Sling Procedures Sling operations are some of the oldest anti-incontinence procedures
performed. They evolved from an attempt to both support the urethra
and recreate or augment urethral sphincter tone lost to injury or atrophy. These
procedures are designed to narrow the urethra, provide urethral
support, increase urethral closure pressure (UCP) by
external compression, and restore posterior urethrovesical angle.7 The concept that sling procedures increase UCP has recently been called
into question because some follow-up studies have failed to show
a significant increase.8,9 Although there is no typical patient when it comes to treating urinary
incontinence, many urogynecologists reserve sling procedures for patients
who have had previous unsuccessful anti-incontinence procedures. These
patients are often severely incontinent and display little
or no urethral mobility during increases in intra-abdominal pressure. Their
UCP is generally low (< 20 cm H2O), and they have low Valsalva leak-point pressures (< 60 cm
H2O). These are the generally accepted diagnostic criteria for intrinsic
sphincter deficiency. The modern sling procedure evolved from an operation described by Giordano
in 1907, in which gracilis muscle flaps were transplanted near the
urethra. In 1917, surgeons Goebell, Frankenheim, and Stoeckel developed
a sling procedure using pyramidalis muscle with attached rectus fascia. After
the muscle bellies were dissected free to the level of the
symphysis, the ends were passed behind the pubic bone and sutured below
the urethra. The vesical neck was also plicated. The 1930s saw the decrease
of musculofascial slings and the advent of slings composed only
of fascia. In 1942, Aldridge described an operation that closely resembles
a variant of the sling procedure still performed today. He dissected
bilateral strips of rectus fascia from the anterior aspect of the
muscle, leaving the medial portions attached to the muscle. The strips
of fascia were then tunneled through the muscle, passed behind the
symphysis, and sutured below the urethra. Ensuing years have brought the
use of synthetic materials for slings and the use of suture bridges
and patch slings.10 Current slings are usually composed of fascia, either cadaveric donor
tissue or tissue harvested from the patient herself at the time of operation. Cure rates with sling procedures are reported to be 70% to 95%.11,12 The results are similar regardless of type of sling material used. The
variability arises from differences in technique, definition of cure, and
length of follow-up. Although reports of cure rates are abundant, documentation
of early and late complications is poor. In addition
to the risks of hemorrhage, infection, and injury to local organs, one
must consider the effect of the procedure on voiding. There is a 2% to 30% risk of severe voiding dysfunction or retention.13 This estimation is based primarily on observation and is in need of further
study. Detrusor instability and various irritative bladder symptoms
such as frequency and urgency occur in anywhere from 2% to 50% of
patients.14 Unfortunately, it is difficult to predict which patients will have these
complications. These symptoms often lessen with time and usually can
be treated pharmacologically. Less common complications include erosion
of the sling material (more common with synthetic slings), fistula
or sinus tract formation, nerve injury or entrapment, and abscess
formation. As previously mentioned, because of the perceived higher
rate of potential complications, many pelvic surgeons continue to
