventilation increases progressively from the first trimester, reaching 20–40% above baseline at term, mediated by an increase in tidal volume of approximately 30–35%.5 These effects are generated by an increased respiratory drive caused by elevated serum progesterone levels. Blood gas measurements in pregnancy demonstrate a respiratory alkalosis (PaCO2 28–32 mmHg) with compensatory renal excretion of bicarbonate (bicarbonate 18–21 mEq/L).6 Although oxygenation is not affected by pregnancy, as FRC diminishes towards terms, mild hypoxemia may develop in the supine position. Oxygen consumption increases significantly due to increased fetal and maternal demands, reaching 20–33% above baseline by the third trimester. Alkalosis (respiratory or metabolic) reduces uterine blood flow, adversely affecting fetal oxygenation.7
AIRWAY MANAGEMENT IN THE PREGNANT PATIENT
Failed intubation of the pregnant patient is more common than in other anesthetic intubations. The incidence of difficult intubation is about 0.7–5% in pregnant patients8 and failed intubation is reported from zero to about 0.4% of intubations.9 A study analysing failed intubations in obstetric patients during the period 1970–2015 reported a stable incidence of 0.23%.8 Maternal deaths occurred in 1.1% of failed intubations, from aspiration, hypoxemia, or esophageal intubation. Increased use of neuraxial analgesia during labor and delivery has decreased the need for general anesthesia and endotracheal intubation. However, intubation may still be required for women with respiratory failure, acute neurological events or those experiencing a complication of local anesthesia. In centers where intubation of pregnant women is less common, for example where general anesthesia is reserved for emergency situations only, intubation failure is more common. Steps to minimize airway failure, such as simulation drills and trouble-shooting algorithms, will be of increased benefit in these institutions.
The obstetric airway
The pregnant patient presents an anatomically and physiologically difficult airway. Anatomic effects include edematous and friable upper airways which can lead to airway bleeding. The diameter of the oropharynx narrows in pregnancy and the Mallampati class may increase further during labor and delivery.10 Airway edema may be exacerbated by intravenous fluid administration, by preeclampsia and by Valsalva maneuvers during labor and delivery. Breast fullness may make insertion of the laryngoscope difficult, necessitating a stubby laryngoscope handle. From a physiological perspective, the diminished FRC and increased oxygen consumption produces a reduced oxygen reserve, resulting in rapid desaturation in response to apnea or hypoventilation.11 The delayed gastric emptying and elevated intraabdominal pressure accompanying pregnancy necessitate precautions for a full stomach in all patients. As a result of these difficulties, the obstetric airway should always be managed by the most experienced operator available, with adequate preparation and appropriate equipment should be available.
Intubating the obstetric patient
Some general guidelines/suggestions can be made to facilitate successful intubation of the pregnant patient, but the management of the obstetric airway should be the responsibility of the person most experienced in this field.
Although maternal death from gastric acid aspiration is now very rare, this complication should always be considered. The pregnant patient should always be considered to have a full stomach, due to the delayed gastric emptying and the decreased lower esophageal sphincter tone. Guidelines for oral intake during labor have been developed.12,13 Oral intake restriction should be individualized – the patient at higher risk of operative delivery should be more restricted. In general, a modest amount of clear liquid is acceptable during labor, but food is discouraged. For elective cesarean section, guidelines suggest discontinuing food 6–8 hours prior, and clear liquids 2 hours prior. Antacid therapy is given prior to surgery, including an antacid solution (e.g. sodium citrate) and/or an H2-receptor antagonist.
As with any difficult intubation, a semi-upright position makes breathing easier prior to intubation. The shoulder and head should be elevated so the sternum and external auditory meatus are in the same plane, which can improve laryngoscopic view.14,15 Due to the risk of aortocaval compression, left uterine displacement of 15 degrees should ideally be used, but may need to be abandoned if intubation would be facilitated by supine positioning.16 Manual displacement of the uterus to the left can be performed.
The decreased FRC and increased oxygen consumption in pregnancy result in a reduced oxygen reserve, and apneic time to oxygen desaturation is much shorter in pregnant women. Oxygen should be administered during preparation for intubation using a tight-fitting oxygen mask. When pre-oxygenation time is limited, 8 deep breaths over 60 seconds can be used.17 It is important to avoid hyperventilation, as respiratory alkalosis can reduce uterine and placental blood flow. Passive apneic oxygenation via high-flow cannulae can be used during laryngoscopy.
