Hysteroscopic Method Although the mechanics of the hysteroscopic methods of endometrial ablation
are discussed in this chapter, the mechanics of operative hysteroscopy
are not. The reader is referred to other chapters for discussion
of general hysteroscopic principles, distention media, and technique. LASER ENDOMETRIAL ABLATION. The Nd: YAG laser emits an invisible beam of light with a wavelength of 1064 nm. The
Nd:YAG laser has three properties that make it well suited
to endometrial ablation: (1) it penetrates the endometrium to a depth
of 5 to 6 mm, (2) it passes unabsorbed through clear liquids, and (3) it
can be directed through flexible quartz fibers. Quartz fibers of different diameters from 600 to 1200 μm have been developed
for use with the Nd:YAG laser The tissue effect with these fibers
is related to the power density produced. Wider fibers have lower
power densities and require a longer contact time to produce the same
effect achieved with a smaller fiber at any given power. Generally, 6000-μm
fibers are recommended for endometrial ablation. The flexible
quartz fibers used for endometrial ablation are surrounded by a protective
plastic coat. When laser energy is directed through these “bare
fibers,” it penetrates the surface of the endometrium and
exerts a maximal thermocoagulation in the superficial myometrium. Thus, a
deep thermocoagulation is achieved (Fig. 2). This is ideal for endometrial ablation but not well suited for other
applications such as intra-abdominal applications. Consequently, shaped “sapphire” ceramic tips that can focus the laser energy
in various configurations, depending on the shape of the tip, have been
developed to fit onto these bare quartz fibers. Although these tips
reduce the depth of tissue destruction and increase the range of applications
in which the Nd:YAG laser can be used, they should not be used
for endometrial ablation. In endometrial ablation, deep tissue destruction
is desirable to destroy the basalis layer. Any reduction in the
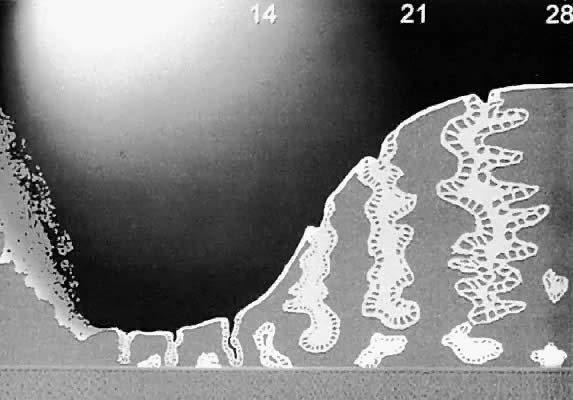
depth of destruction potentially reduces the effectiveness of the procedure.  Fig. 2. Diagrammatic representation of the zones of tissue destruction around an
Nd:YAG laser fiber. Fig. 2. Diagrammatic representation of the zones of tissue destruction around an
Nd:YAG laser fiber.
|
Sapphire tips by the nature of their design are subjected to very high
temperatures and must be cooled by a secondary cooling system to prevent
overheating. One method of cooling involves directing carbon dioxide (CO2) gas under pressure through a coaxial channel. This gas exits a small
opening near the base of the sapphire tip and cools the tip. The combined
effect of a large volume of gas under pressure instilled into the
closed uterine cavity may produce a gas embolus. Several deaths from this
effect have been reported.12 Therefore, the use of gas-cooled tips in the uterine cavity is absolutely
contraindicated. The use of a bare quartz fiber can be used in two different manners to
perform endometrial ablation. These two methods are the contact, or dragging, method
and the noncontact, or blanching, method. The contact method was the original method used by Goldrath and associates
to perform the first laser endometrial ablation.2 This method involves “dragging” the bare fiber across the
endometrial surface. In this technique, the fiber is kept in constant
contact with the endometrial surface. The principal advantages of the
dragging technique is that it is very easy to identify the treated areas
from the untreated areas and the technique can be easily used for all
areas of the uterine cavity. In the noncontact, or blanching, technique, the quartz fiber is held at
right angles to and a short distance above the endometrial surface. This
method is believed by its supporters to be less likely to interrupt
underlying blood vessels, thus reducing the risk of fluid intravasation. The