perform a retropubic urethropexy as their primary anti-incontinence
surgery. Retropubic Operations Both operations described in this section have as their common goal the
identification of strong periurethral tissues near the vesicle neck and
the suturing of these tissues to a supportive structure attached to
the pubis. This serves to return the bladder neck to an intra-abdominal
location so that it sees the same transmural pressures as the
bladder. Urethral closure pressure has been shown both to increase and
decrease after these procedures and is thus not thought to play a role
in their mechanism of achieving continence.15,16 MARSHALL-MARCHETTI-KRANTZ In 1949, Marshall reported the empirical observation that suturing the
periurethral tissues to the pubic bone alleviated urinary stress incontinence
after examining a patient with iatrogenic incontinence after vesical
neck resection.17 The original description called for number-1 chromic suture, but
both the MMK and Burch procedures are now typically performed with permanent
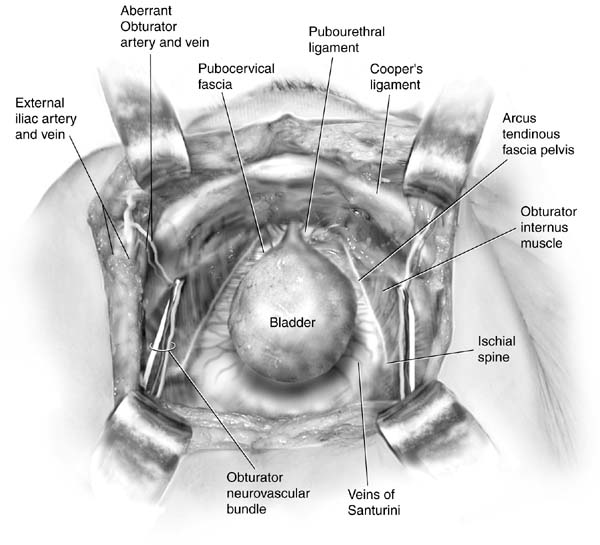
sutures. Access to the retropubic space is obtained as described. The
bladder neck is identified by placing the nondominant hand in
the vagina and palpating the Foley bulb with the index and middle fingers. While
elevating the vaginal fingers, a Kittner dissector is used
to place countertraction on the fatty tissue overlying the periurethral
fascia (Fig. 2A inset). A gentle sweeping motion easily cleans the fat away, revealing
the white fascia below. This dissection permits the surgeon to take
good bites of tissue and fosters adherence of the periurethral tissue
to the back of the symphysis. Elevation of the vaginal finger permits
the operator to place a figure-eight, full-thickness (excluding
the vaginal epithelium if possible) bite of tissue (see
inset of Fig. 2B). A single suture is placed on both sides of the urethrovesical junction
in this fashion. Each suture is then fixed to the periosteum or
fibrocartilage of the pubic bone in such a way that the vesical neck
is barely brought in contact with the pubic symphysis (Fig. 3). Injury to the bladder and ureters is ruled out with cystoscopy, suprapubic
telescopy, or intentional cystotomy. Because postoperative
voiding efficiency is unpredictable, a suprapubic catheter is the preferred
method of bladder drainage. |
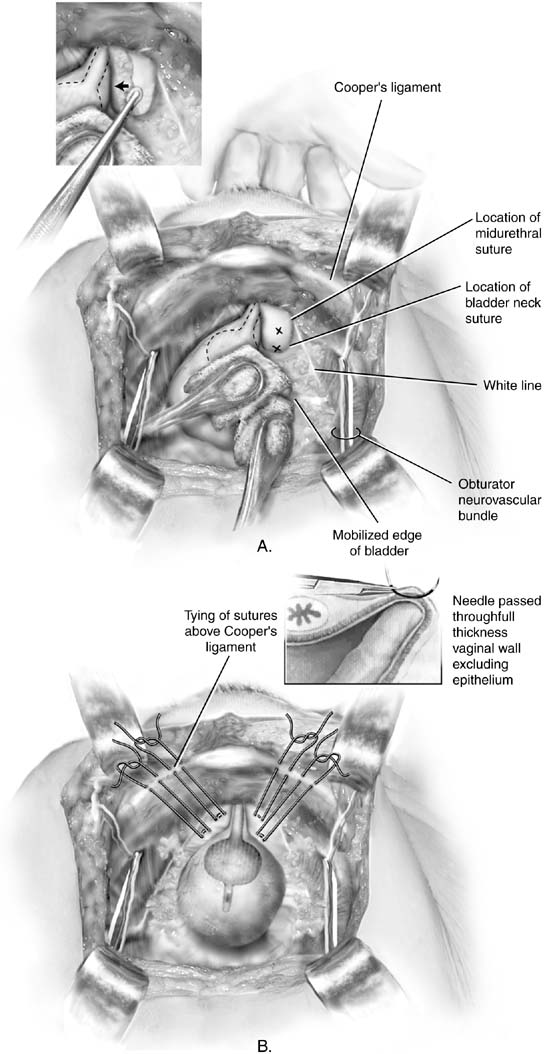 Fig. 2. A. Burch colposuspension. The bladder is gently mobilized to the opposite
side using sponge sticks. The anterior vaginal wall is elevated by the
middle finger of the surgeon's nondominant hand. The position of
the sutures should be at least 2 cm lateral to the proximal urethra
and bladder neck. Xs mark the ideal placement of the Burch colposuspension
sutures. Inset: The anterior vaginal wall on the right side is being
elevated by a vaginal finger. A Kittner dissector is passed on top
of the finger, mobilizing the fat medially. B. Burch colposuspension. Sutures have been appropriately placed on each
side of the proximal urethra and bladder neck. Figure-eight bites
are taken through the vagina. Double-armed sutures are used
so that the end of each suture can be brought up through the ipsilateral
Cooper's ligament, thus allowing the sutures to be tied above
the ligament. Inset: Detail of the suture being placed over the surgeon's
vaginal finger. The suture should include full-thickness
vaginal wall, excluding the epithelium. (Baggish MS, Karram MM, [eds]: Atlas
of Pelvic Anatomy and Gynecologic Surgery. New
York, Harcourt, 2001.)