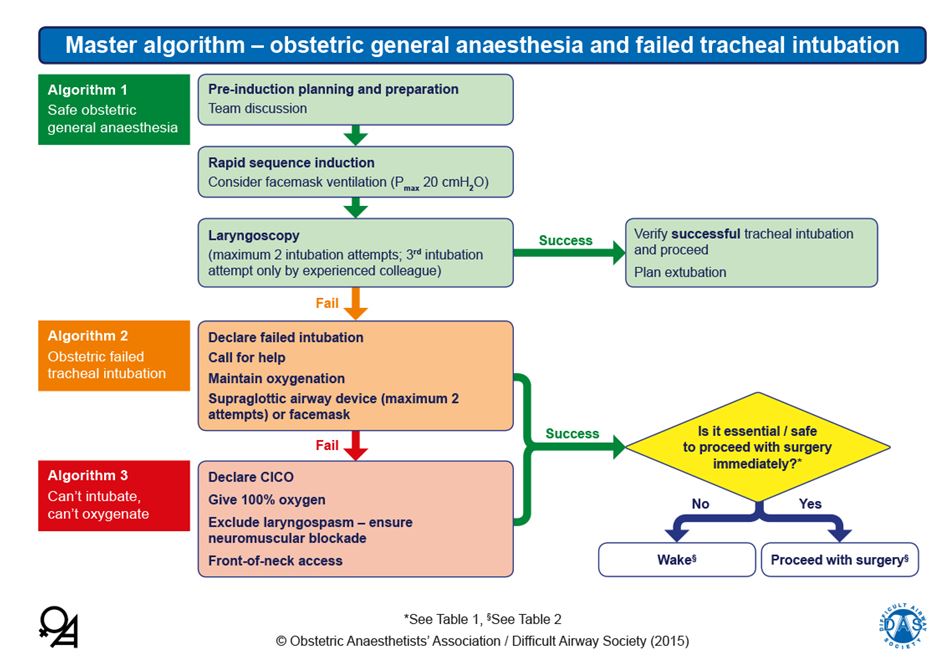
Equipment for management of the difficult airway should be easily accessible, including a video laryngoscope, supraglottic airways and facilities for emergent front-of-neck access. Several obstetric-specific algorithms have been developed for failed intubation, for example from the United Kingdom Obstetric Anaesthetists’ Association (OAA) and Difficult Airway Society (DAS) (Figure 1).18,19
1
Algorithm for obstetric intubation. Reproduced from Mushambi MC, Kinsella SM, Popat M, Swales H, Ramaswamy KK, Winton AL, Quinn AC. Obstetric Anaesthetists' Association and Difficult Airway Society guidelines for the management of difficult and failed tracheal intubation in obstetrics. Anaesthesia 2015;70:1286–1306, with permission from Obstetric Anaesthetists’ Association and Difficult Airway Society.
MECHANICAL VENTILATION
Acute respiratory distress syndrome and acute lung injury in pregnancy
The pregnant patient is at risk of developing respiratory failure from pregnancy-associated complications or other conditions. Acute respiratory distress syndrome (ARDS) is not uncommon in pregnancy and is a leading cause of maternal death.20 The pregnant state may predispose to the development of pulmonary edema by a number of mechanisms, including the increased circulating blood volume, the reduced serum albumin level, a possible upregulation of components of the acute inflammatory response and increased capillary leak.21,22
In addition to the usual causes of ARDS in the non-pregnant patient, such as pneumonia and sepsis, some conditions are more common in pregnancy.
Gastric acid aspiration is an important cause of maternal acute lung injury, due to the increased intra-abdominal pressure caused by the enlarged uterus, the lowered the tone of the esophageal sphincter, and use of the supine position for delivery. Aspiration of gastric contents with pH 2.5 or lower causes chemical pneumonitis and permeability edema. All pregnant patients should be considered to have a full stomach.
Transfusion-related acute lung injury is an important complication of blood component therapy in the pregnant and non-pregnant patient.23 The clinical presentation is of sudden onset of dyspnea and tachypnea during, or within 6 hours, of transfusion of plasma-containing blood products. The clinical picture is difficult to distinguish from other causes of ARDS and the differential diagnosis includes circulatory fluid overload.
Amniotic fluid embolism is a relatively rare but serious condition occurring in about 8 per 100,000 births, with a case fatality rate of 22%.24 The acute event may result in cardiac or respiratory arrest, and those patients who survive the initial event may develop disseminated intravascular coagulation and ARDS.25 Treatment is supportive, involving mechanical ventilation and hemodynamic management.
Preeclampsia may be associated with the development of pulmonary edema in about 2.9% of patients with this condition.26 Pulmonary edema may occur in the postpartum period, related to return of blood from the contracting uterus to the central circulation as well as fluid administration during delivery. Low colloid oncotic pressure and abnormal vascular permeability likely also contribute.
Blood gas targets
While it seems likely that maternal hypoxemia or hypercapnia may be harmful to the fetus, few data exist to identify the optimal oxygen and carbon dioxide goals for ventilatory support during pregnancy. While some similar controversy exists for the non-pregnant patient, pregnancy is complicated by the unique requirements of the developing fetus. It is important to bear in mind that fetal blood gases depend not only on maternal blood gases, but also on placental perfusion and placental function. Decreased uterine blood flow significantly impacts oxygen delivery to the fetus. Uterine blood flow may be reduced by the presence of catecholamines, maternal alkalosis, uterine contractions, or the reduced venous return produced by elevated intrathoracic pressure.27
Oxygen target
It has been suggested that maternal arterial oxygenation should be maintained at greater than 70 mmHg (or saturation >95%),28 but this recommendation is not based on any evidence. Furthermore, maternal oxygen saturation is only one of many factors affecting fetal oxygen supply.
