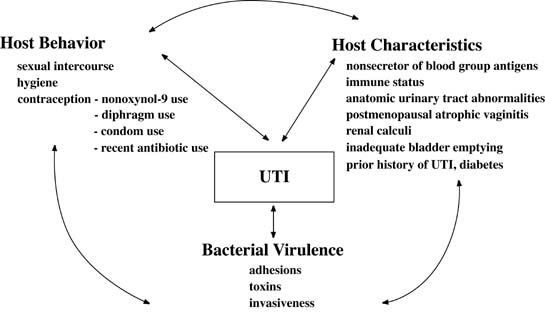
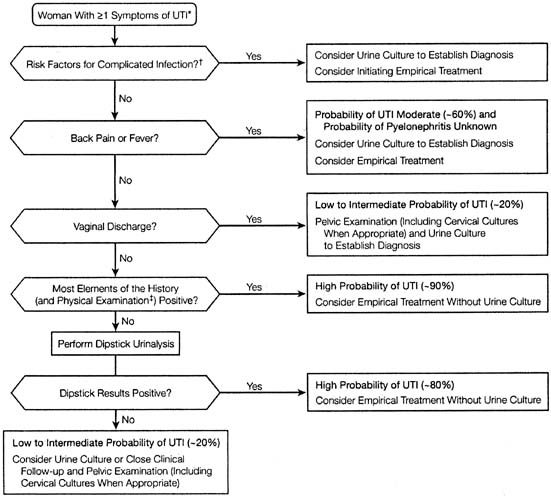
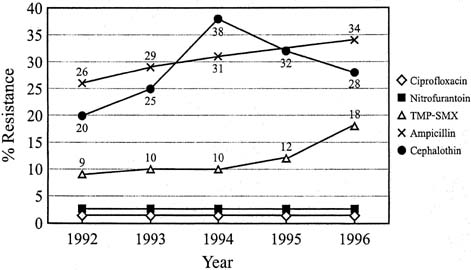
UTIs are the most common medical complication of pregnancy. The prevalence
of asymptomatic bacteriuria in pregnancy is 4% to 7%.70,71,72 Acute cystitis occurs in 1.3%73 and acute pyelonephritis in 1% of pregnant women.74 The risk of asymptomatic bacteriuria increases with increasing parity, lower
socioeconomic status, increased age, sexual activity, sickle cell
trait or disease, diabetes, and previous UTI. The etiologic agents
of UTIs in pregnancy are the same as those of acute uncomplicated UTI
in nonpregnant women. E. coli causes 80% to 90% of UTIs in pregnancy, and P. mirabilis, K. pneumoniae, S. saprophyticus, and enterococci are the usual isolates from the remainder of patients with uncomplicated
infections. In pregnancy, group B b-hemolytic streptococci are
also potential urinary tract pathogens.75 During pregnancy, the female urinary tract undergoes profound physiologic
changes that facilitate the development of acute pyelonephritis. As
early as 7 weeks' gestation, a physiologic hydroureter develops
from the hormonal effects of progesterone, which causes ureteral atony, increased
ureteral volume, and urinary stasis. Mechanical obstruction
from the enlarging uterus also delays ureteral emptying function. Physiologic
bladder changes, including decreased smooth muscle tone, increased
capacity, and incomplete emptying, predispose pregnant women to
vesicoureteral reflux and ascending pyelonephritis. The urinary tract
returns to normal size and function by 6 weeks postpartum. An astoundingly high rate of 30% to 40% of untreated pregnant
women with bacteriuria have pyelonephritis76 in contrast to less than 1% of those without bacteriuria in early
pregnancy.77 Asymptomatic bacteriuria is the most significant factor in the development
of pyelonephritis during pregnancy.76 Treatment of asymptomatic bacteriuria reduces the risk of pyelonephritis
to that of a nonbacteriuric population. Early pregnancy screening and
treatment of asymptomatic bacteriuria prevents 70% to 80% of
cases of pyelonephritis. Pyelonephritis is a potentially serious threat for both the mother and
the fetus. Pyelonephritis during pregnancy can lead to multiorgan dysfunction
with hemodynamic (shock), hematologic (anemia, thrombocytopenia), renal (decreased creatinine clearance), and
pulmonary (adult respiratory distress syndrome) effects. Approximately 1% of pregnant women with acute pyelonephritis
have respiratory insufficiency from alveolar capillary leak. Most cases
result in tachycardia, increased respiratory rate, and dyspnea.78 Prompt chest x-ray and arterial blood gas analyses are indicated. Although
most patients respond to increased oxygen, occasionally full-blown
adult respiratory distress requiring intubation and intense