Fig. 2. A. Burch colposuspension. The bladder is gently mobilized to the opposite
side using sponge sticks. The anterior vaginal wall is elevated by the
middle finger of the surgeon's nondominant hand. The position of
the sutures should be at least 2 cm lateral to the proximal urethra
and bladder neck. Xs mark the ideal placement of the Burch colposuspension
sutures. Inset: The anterior vaginal wall on the right side is being
elevated by a vaginal finger. A Kittner dissector is passed on top
of the finger, mobilizing the fat medially. B. Burch colposuspension. Sutures have been appropriately placed on each
side of the proximal urethra and bladder neck. Figure-eight bites
are taken through the vagina. Double-armed sutures are used
so that the end of each suture can be brought up through the ipsilateral
Cooper's ligament, thus allowing the sutures to be tied above
the ligament. Inset: Detail of the suture being placed over the surgeon's
vaginal finger. The suture should include full-thickness
vaginal wall, excluding the epithelium. (Baggish MS, Karram MM, [eds]: Atlas
of Pelvic Anatomy and Gynecologic Surgery. New
York, Harcourt, 2001.)
|
|
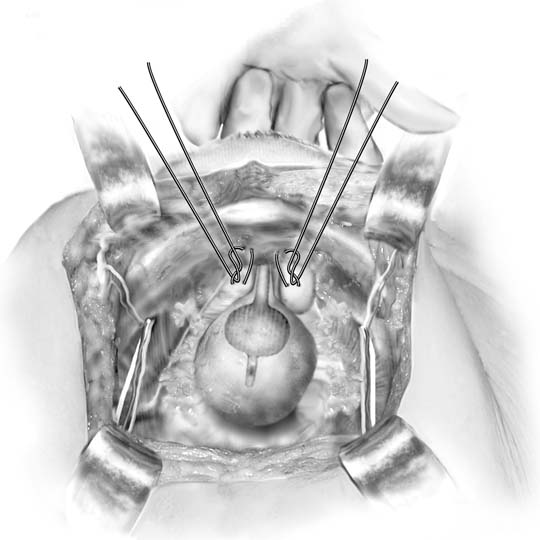 Fig. 3. Marshall-Marchetti-Krantz procedure. One suture is placed
bilaterally at the level of the bladder neck and then into the periosteum
of the pubic symphysis. (Baggish MS, Karram MM, [eds]: Atlas of Pelvic Anatomy and Gynecologic Surgery. New York, Harcourt, 2001.)
Fig. 3. Marshall-Marchetti-Krantz procedure. One suture is placed
bilaterally at the level of the bladder neck and then into the periosteum
of the pubic symphysis. (Baggish MS, Karram MM, [eds]: Atlas of Pelvic Anatomy and Gynecologic Surgery. New York, Harcourt, 2001.)