Several different approaches have been used to study the issue of maternal oxygenation. A mathematical model approach used animal data to show that decreasing maternal arterial oxygen saturation from 96% to 85% would reduce fetal umbilical vein saturation from about 70% to 55%.29 A clinical study used short-term inhalation of 10% oxygen in near-term pregnant women with a healthy fetus, to generate controlled maternal hypoxemia (saturation <85%). No adverse fetal effects were noted on fetal heart rate monitoring and fetal middle cerebral arterial Doppler assessment.30 Optimizing maternal oxygenation seems a reasonable clinical approach; however, harm from mild maternal hypoxemia has not been clearly established and data in non-pregnant patients suggests that hyperoxia could be harmful.31,32 Supplemental oxygen therapy should be provided for maternal hypoxia and not routinely for non-reassuring fetal status.33 A single study in pregnant patients suggests a small increase in neonatal morbidity associated with maternal hyperoxia in acidemic neonates.34
Carbon dioxide target
Maternal PaCO2 levels are normally decreased during pregnancy to about 28–32 mmHg, providing a gradient between the fetal and maternal blood to facilitate CO2 removal. This change is mediated largely by the increased respiratory drive generated by progesterone. Marked hypocapnia and respiratory alkalosis may cause fetal hypoxia by reducing placental perfusion.35 Furthermore, studies suggest that fetal hypocapnia may impair fetal cerebral perfusion.36 It is unclear whether permissive hypercapnia, an accepted approach in the ventilation of patients with severe ARDS, is harmful to the fetus. The potential concerns with maternal hypercapnia include reducing the gradient for excretion of fetal CO2, and transfer of CO2 to the fetus producing a fetal respiratory acidosis. This fetal acidosis may affect oxygenation by reducing fetal hemoglobin affinity for oxygen (right-shifting the oxyhemoglobin dissociation curve). However, isolated fetal respiratory acidosis does not have the same ominous implications for the fetus as lactic acidosis secondary to asphyxia.37 Two small clinical studies from the 1970s evaluated the immediate neonatal effects of delivery in women with mild hypercapnia (40–60 mmHg), in those with normal CO2 (for pregnancy) or mild hypocapnia (23–26 mmHg).38,39 Hypercapnia was associated with better APGAR scores than the other groups. A study in healthy pregnant women evaluated fetal well-being by fetal heart rate monitoring, during a maternal rebreathing procedure increasing PaCO2 levels from about 23 mmHg to 60 mmHg, with normoxia.40 No adverse effects were noted in this short-term study. A report of five pregnant women with status asthmaticus describes severe maternal hypercapnia (with PaCO2levels greater than 100 mmHg in two women), with good maternal and neonatal outcomes.41 While respiratory alkalosis should clearly be avoided, transient hypercapnia may be tolerated by the fetus.
Ventilation management
Non-invasive ventilation
Non-invasive ventilation (NIV) is used in non-pregnant patients for short-term ventilatory support and has the advantages of avoiding the complications associated with endotracheal intubation and sedation. In pregnancy there is often a concern about use of NIV due to the risk of aspiration resulting from the decreased gastric emptying, increased intra-gastric pressure and decreased gastroesophageal sphincter tone in pregnancy.42 However, NIV has a role in obstetric respiratory complications, particularly those which reverse rapidly.43 This modality should be restricted to patients who are alert, protecting their airway and with good spontaneous breathing efforts. Risk versus benefit needs to be assessed in the individual patient.
In terms of specific diagnoses appropriate for NIV in the pregnant patient, most reports are for chronic respiratory failure in the context of neuromuscular disease,44 and few reports address initiation of NIV in the acute setting. NIV is most successful in cases requiring short-term ventilation, such as hypoxemic respiratory failure due to pulmonary edema. A report of 186 pregnant women who required ventilator support for H1N1 influenza pneumonitis describes 83 (45%) who received initial NIV, and intubation was avoided in 38 (46% of the NIV group).45 In this group, septic shock and higher severity of illness was associated with NIV failure.
Invasive mechanical ventilation
The evidence-base to inform an approach to mechanical ventilation in the pregnant patient is very limited; pregnant women were largely excluded from or not reported as a subgroup in recent major mechanical ventilation trials. The following recommendations are based on physiological concepts, small observational studies and expert opinions (Table 1). The major physiological changes in pregnancy impacting mechanical ventilation are the reduced chest wall compliance generated by the enlarging uterus, and the increased tidal volume and respiratory alkalosis.
1
Suggested ventilator management of the pregnant patient.