ventilatory support will ensue.79 Acute pyelonephritis in the late second and third trimesters is associated
with a 20% to 50% risk of preterm delivery. Thus, acute
pyelonephritis in the mother can lead to severe neonatal consequences
from early preterm delivery. Potential mechanisms involved in preterm
labor include maternal bacteremia and endotoxemia, which result in
systemic levels of inflammatory mediators, including cytokines, which
in turn lead to myometrial activity. Whether asymptomatic bacteriuria alone leads to premature labor or other
adverse perinatal outcomes in women without symptomatic pyelonephritis
have remained a controversial subject. Kass initially reported a two-fold
to three-fold increase in the neonatal death rate
and prematurity rate of pregnant patients with asymptomatic bacteriuria;76 these findings were supported by others.80 However, not all studies have found an association between bacteriuria
and poor perinatal outcome.81,82 Using meta-analysis, evidence combined from multiple studies suggests
that asymptomatic bacteriuria is associated with both preterm delivery
and low birth weight.83 Table 5 summarizes studies that associate bacteriuria with low birth weight, and Table 6 summarizes studies that associate bacteriuria with preterm delivery. In
the meta-analysis, nonbacteriuric pregnant patients had a 35% reduction
in the risk of low birth weight (relative risk [RR] = .65) and a 50% reduction in the risk
of preterm delivery (RR = .50) compared with patients
with untreated asymptomatic bacteriuria.83 Table 5. Meta-Analysis of Cohort Studies of Low-Birth-Weight (LBW) Patients With or Without Bacteriuria (n = 17)
| Author |
Untreated Bacteriuria LBW/Total |
Nonbacteriurics LBW/Total |
RR |
95% CI |
| Kass |
26/95 |
88/1000 |
0.3 |
0.2–0.5 |
| Layton |
10/59 |
10/112 |
0.5 |
0.2–1.3 |
| Kincaid-Smith and Bullen55 |
12/56 |
25/500 |
0.2 |
0.1–10.5 |
| Stuart et al. |
20/88 |
83/729 |
0.5 |
0.3–0.8 |
| Little |
13/141 |
360/4735 |
0.8 |
0.5–1.4 |
| Wilson et al. |
14/137 |
592/6079 |
0.95 |
0.6–1.6 |
| Dixon & Brant |
4/71 |
68/1309 |
0.9 |
0.3–2.5 |
| Savage et al. |
21/98 |
67/496 |
0.6 |
0.4–1.0 |
| Patrick64 |
7/75 |
37/500 |
0.8 |
0.4–1.8 |
| Robertson et al. |
16/204 |
120/1980 |
0.8 |
0.5–1.3 |
| Wren |
14/90 |
140/3009 |
0.3 |
0.2–0.5 |
| Elder et al. |
15/122 |
20/107 |
1.5 |
0.8–3.0 |
| Brumfitt |
21/178 |
32/477 |
0.6 |
0.3–0.98 |
| Bryant et al. |
2/32 |
4/44 |
1.5 |
0.3–7.9 |
| Sleigh et al. |
7/100 |
7/100 |
1.0 |
0.35–2.8 |
| Norden and Kilpatrick |
17/114 |
14/109 |
0.9 |
0.4–1.7 |
| Whalley |
26/176 |
21/176 |
0.8 |
0.5–1.4 |
Typical RR = 0.65; 95% CI, 0.57–0.75.
RR, relative risk; CI, confidence interval.
(Adapted from Romero R, Oyarzun E, Mazor M, et al. Meta-analysis
of the relationship between asymptomatic bacteriuria and preterm
delivery/low birth weight. Obstet Gynecol 1989;73:576.)