primary disadvantage to this method is the difficulty in getting
the fiber at the correct angle over the endometrial surface. There
is also occasional difficulty in differentiating photocoagulated tissue
from the pale suppressed endometrium. Because the light produced by the Nd:YAG laser is invisible to the human
eye, a helium neon (HeNe) laser is used to illuminate the distal 5 mm
of the tip. Thus, the tip glows red not because it is hot but because
of the light produced by the HeNe laser. Technique of Laser Endometrial Ablation. The contact, or dragging, technique is more commonly used than the noncontact
method, and a description of it follows. However, the basic principles
of the technique still apply to the noncontact method. After placement of the hysteroscope, the laser is activated at a power
of 50 to 80 W. A power of 50 W is probably the lowest power setting that
will provide adequate endometrial ablation. Higher powers are inherently
more dangerous but also permit shorter treatment times and reduce
the potential for fluid absorption. Powers up to 80 W are recommended
by some authors. Although several different variations in technique are available, most
laser procedures start at the tubal ostium area. A series of circular
parallel furrows are performed until the ostium is completely ablated. The
procedure is repeated on the opposite side (Fig. 3). Then the anterior wall, the lateral walls, and finally the posterior
wall are ablated.  Fig. 3. Diagrammatic representation of a continuous flow hysteroscope. Fig. 3. Diagrammatic representation of a continuous flow hysteroscope.
|
The laser is only activated as the fiber is being pulled toward the surgeon
and never as the fiber is being pushed back toward the fundus. This
is a safety measure because the fiber is more likely to perforate the
uterus while being advanced. If a perforation should occur while the
laser is activated, serious damage can occur. The laser also should
not be activated unless the glowing red tip can be seen, ensuring that
the tip is within the endometrial cavity and not outside the uterus. The
laser should also be fired only when the fiber is moving. Otherwise, the
depth of destruction will be much greater than desired. ELECTROSURGICAL ENDOMETRIAL ABLATION. The development of electrosurgical methods of endometrial ablation has
resulted in decline in the use of the laser ablation techniques. Many
physicians find the electrosurgical methods to be easier and to require
less operator skill, and the equipment cheaper to acquire and maintain. With laser ablation methods, the procedure is generally more prolonged, lasting 30 to 40 minutes
compared with 20 to 30 minutes with electrosurgery
when performed by an experienced operator. The overall reported
cure rate with electrosurgical methods is slightly less than that reported
with laser but the difference does not appear to be clinically significant. Unlike
laser energy, electricity is conducted by electrolyte-containing
fluids. Thus, nonelectrolyte fluids must be used when working
with unipolar electrosurgical systems. Use of these fluids increases
the likelihood of fluid overload and hyponatremia. With the use of endometrial resection, the risk of uterine perforation
is increased over that of rollerball techniques. Furthermore, resection
of the superficial portion of myometrium increases the likelihood of
bleeding and exposes the venus sinuses. These open sinuses increase the
absorption of the irrigation fluid and increase the possibility of
hyponatremia. With rollerball coagulation, vessels are coagulated rather
than opened, thus decreasing fluid absorption and bleeding compared
with resection methods. Although endometrial resection can resect small submucous fibroids, there
is an increased risk of uterine perforation with resection methods. One
theoretical benefit with endometrial resection is that pieces of