Pregnant patient | Comparison with the non-pregnant patient | |
Patient position | Left lateral position for hemodynamic benefit | Head of bed elevation is used to reduce the incidence of ventilator-associated pneumonia |
Tidal volume | 6 ml/kg PBW | Not different |
Plateau pressure limit | Higher than non-pregnant patient, e.g. 35 cmH2O | Usually = 30 cmH2O |
PEEP level | Be aware of basal atelectasis due to elevated diaphragms | Not different |
Oxygen saturation goal | Usually >94% – unclear if this is necessary | Often accept lower |
Carbon dioxide goal | Hypocapnia is harmful Limited data on hypercapnia. Usually avoided but moderate levels (e.g. <50 mmHg) may be safe | Permissive hypercapnia is often utilized in the non-pregnant patient with ARDS who is difficult to ventilate |
Rescue interventions for intractable hypoxemia |
|
|
PEEP, positive end-expiratory pressure; PBW, predicted body weight; ECLS, extracorporeal life-support
Small studies have addressed the ventilator mode to be used in the pregnant patient. A potential benefit has been suggested from the use of airway pressure release ventilation (APRV) in pregnant women with lung injury.46,47 This mode potentially provides non-injurious ventilation with optimal lung volumes, but its role even in the non-pregnant patient remains to be elucidated. No difference in mode or ventilator settings was noted comparing pregnant and non-pregnant patients in a case control study of patients in Australia during the 2009 influenza pandemic.48
Current guidelines for ventilation of the non-pregnant patient suggest low tidal volume ventilation (6 ml/kg of ideal body weight).49,50 The use of ideal body weight based on height seems reasonable in pregnancy. Aiming for 6 ml/kg tidal volume is likely the safest approach, despite the fact that tidal volume increases in pregnancy.51 Low tidal volume ventilation may result in permissive hypercapnia and the safety of this in pregnancy is unclear (see discussion above). The risk/benefits of increasing the tidal volume in the individual patient need to be considered.
Airway pressures in pregnant women will likely be higher for a given tidal volume, as the chest wall compliance is reduced. The actual change in compliance has not been accurately quantified, and the increase in airway pressures expected during pregnancy is therefore unknown. This increased pressure (e.g. plateau pressure at end-inspiratory occlusion) is not necessarily harmful as it does not represent the transpulmonary pressure applied to the alveolus and does not necessarily create lung strain.52,53 Routine monitoring of airway pressures on the ventilator does not differentiate between a stiff lung (a risk for lung injury) and a stiff chest wall (as occurs in pregnancy). This differentiation can be achieved by measuring esophageal pressure which acts as a surrogate for pleural pressure. The difference between the alveolar pressure and pleural pressure is the transpulmonary pressure, which is the pressure responsible for alveolar inflation and from which lung compliance can be calculated.53 This method can also be used to set positive end-expiratory pressure (PEEP) in ARDS.54 Esophageal pressure measurement requires insertion of a special balloon in the esophagus and has rarely been described in pregnancy. A single study evaluating normal pregnant women found that pregnancy increases measured esophageal pressure.55
The enlarged uterus leads to a decreased FRC which renders pregnant women susceptible to alveolar derecruitment and atelectasis.51 Recruitment maneuvers and higher PEEP levels have been shown to be effective in improving lung compliance and oxygenation, during cesarean delivery under general anesthesia.56 It should be remembered, however, that increased intrathoracic pressure may cause hypotension and decreased cardiac output, which is potentially deleterious to the fetus.
Non-conventional and rescue interventions
Neuromuscular blockade (NMB), i.e. paralysis, is often used to improve patient-ventilator synchrony and avoid high tidal volumes in patients with refractory hypoxemia and ARDS. Although this intervention carries potential risks, a randomized controlled trial demonstrated a significant mortality benefit in patients with early ARDS using a 48 hour infusion of a specific NMB.57 The trial, however, excluded pregnant patients due to safety concerns regarding the use of NMBs during pregnancy. Intermittent doses of NMBs seem to be safe during pregnancy, but a 48 hour infusion may carry some risks (see below).58
Prone positioning of the patient with ARDS improves lung recruitment and provides a mortality benefit in ARDS patients with a PaO2/FiO2 ratio <150.59 Again, pregnant women were excluded from this study. However, case reports have described successful use of prone positioning in pregnancy,60,61 and a prospective study demonstrated that the prone position relieves uterine compression of the large maternal vessels and may improve placental blood flow.62
Other interventions, such as inhaled nitric oxide (iNO) and high frequency oscillation (HFOV), have been reported as rescue therapies during pregnancy.63,64 Negative trial data in the non-pregnant patient65,66 are making these procedures less common in clinical practice, although HFOV may still have a role as a rescue intervention in severe ARDS.67 Extracorporeal life-support (ECLS), i.e. extracorporeal membrane oxygenation (ECMO), provides oxygenation and CO2 removal via an external circuit with pump and oxygenator. A number of pregnant women were managed with this technique during the 2009 H1N1 influenza with reasonably good outcome.68 A metaanalysis of five retrospective studies demonstrates a maternal survival of 75% with 70% live birth rate.69
Role of delivery
Management of the pregnant woman with severe ARDS may bring up the question of whether urgent delivery would improve the mother’s respiratory status. This beneficial effect has been documented in a case report.70 However, this benefit to the mother certainly does not occur in all cases.71,72 We retrospectively assessed the respiratory effects of delivery during mechanical ventilation in 10 women with respiratory failure.73 Improvement in oxygenation (50% decrease in Oxygenation Index) or improved compliance (50% increase) occurred in only 6 of 10 patients.73 A case series of 71 pregnant women with respiratory failure documented the maternal effects of cesarean section within 48 hours of intubation. Women with an obstetric cause for ARDS (e.g. preeclampsia) demonstrated an improvement in oxygenation and shorter duration of ventilation, but this was not observed in women with non-obstetric causes for ARDS.74,74 If the fetus is at risk due to irreversible maternal hypoxemia and is at a gestation with a reasonable expectation of survival, delivery would be appropriate. However, delivery purely in the hope of improving maternal respiratory function is not well supported by the literature, unless delivery will reverse an obstetric complication. This decision will require multidisciplinary input including critical care, maternal–fetal medicine and neonatology. The mode of delivery is based on usual obstetric principles and should be determined by the obstetrician. Although cesarean delivery is quicker and more controlled, the increased physiological stress may be associated with higher mortality in these patients.75 When a pregnant woman at a viable gestation is managed in the ICU all necessary equipment and drugs for vaginal delivery, operative delivery, and neonatal resuscitation should be immediately available, as well as contact details for obstetric and neonatal support.