|
BURCH COLPOSUSPENSION The Burch procedure was described in 1962 after the originator of the procedure
was unable to find adequate periosteum in an elderly patient
in whom he was trying to perform an MMK procedure.18 The retropubic space is entered and prepared as described for the MMK
procedure. Two permanent figure-eight sutures are placed on either
side of the bladder neck. The proximal sutures are placed 2 cm lateral
to the bladder neck, and the distal sutures are placed 2 cm lateral
to the proximal third of the urethra (see Fig. 2A). The ends of each suture are then passed through Cooper's ligament
either by the use of a curved Mayo needle or by using double-armed
suture. Once all sutures are placed, the surgeon elevates
the vagina while an assistant ties the sutures down with the knots on
top of Cooper's ligament (see Fig. 2B). The distal sutures are tied first. When complete, the surgeon should
be able to easily pass two fingers between the pubic bone and the
urethra. Suture bridges are not problematic and are commonly present. An
intravesical assessment is recommended to ensure that no bladder
or ureteral injury has occurred. Laparoscopic approaches to the Burch procedure have also been described. Retrospective
and observational studies suggest cure rates are similar
to open procedures.19 Three prospective trials comparing these two techniques have been published. Burton
in 1994 and Su in 1997 found the open approach to be superior (97% vs. 73% and 96% vs. 80%, respectively).20,21 Fatthy and associates reported similar cure rates for the open procedure
compared to a modified laparoscopic approach followed-up to 18 months (85% vs. 88%) and found less morbidity
and a shorter hospital stay in the laparoscopic group.22 Unfortunately, comparisons are difficult to make between the open and
laparoscopic approaches secondary to a multitude of technical variations (aside
from the actual approach) from the traditional procedure. Cure rates for the retropubic procedures are similar, 65% to 90%, at 1 to 10 years.23,24 Indeed, the single randomized prospective trial that compared the Burch
with the MMK procedures found no significant difference in cure rate.23 These procedures have stood the test of time, and there is long-term
success rate data. This is particularly true for the Burch procedure, which
is the more studied of the two operations. It seems that over
time, the cure rate of the retropubic suspensions decreases steadily
from 90% at 1 year to about 70% by 10 years postoperatively, before
reaching a plateau at 65% to 70% in patients
who have been followed-up more than 20 years.24 Complications of retropubic procedures are similar to sling procedures
with some differences in incidence. Because more dissection is needed
for retropubic procedures compared with sling procedures, one would anticipate
a higher incidence of infectious and hemorrhagic complications, but
less worry about erosions and sinus tract formation. The risk
of de novo detrusor instability is reported from 5% to 27%, but Alcalay
and associates have reported on patients with 10-year follow-up
with an incidence of 14%. They have also reported
voiding dysfunction in 22%.25 One complication unique to retropubic suspensions is the occurrence of
osteitis pubis, which occurs in up to 2.5% of patients undergoing
the MMK procedure. Long-term studies of the Burch procedure
have shown a significant incidence of prolapse formation. Rectocele has
been noted in 11% to 25% and enterocele in 4% to 10% of
patients followed-up 10 to 20 years.24 Paravaginal Repair A discussion of the paravaginal repair is included here because it is a