the endometrium are available for examination by the pathologist. With
coagulation methods, no material is available for review. However, with
appropriate evaluation of patients before ablation procedures, along
with the possibility of target biopsy at the time of the procedure, the
likelihood of missed carcinoma is quite rare. Procedure for Rollerball Ablation. Generally, the electrosurgical generator is set at 60 to 100 W. Although
we generally use pure cutting current, many authors use some form of
blended current during ablation. Whatever the current modulation, the
lower wattage should be chosen initially and then increased depending
on the tissue effect observed. With the commonly used 3-mm ball and
a drag speed of 1 cm/second, the crater produced is approximately 1 mm
in depth with thermal damage extending for 1 to 2 more mm. Slower drag
speeds or higher wattage are associated with greater tissue penetration. The
use of a rollerbar instead of the 3-mm ball allows a greater
surface to be covered in a smaller amount of time but results in a lower
current density and significantly less tissue penetration than with
the rollerball, if all other factors are kept equal. As with other techniques, as a safety measure, the electrode should be
activated only when it is being withdrawn toward the operator. Most surgeons
prefer to begin coagulation with the anterior wall because accumulation
of bubbles and debris in this area over time makes coagulation
later more difficult. However, some surgeons coagulate the tubal ostia
initially, with the belief that this decreases fluid loss through the
fallopian tubes and, subsequently, the lateral walls and finally the
posterior walls (Fig. 4). As the ablation is carried downward, care should be exercised not to
treat the cervical canal itself because this could result in sealing
off of the endometrial cavity with subsequent development of a hematometrium
or pyometrium. 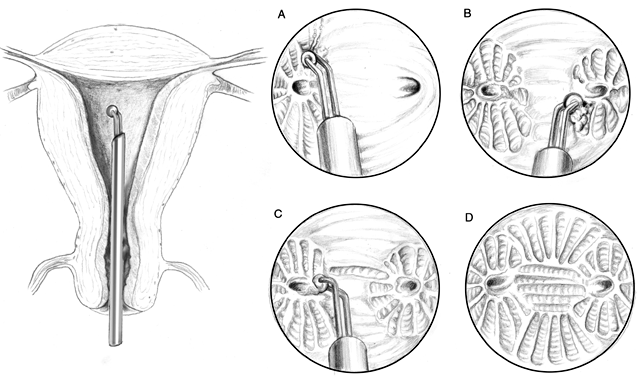 Fig. 4. Schema of rollerball ablation of the endometrium by resectoscope. Note
the end results of ball versus loop are similar if depth of injury is
kept constant.(Adapted from Baggish MS, Valle RF, Barbot J: Endometrial ablation. In
Baggis MS, Barbot J, Valle RF [eds]: Diagnostic and Operative Hysteroscopy. St
Louis, Mosby, 1999.) Fig. 4. Schema of rollerball ablation of the endometrium by resectoscope. Note
the end results of ball versus loop are similar if depth of injury is
kept constant.(Adapted from Baggish MS, Valle RF, Barbot J: Endometrial ablation. In
Baggis MS, Barbot J, Valle RF [eds]: Diagnostic and Operative Hysteroscopy. St
Louis, Mosby, 1999.)
|
Endometrial Resection. The technique for endometrial ablation is essentially the same as that
for rollerball coagulation as noted previously (Fig. 5). Because the risk of perforation is higher with this technique, special
care should be taken in the thinner area around the tubal ostia. 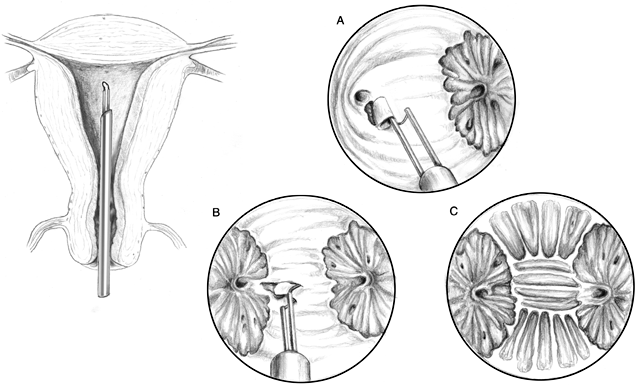 Fig. 5. Schema of endometrial resection via resectoscope. The cornual areas are
resected following completion of the anterior wall. The power is reduced
to approximately 50 W of pure cut or blend one current, and the depth
adjusted to 1 to 3 mm. Next, the cornu is resected. Specimens are
sent for pathologic examination.(Adapted from Baggish MS, Valle RF, Barbot J: Endometrial ablation. In
Baggis MS, Barbot J, Valle RF [eds]: Diagnostic and Operative Hysteroscopy. St
Louis, Mosby, 1999.) Fig. 5. Schema of endometrial resection via resectoscope. The cornual areas are
resected following completion of the anterior wall. The power is reduced
to approximately 50 W of pure cut or blend one current, and the depth
adjusted to 1 to 3 mm. Next, the cornu is resected. Specimens are
sent for pathologic examination.(Adapted from Baggish MS, Valle RF, Barbot J: Endometrial ablation. In
Baggis MS, Barbot J, Valle RF [eds]: Diagnostic and Operative Hysteroscopy. St
Louis, Mosby, 1999.)