Drug therapy
Patients on mechanical ventilation often require sedation, for comfort and to facilitate synchrony between the patient’s breathing efforts and the ventilator. Current practice in the non-pregnant patient is to minimize sedation, using a sedation administration algorithm or by daily awakening. These protocols have not been studied in the pregnant patient. The safety of continuous analgesia and sedation in pregnant patients and on their fetuses is unknown. In 2017, the Food and Drug Administration (FDA) of the United States warned that repeated or prolonged administration of general anesthetic or sedative drugs in pregnant women during their third trimester may affect the development of children’s brains.76 Human data are sparse and inconclusive and this statement was based largely on animal studies, generating considerable controversy. Nevertheless, minimizing sedation seems even more relevant in the pregnant patient, but further studies on the cognitive and developmental impact on human offspring are needed.
Non-depolarizing neuromuscular blocking agents (NMB) cross the placenta in variable amounts. The fetal–maternal drug concentration ratio for atracurium, vecuronium, rocuronium and pancuronium varies between 0.07 and 0.2677 and pharmacokinetics and dynamics are affected by the physiological changes occurring in pregnancy.78 Few human data exist for cisatracurium use in pregnancy except occasional case reports.79 Data on NMB use in pregnancy are derived largely from cesarean delivery or other surgery during the second and third trimester.77 Longer-term infusions, as used in the ICU, have not been studied. A single report described NMB administration for 10 days associated with fetal paralysis and development of neonatal arthrogryposis.80 Another report describes good outcomes after a 10 hour pancuronium infusion during the third trimester.81 It should also be noted that the neuromuscular blockade effect may be exacerbated with hypermagnesemia induced for prevention of seizures in preeclampsia.82
The obstetric team and neonatal team should always be informed of the potential risk of neonatal respiratory depression and drug withdrawal after delivery.
Outcome after mechanical ventilation during pregnancy
Few studies have addressed maternal and neonatal outcomes after prolonged (i.e. non-surgical) mechanical ventilation. A study using an administrative database reports a maternal mortality of 9% for pregnant women mechanically ventilated for ARDS.83 Increased duration of ventilation was associated with an increased mortality, from 6.9% for less than 96 hours to 14.0% when greater than 96 hours. Only one study has provided data on long-term neonatal outcome following maternal ventilation in the ICU. In this retrospective study of 71 women with gestation greater than 25 weeks ventilated for respiratory failure, hospital maternal mortality was 5.6%. All neonates survived to hospital discharge, although 20% of babies required mechanical ventilation and 20% demonstrated neurological impairment at 6 months.74
PRACTICE RECOMMENDATIONS
- Endotracheal intubation is more difficult in the pregnant patient owing to upper airway edema and friability, and rapid oxygen desaturation during apnea.
- Intubation should be performed by the most experienced operator available, with consideration of failed intubation algorithms and access to all appropriate equipment.
- Oxygen targets during pregnancy should provide adequate maternal oxygenation but avoid unnecessary hyperoxia.
- Hyperventilation and hypocapnia reduce placental perfusion, but mild maternal hypercapnia is probably not harmful to the fetus.
- There are limited data to inform an approach for mechanical ventilation specific to the pregnant patient, and usual recommendations applicable to the non-pregnant patient should be utilized.
- Delivery of the fetus may not necessarily improve the mother’s respiratory status, except in those patients with an obstetric cause of maternal illness.
CONFLICTS OF INTEREST
Author(s) statement awaited.
Feedback
Publishers’ note: We are constantly trying to update and enhance chapters in this Series. So if you have any constructive comments about this chapter please provide them to us by selecting the "Your Feedback" link in the left-hand column.