retropubic procedure. It should not be considered a primary anti-incontinence
operation. The goal of this operation is to repair a specific
anatomic defect: separation of one or both sides of the endopelvic
fascial hammock that normally inserts at the arcus tendineus fasciae
pelvis (white line) at the pelvic sidewall. In the past, it
has been used as a means to treat stress incontinence.26 Although it will make some women continent, presumably by elevation of
the bladder neck, it does not produce a lasting result. As Colombo and
colleagues have shown, the Burch procedure is clearly superior for the
treatment of incontinence.27 In patients who have paravaginal defects with resultant cystocele associated
with stress incontinence, a procedure called paravaginal plus has
been described. In this procedure, the paravaginal defects are repaired
as described in the next paragraph, and Burch colposuspension sutures
are placed as described previously (Fig. 4). To perform the abdominal paravaginal repair, one gains access to
the retropubic space as described previously. The ischial spine and
attached arcus tendineus fascia should be identified. Paravaginal defects
typically are readily apparent as a detached portion of the vagina
from the white line (Figs. 5A and 5C). Using the nondominant hand, the surgeon elevates the anterolateral
vaginal sulcus on the side of the defect. A full-thickness (excluding
epithelium) figure-eight bite of vaginal tissue
is taken with permanent suture near the vaginal apex and then fixed
to the white line or fascia of the obturator internus muscle 1 to 2 cm
from the ischial spine. This is tied down. Then, proceeding distally, three
or four similar sutures are placed such that the final suture
is as close as possible to the pubic ramus (see Fig. 5B). |
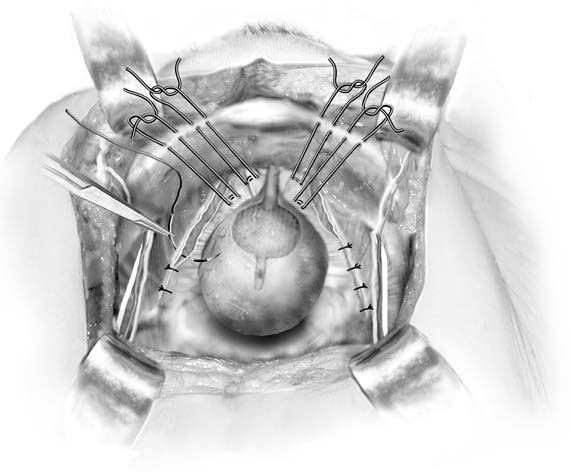 Fig. 4. Paravaginal plus. In patients with paravaginal defects and urinary stress
incontinence, the paravaginal defects are repaired and then Burch colposuspension
sutures are placed. (Baggish MS, Karram MM, [eds]: Atlas of Pelvic Anatomy and Gynecologic Surgery. New York, Harcourt, 2001.)
Fig. 4. Paravaginal plus. In patients with paravaginal defects and urinary stress
incontinence, the paravaginal defects are repaired and then Burch colposuspension
sutures are placed. (Baggish MS, Karram MM, [eds]: Atlas of Pelvic Anatomy and Gynecologic Surgery. New York, Harcourt, 2001.)
|
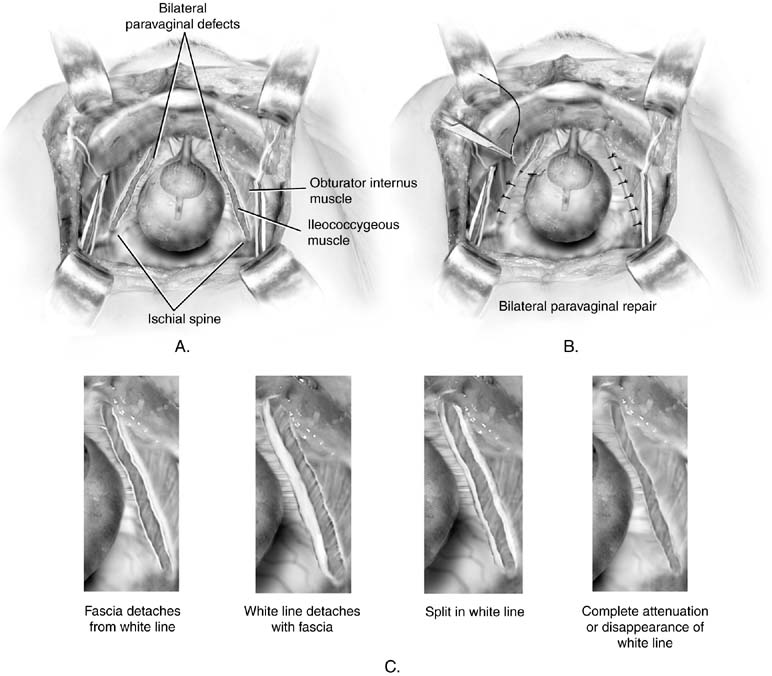 Fig. 5. A. Paravaginal defect. Bilateral defects are illustrated. B. Retropubic paravaginal defect repair. The defects are repaired by placing
the first suture just distal to the ischial spine and working toward
the symphysis. C. Paravaginal defect. Four potential anatomic findings in patients with
paravaginal defects are illustrated. All result in a falling away of the
vagina with its underlying fascia from the lateral pelvic side wall. (Baggish MS, Karram MM, [eds]: Atlas of Pelvic Anatomy and Gynecologic Surgery. New York, Harcourt, 2001.) Fig. 5. A. Paravaginal defect. Bilateral defects are illustrated. B. Retropubic paravaginal defect repair. The defects are repaired by placing
the first suture just distal to the ischial spine and working toward
the symphysis. C. Paravaginal defect. Four potential anatomic findings in patients with
paravaginal defects are illustrated. All result in a falling away of the
vagina with its underlying fascia from the lateral pelvic side wall. (Baggish MS, Karram MM, [eds]: Atlas of Pelvic Anatomy and Gynecologic Surgery. New York, Harcourt, 2001.)