|
Most surgeons prefer an 8-mm diameter loop for endometrial ablation by
resection. With this loop, 4 mm of tissue are resected with each pass. However, some
surgeons prefer a 4-mm diameter loop because it is easier
to place in restricted areas such as the ostia and because the risk
of perforation is less. For this reason, this diameter loop is frequently
chosen by those just learning the technique and for teaching purposes. Disadvantages
to this size loop include that the loop is more easily
damaged and requires more passes to obtain adequate tissue destruction. Nonhysteroscopic Ablation Systems THERMAL BALLOON. The first balloon system granted Food and Drug Administration (FDA) approval
in the United States in December 1997 was the ThermaChoice intrauterine
hot water balloon (Gynecare, Inc, a division of Ethicon, Inc, Somerville, NJ). This
device uses an intrauterine balloon to deliver
destructive heat to the endometrial layer to destroy the blood supply (Fig. 6). The ThermaChoice procedure uses a water-filled balloon that conforms
to the shape of the uterus. Once activated, the water is heated by a
heating element to a temperature of approximately 87°C, or 187°F. The
system is composed of a balloon connected to a catheter that is
connected into a central control unit. The balloon is manually inflated
with 5% dextrose in water (D5W) to a pressure of 160 to 170 mmHg. Inability to obtain suitable initial
pressure may indicate a uterine perforation or a uterine cavity too
large for the balloon. A heating element inside the balloon then heats
the solution. In the recently released ThermaChoice II system, a fluid
mixing impeller distributes the heat more evenly. The heating interval
is set at 8 minutes. If the pressure or temperature deviates outside
prescribed parameters, the system automatically shuts down. If a uterine
perforation occurs, the pressure decreases and deactivates the system. At
the completion of the procedure, the balloon is deflated and
removed. 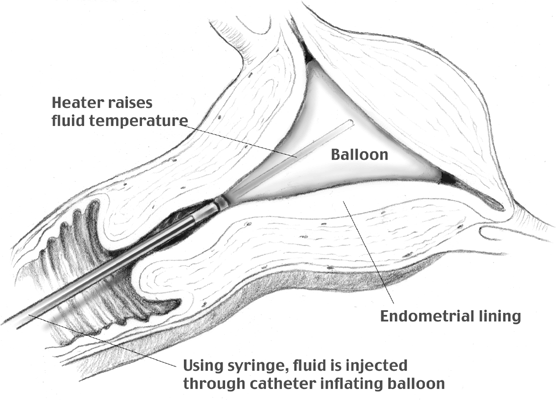 Fig. 6. Mechanisms of endometrial ablation using intrauterine balloon. Fig. 6. Mechanisms of endometrial ablation using intrauterine balloon.
|
Initial studies in women undergoing hysterectomy have indicated that the
temperature of the uterine serosa remains unchanged during balloon ablation.13 These same studies also indicate that cell death could be expected up
to 5 mm from the surface of the balloon. RADIO-WAVE ELECTRODE INTRAUTERINE BALLOON. The radio-wave balloon consists of an inflatable balloon with electrodes
attached to its surface (Fig. 7). One such device, the Vesta radio-wave balloon (Valleylab, Inc, Boulder, CO), has
been undergoing FDA testing in the United States. The Vesta
balloon contains 12 electrodes, each of which is controlled by a separate
thermistor. The system works on the same principle as other electrosurgical
devices. The system delivers radiofrequency current to the
endometrial tissue. This is converted into intracellular heat, producing
coagulation. After the device is inserted into the endometrial cavity, the
balloon is inflated and activated. Unless all electrodes make
contact with the uterine wall, the device will shut down. Once activated, the
electrodes are kept at 75°C for 4 minutes. The surface thermistors
control the tissue effect to obtain a predetermined depth of
coagulation. 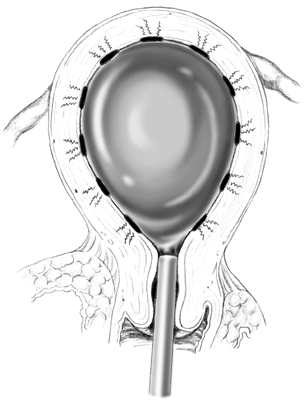 Fig. 7. The uterus is distended by means of a balloon onto which electrodes are
placed. Activation of electric current produces monopolar current flowing
into and destroying the endometrium.(Adapted from Baggish MS, Valle RF, Barbot J: Endometrial ablation. In
Baggish MS, Barbot J, Valle RF [eds]: Diagnostic and Operative Hysteroscopy. St
Louis, Mosby, 1999.) Fig. 7. The uterus is distended by means of a balloon onto which electrodes are
placed. Activation of electric current produces monopolar current flowing
into and destroying the endometrium.(Adapted from Baggish MS, Valle RF, Barbot J: Endometrial ablation. In
Baggish MS, Barbot J, Valle RF [eds]: Diagnostic and Operative Hysteroscopy. St
Louis, Mosby, 1999.)