REFERENCES
Pollock W, Rose L, Dennis CL. Pregnant and postpartum admissions to the intensive care unit: a systematic review. Intensive Care Med 2010;36:1465–74 | |
Cantwell R, Clutton-Brock T, Cooper G, et al. Saving Mothers' Lives: Reviewing maternal deaths to make motherhood safer: 2006–2008. The Eighth Report of the Confidential Enquiries into Maternal Deaths in the United Kingdom. BJOG 2011;118(1):1–203 | |
Elkus R, Popovich J. Respiratory physiology in pregnancy. Clin Chest Med 1992;13:555–65. | |
Marx GF, Murthy PK, Orkin LR. Static compliance before and after vaginal delivery. Br J Anaesth 1970;42:1100–1104. | |
Rees GB, Pipkin FB, Symonds EM, et al. A longitudinal study of respiratory changes in normal human pregnancy with cross-sectional data on subjects with pregnancy-induced hypertension. Am J Obstet Gynecol 1990;162:826–30. | |
Lucius H, Gahlenbeck HO, Kleine O, et al. Respiratory functions, buffer system, and electrolyte concentrations of blood during human pregnancy. Respir Physiol 1970;9:311–7. | |
Buss DD, Bisgard GE, Rawlings CA, et al. Uteroplacental blood flow during alkalosis in the sheep. Am J Physiol 1975;228:1497–500. | |
Kinsella SM, Winton AL, Mushambi MC, et al. Failed tracheal intubation during obstetric general anaesthesia: a literature review. Int J Obstet Anesth 2015;24:356–74 | |
Djabatey EA, Barclay PM. Difficult and failed intubation in 3430 obstetric general anaesthetics. Anaesthesia 2009;64:1168–71. | |
Kodali BS, Chandrasekhar S, Bulich LN, et al. Airway changes during labor and delivery. Anesthesiology 2008;108:357–62. | |
Archer GW, Marx GF. Arterial oxygen tension during apnoea in parturient women. Br J Anaesth 1974;46:358–60. | |
Practice Guidelines for Obstetric Anesthesia: An Updated Report by the American Society of Anesthesiologists Task Force on Obstetric Anesthesia and the Society for Obstetric Anesthesia and Perinatology. Anesthesiology 2016;124:270–300. | |
Committee on Obstetric Practice ACOG. ACOG Committee Opinion No. 441: Oral intake during labor. Obstet Gynecol 2009;114:714. | |
Lebowitz PW, Shay H, Straker T, et al. Shoulder and head elevation improves laryngoscopic view for tracheal intubation in nonobese as well as obese individuals. J Clin Anesth 2012;24:104–8. | |
Rucklidge MW, Yentis SM. Obstetric difficult airway guidelines – decision-making in critical situations. Anaesthesia 2015;70:1221–5. | |
Lee AJ, Landau R, Mattingly JL, et al. Left Lateral Table Tilt for Elective Cesarean Delivery under Spinal Anesthesia Has No Effect on Neonatal Acid-Base Status: A Randomized Controlled Trial. Anesthesiology 2017;127:241–9. | |
Chiron B, Laffon M, Ferrandiere M, et al. Standard preoxygenation technique versus two rapid techniques in pregnant patients. Int J Obstet Anesth 2004;13:11–4. | |
Mushambi MC, Kinsella SM, Popat M, et al. Obstetric Anaesthetists' Association and Difficult Airway Society guidelines for the management of difficult and failed tracheal intubation in obstetrics. Anaesthesia 2015;70:1286–306. | |
Apfelbaum JL, Hagberg CA, Caplan RA, et al. Practice guidelines for management of the difficult airway: an updated report by the American Society of Anesthesiologists Task Force on Management of the Difficult Airway. Anesthesiology 2013;118:251–70. | |
Perry KG, Martin RW, Blake PG, et al. Maternal mortality associated with the adult respiratory distress syndrome. South Med J 1998;91:441–4 | |
Bandi VD, Munnur U, Matthay MA. Acute lung injury and acute respiratory distress syndrome in pregnancy. Crit Care Clin 2004;20:577–607. | |
Lapinsky SE. Pregnancy joins the hit list. Crit Care Med 2012;40:1679–80. | |
Cantwell R, Clutton-Brock T, Cooper G, et al. The Eighth Report on Confidential enquiries into Maternal Deaths in the United Kingdom. BJOG 2011;118(1):1–203 | |
Abenhaim HA, Azoulay L, Kramer MS, et al. Incidence and risk factors of amniotic fluid embolisms: a population-based study on 3 million births in the United States. Am J Obstet Gynecol 2008;199:49.e1–8 | |
Shamshirsaz AA, Clark SL. Amniotic Fluid Embolism. Obstet Gynecol Clin North Am 2016;43:779–90. | |
Sibai BM, Mabie BC, Harvey CJ, et al. Pulmonary edema in severe preeclampsia-eclampsia: Analysis of thirty-seven consecutive cases. Am J Obstet Gynecol 1987;156:1174–9. | |
Aoyama K, Seaward PG, Lapinsky SE. Fetal outcome in the critically ill pregnant woman. Crit Care 2014;18:307. | |
Cole DE, Taylor TL, McCullough DM, et al. Acute respiratory distress syndrome in pregnancy. Crit Care Med 2005;33:S269–78. | |
Meschia G. Fetal oxygenation and maternal ventilation. Clin Chest Med 2011;32:15–9. | |