|
Artificial Sphincter The use of an artificial sphincter to treat urinary stress incontinence
may be appropriate in some cases of severe urine loss. It is an implantable
device that occludes the urethra but can be voluntarily opened, permitting
the patient to empty her bladder. Because of the technical
difficulty encountered in placing such a device and the fairly limited
pool of appropriate patients, this means of treatment has not gained
wide acceptance. Artificial urinary sphincters were first used in 1972. Several modifications
have resulted in advanced devices consisting of a cuff, a pressure-regulating
balloon, and a control pump (Fig. 6). The cuff is placed around the bladder neck, and the balloon is
fixed into the retropubic space. The pump is placed subcutaneously into
one of the labia majora (Fig. 7). The cuff is normally in the activated state, in which it is inflated, thus
squeezing the bladder neck closed. The balloon sees changes
in intra-abdominal pressure and incrementally regulates the pressure
applied to the cuff. When the patient needs to void, she squeezes
the pump located in her labium, which deactivates the cuff. The cuff
automatically begins to reinflate, but takes 3 minutes to do so, permitting
the patient to empty. 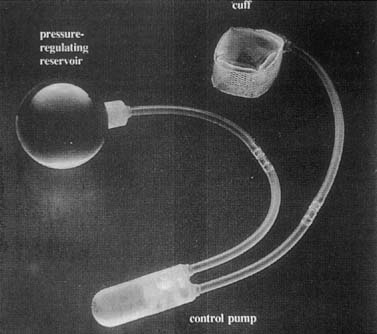 Fig. 6. AMS 800 artificial urinary sphincter. There is a small button on control
pump for activation and deactivation of device . (Walters MD, Karram MM, [eds]: Urogynecology and Reconstructive Pelvic Surgery, 2nd ed. St Louis, Mosby, 1999.) Fig. 6. AMS 800 artificial urinary sphincter. There is a small button on control
pump for activation and deactivation of device . (Walters MD, Karram MM, [eds]: Urogynecology and Reconstructive Pelvic Surgery, 2nd ed. St Louis, Mosby, 1999.)
|
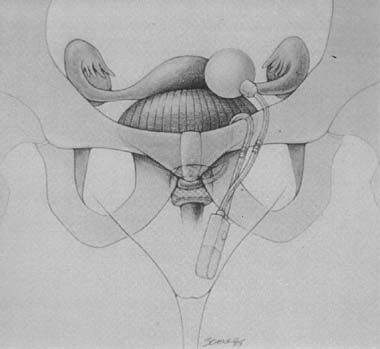 Fig. 7. Implanted artificial urinary sphincter. (Walters MD, Karram MM, [eds]: Urogynecology and Reconstructive Pelvic Surgery, 2nd ed. St Louis, Mosby, 1999.) Fig. 7. Implanted artificial urinary sphincter. (Walters MD, Karram MM, [eds]: Urogynecology and Reconstructive Pelvic Surgery, 2nd ed. St Louis, Mosby, 1999.)
|
The complex nature of the device makes unmotivated and nondexterous patients
poor candidates for this intervention. Other contraindications include
bladder overactivity that cannot be controlled with medication
or biofeedback and high-grade vesicoureteral reflux. There is also
the risk of infection, erosion, and device malfunction. Short-term
success rates with the artificial sphincter are reported to be 68% to 100%, but mechanical complication rates are as high
as 21%.14,28 Furthermore, women appear to be more susceptible to erosions with this
procedure than men, with up to 56% of women experiencing this
complication compared to 23% in men.29 A recent series of 68 women who were followed-up for a median of 12 years
reported an overall continence rate of 81%, but only 25 (37%) had the original device still in place, 17% had
the device replaced for mechanical failure, and 46% had
the device removed for infection of erosion.30 |