|
Currently, further development of the Vesta system in the United States
has been put on hold by the manufacturer while they consider design changes
requested by the FDA. Another device using radiofrequency current is Novasure (Novocept, Palo
Alto, CA). This device, which has not undergone US trials, uses an intrauterine
fan made of two layers of copper mesh. After placement in the
uterine cavity, the fan is deployed. This action creates a suction
to bring the endometrial walls and the mesh into close contact. Bipolar
radiofrequency current is then used to ablate the endometrium in less
than 2 minutes. The radiofrequency generator monitors the tissue impedance
and automatically terminates the procedure at the desired depth
of coagulation. BALLOONLESS HOT WATER SYSTEMS. Several companies have developed endometrial ablation systems that use
unconfined intracavitary hot water as the source of heat for endometrial
ablation. In the United States, only one manufacturer (BEI Corporation) has
began patient trials. This HydoTermAblator, or HTA, system uses
externally heated saline rather than an internal source of energy (Fig. 8). Unlike the previously discussed systems, the uterine cavity is irrigated
with the heated solution rather than being contained within a balloon. This
irrigation is performed under constant hysteroscopic monitoring. Preliminary
studies indicate that uniform endometrial destruction
to a depth of 3 to 4 mm in the uterine cavity and 2 to 3 mm in the cornual
region is easily obtained.14 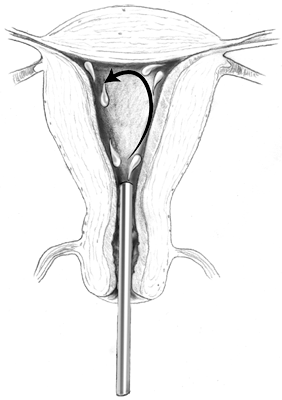 Fig. 8. Mechanisms of endometrial ablation using unconfined circulating hot water. Fig. 8. Mechanisms of endometrial ablation using unconfined circulating hot water.
|
Potential advantages include complete coverage of the endometrial cavity
despite surface irregularities or uterine anomalies such as uterine
septa. Other proposed advantages include low likelihood of unrecognized
perforation because the procedure is performed under direct visualization
and the low risk of fluid and electrolyte imbalance that can be
associated with laser or electrosurgical methods. Unpublished data from
the ongoing US trial reportedly show that the device is statistically
comparable to the rollerball ablation control arm. CRYOSURGICAL ENDOMETRIAL ABLATION. A different method from the thermal methods of endometrial ablation is
taken by the manufacturers of a cryogenic probe for endometrial ablation. The
CryoGen First Option system (Cryogen, Inc, San Diego, CA) recently
began undergoing clinical evaluation in FDA trials. With this system, the
probe is placed and monitored under ultrasonic guidance. The
cryoablation is capable of producing profound tissue destruction, averaging 9 to 12 mm
in depth. The as-yet unproven assumption is that this
deeper ablation should be associated with higher amenorrhea rates. Early
unpublished data from the first 6 months of the FDA clinical trial
have indicated the outcomes to be comparable to the rollerball control
arms. The cryoablation procedure is a 10- to 12-minute procedure. Because
the freezing has a partial anesthetic effect, it may prove to be
an effective office procedure performed under paracervical block. Other Devices Two other devices being investigated in Europe include a diode laser and
a microwave device marketed under the name MEA (Microwave Endometrial
Ablation). Preliminary data available indicate that these devices have
similar outcomes to the other products previously described. |