Polvi HJ, Pirhonen JP, Erkkola RU. The hemodynamic effects of maternal hypo- and hyperoxygenation in healthy term pregnancies. Obstet Gynecol 1995;86:795–9. | |
Stub D, Smith K, Bernard S, et al. Air Versus Oxygen in ST-Segment-Elevation Myocardial Infarction. Circulation 2015;131:2143–50. | |
Girardis M, Busani S, Damiani E, et al. Effect of Conservative vs Conventional Oxygen Therapy on Mortality Among Patients in an Intensive Care Unit: The Oxygen-ICU Randomized Clinical Trial. JAMA 2016;316:1583–9. | |
Hamel MS, Anderson BL, Rouse DJ. Oxygen for intrauterine resuscitation: of unproved benefit and potentially harmful. American Journal of Obstetrics and Gynecology 2014;211:124–7. | |
Raghuraman N, Temming LA, Stout MJ, et al. Intrauterine Hyperoxemia and Risk of Neonatal Morbidity. Obstet Gynecol 2017;129:676–82. | |
Buss DD, Bisgard GE, Rawlings CA, et al. Uteroplacental blood flow during alkalosis in the sheep. Am J Physiol 1975;228:1497–500. | |
Tomimatsu T, Kakigano A, Mimura K, et al. Maternal carbon dioxide level during labor and its possible effect on fetal cerebral oxygenation: mini review. J Obstet Gynaecol Res 2013;39:1–6 | |
Low JA, Panagiotopoulos C, Derrick EJ. Newborn complications after intrapartum asphyxia with metabolic acidosis in the term fetus. Am J Obstet Gynecol 1994;170:1081–7. | |
Peng AT, Blancato LS, Motoyama EK. Effect of maternal hypocapnia v. eucapnia on the foetus during Caesarean section. Br J Anaesth 1972;44:1173–8.294. | |
Ivankovic AD, Elam JO, Huffman J. Effect of maternal hypercarbia on the newborn infant. Am J Obstet Gynecol 1970;107:939–46. | |
Fraser D, Jensen D, Wolfe LA, et al. Fetal heart rate response to maternal hypocapnia and hypercapnia in late gestation. J Obstet Gynaecol Can 2008;30:312–6. | |
Elsayegh D, Shapiro JM. Management of the obstetric patient with status asthmaticus. J Intensive Care Med 2008;23:396–402. | |
Rowe TF. Acute gastric aspiration: prevention and treatment. Semin Perinatol 1997;21(4):313–9. | |
Al-Ansari MA, Hameed AA, Al-Jawder SE, et al. Use of noninvasive positive pressure ventilation in pregnancy: Case series. Ann Thoracic Medicine 2007;2:23–5. | |
Allred CC, Matías Esquinas A, Caronia J, et al. Successful use of noninvasive ventilation in pregnancy. Eur Respir Rev 2014;23:142–4. | |
Zhang PJ, Li XL, Cao B, et al. Clinical features and risk factors for severe and critical pregnant women with 2009 pandemic H1N1 influenza infection in China. BMC Infect Dis 2012;12:29. | |
Hirani A, Marik PE, Plante LA. Airway pressure-release ventilation in pregnant patients with acute respiratory distress syndrome: a novel strategy. Respir Care 2009;54:1405–8. | |
Folk JJ, Landsberg DM, Robinson KA, et al. Airway pressure release ventilation and respiratory failure during pregnancy. A report of three cases. J Reprod Med 2015;60(1–2):65–70. | |
Pollock WE, Bellomo R, Webb S, et al. Provision of mechanical ventilation to pregnant/postpartum women with H1N1 influenza: A case-control study. Aust Crit Care 2013;26:83. | |
Acute Respiratory Distress Syndrome Network, Brower RG, Matthay MA, et al. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 2000;342:1301–8. | |
Serpa Neto A, Cardoso SO, Manetta JA, et al. Association between use of lung-protective ventilation with lower tidal volumes and clinical outcomes among patients without acute respiratory distress syndrome: a meta-analysis. JAMA 2012;308:1651–9. | |
LoMauro, A., Aliverti, A. Respiratory physiology of pregnancy: Physiology masterclass. Breathe 2015;11:297–301. | |
Ranieri VM, Brienza N, Santostasi S, et al. Impairment of lung and chest wall mechanics in patients with acute respiratory distress syndrome: role of abdominal distension. Am J Respir Crit Care Med 1997;156:1082–91. | |
Akoumianaki E, Maggiore SM, Valenza F, et al. The application of esophageal pressure measurement in patients with respiratory failure. Am J Respir Crit Care Med 2014;189:520–31. | |
Talmor D, Sarge T, Malhotra A, et al. Mechanical ventilation guided by esophageal pressure in acute lung injury. N Engl J Med 2008;359:2095–104. | |
Gee JB, Packer BS, Millen JE, et al. Pulmonary mechanics during pregnancy. J Clin Invest 1967;46:945–52. | |
Aretha D, Fligou F, Kiekkas P, et al. Safety and effectiveness of alveolar recruitment maneuvers and positive end-expiratory pressure during general anesthesia for cesarean section: a prospective, randomized trial. Int J Obstet Anesth 2017;30:30–8. | |
Papazian L, Forel JM, Gacouin A, et al. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med 2010;363:1107–16. | |
Guay J, Grenier Y, Varin F. Clinical pharmacokinetics of neuromuscular relaxants in pregnancy. Clin Pharmacokinet 1998;34:483. | |
Guérin C, Reignier J, Richard JC, et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med 2013;368:2159–68. | |
Samanta S, Samanta S, Wig J, et al. How safe is the prone position in acute respiratory distress syndrome at late pregnancy? Am J Emerg Med 2014;32:687e1–3. | |
Kenn S, Weber-Carstens S, Weizsaecker K, et al. Prone positioning for ARDS following blunt chest trauma in late pregnancy. Int J Obstet Anesth 2009;18:268–71. | |
Nakai Y, Mine M, Nishio J, et al. Effects of maternal prone position on the umbilical arterial flow. Acta Obstet Gynecol Scand 1998;77:967–9. | |
Brown CM. Severe influenza A virus (H1N1) infection in pregnancy. Obstet Gynecol 2010;115:412–4. | |
Ellington SR, Hartman LK, Acosta M, et al. Pandemic 2009 influenza A (H1N1) in 71 critically ill pregnant women in California. Am J Obstet Gynecol 2011;204:S21–30 | |
Ferguson ND, Cook DJ, Guyatt GH, et al. High-frequency oscillation in early acute respiratory distress syndrome. N Engl J Med 2013;368:795–805 | |
Karam O, Gebistorf F, Wetterslev J, et al. The effect of inhaled nitric oxide in acute respiratory distress syndrome in children and adults: a Cochrane Systematic Review with trial sequential analysis. Anaesthesia 2017;72:106–17. | |
Meade MO, Young D, Hanna S, et al. Severity of Hypoxemia and Effect of High Frequency Oscillatory Ventilation in ARDS. Am J Respir Crit Care Med 2017;196:727–33 | |
Nair P, Davies AR, Beca J, et al. Extracorporeal membrane oxygenation for severe ARDS in pregnant and postpartum women during the 2009 H1N1 pandemic. Intensive Care Med 2011;37:648–54. | |
Saad AF, Rahman M, Maybauer DM, et al. Extracorporeal Membrane Oxygenation in Pregnant and Postpartum Women With H1N1-Related Acute Respiratory Distress Syndrome: A Systematic Review and Meta-analysis. Obstet Gynecol 2016;127:241–7. | |
Daily WH, Katz AR, Tonnesen A, et al. Beneficial effect of delivery in a patient with adult respiratory distress syndrome. Anesthesiology 1990;72:383–6. | |
Tomlinson MW, Caruthers TJ, Whitty JE, et al. Does delivery improve maternal condition in the respiratory-compromised gravida? Obstet Gynecol 1998;91:108–11. | |
Mabie WC, Barton JR, Sibai BM. Adult respiratory distress syndrome in pregnancy. Am J Obstet Gynecol 1992;167:950–7. | |
Lapinsky SE, Rojas-Suarez JA, Crozier TM, et al. Mechanical ventilation in critically-ill pregnant women: a case series. Int J Obstet Anesth 2015;24:323–8. | |
Hung CY, Hu HC, Chiu LC, et al. Maternal and Neonatal outcomes of respiratory failure during pregnancy. J Formosan Med Assoc 2018;117:413–20 | |
Jenkins TM, Troiano NH, Graves CR, et al. Mechanical ventilation in an obstetric population: characteristics and delivery rates. Am J Obstet Gynecol 2003;188:549–52. | |
Federal Drug Agency. FDA Drug Safety Communication: FDA review results in new warnings about using general anesthetics and sedation drugs in young children and pregnant women. 2016. Available at: https://www.fda.gov/drugs/drugsafety/ucm532356.htm Accessed June 12, 2017. | |
Guay J, Grenier Y, Varin F. Clinical pharmacokinetics of neuromuscular relaxants in pregnancy. Clin Pharmacokinet 1998;34:483. | |
Briggs GG, Freeman RK, Yaffe SJ. Drugs in pregnancy and lactation: a reference guide to fetal and neonatal risk 10th edn. Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins Health, 2015. | |
Gavand Y, Krausz-Grignard M, Barrucand B, et al. [Anaesthesia for caesarean section in a pregnant woman with cor triatriatum]. Ann Fr Anesth Reanim 2011;30:688–91. | |
Jago RH. Arthrogryposis following treatment of maternal tetanus with muscle relaxants. Arch Dis Child 1970;45:277–9. | |
Eisenberg VH, Eidelman LA, Arbel R, et al. Legionnaire's disease during pregnancy: a case presentation and review of the literature. Eur J Obstet Gynecol Reprod Biol 1997;72:15–8. | |
Yoshida A, Itoh Y, Nagaya K, et al. Prolonged relaxant effects of vecuronium in patients with deliberate hypermagnesemia: time for caution in cesarean section. J Anesth 2006;20:33–5. | |
Rush B, Martinka P, Kilb B, et al. Acute Respiratory Distress Syndrome in Pregnant Women. Obstet Gynecol 2017;129:530–5. |
Online Study Assessment Option
All readers who are qualified doctors or allied medical professionals can now automatically receive 2 Continuing Professional Development credits from FIGO plus a Study Completion Certificate from GLOWM for successfully answering 4 multiple choice questions (randomly selected) based on the study of this chapter.
Medical students can receive the Study Completion Certificate only.
(To find out more about FIGO’s Continuing Professional Development awards programme CLICK HERE)