Table 6. Meta-Analysis of Cohort Studies of Preterm Delivery
(PTD) Patients With or Without Bacteriuria (n = 17)
| Author |
Untreated Bacteriuria PTD/Total |
Nonbacteriurics PTD/Total |
RR |
95% CI |
| Layton |
4/63 |
9/113 |
1.3 |
0.4–4.1 |
| Patrick |
7/75 |
21/500 |
0.5 |
0.2–1.1 |
| Robertson et al. |
13/204 |
62/1980 |
0.5 |
0.3–0.9 |
| Wren |
15/90 |
204/3009 |
0.5 |
0.2–0.7 |
Typical RR = 0.5; 95% CI, 0.365–0.698.
RR, relative risk; CI, confidence interval.
(Adapted from Romero R, Oyarzun E, Mazor M, et al. Meta-analysis of the
relationship between asymptomatic bacteriuria and preterm delivery/low birth
weight. Obstet Gynecol 1989;73:576.)
In Table 7, meta-analysis of low birth weight in bacteriuric patients administered
placebo versus antibiotic therapy in randomized clinical trials
showed a highly significant reduction of low-birth-weight
infants with antibiotic treatment of bacteriuria (RR = .56).83 Women with chronic renal disease have been shown to have low-birth-weight
infants.84 However, few bacteriuric pregnant patients, even those with pyelonephritis, have
chronic renal disease. The link between bacteriuria and prematurity
remains largely unexplained. A greater frequency of amniotic
fluid infection has been noted in women with bacteriuria compared with
women without infection.85,86 It is possible that cervicovaginal uropathic microorganisms can colonize
and infect both the urinary and amniotic fluid compartments in the
same patient, causing both UTI and premature labor and delivery. In support
of this theory, one report of antibiotic treatment of women with
group B streptococcus bacteriuria reduced the incidence of preterm delivery.87
Table 7. Meta-Analysis of Randomized Clinical Trials of Low-Birth-Weight (LBW) Patients With Bacteriuria
| Author |
Placebo LBW/Total |
Antibiotic LBW/Total |
RR |
95% CI |
| Kass |
26/95 |
6/84 |
0.3 |
0.1–0.6 |
| LeBlanc and McGanity |
6/27 |
7/101 |
0.3 |
0.1–0.9 |
| Kincaid-Smith and Bullen55 |
12/56 |
9/61 |
0.7 |
0.3–1.6 |
| Little |
13/141 |
10/124 |
0.9 |
0.4–2.0 |
| Savage et al. |
21/98 |
7/93 |
0.4 |
0.2–0.8 |
| Wren |
14/190 |
4/83 |
0.3 |
0.1–0.9 |
| Elder et al. |
16/122 |
17/107 |
1.2 |
0.6–2.4 |
| Brumfitt |
21/178 |
18/235 |
0.7 |
0.4–1.2 |
Typical RR = 0.56; 95% CI, 0.43–0.73.
RR, relative risk; CI, confidence interval.
(Adapted from Romero R, Oyarzun E, Mazor M, et al. Meta-analysis
of the relationship between asymptomatic bacteriuria and preterm
delivery/low birth weight. Obstet Gynecol 1989;73:576.)
Women should be screened for bacteriuria at their first prenatal visit. A
midstream clean-catch voided urine specimen for culture remains
the standard for initial urine testing. Measures to detect leukocyte
activity, either by urine dipstick test or by urinalysis, are not sufficiently
sensitive to identify asymptomatic bacteriuria.88 Urine Gram stain is effective in detecting asymptomatic UTIs as a rapid
same-day test.88 The treatment goals of UTI in pregnancy are to eradicate the infection
with the shortest possible course of antibiotics and to maintain sterile
urine for the remainder of pregnancy. Sulfonamides, nitrofurantoin, ampicillin, cephalexin, and nalidixic acid are all considered safe for use in pregnancy. Sulfonamides should not
be used at term or during labor because of the theoretical risk of kernicterus
in the infant. The sulfonamide can displace bilirubin from plasma albumin binding sites and lead to hyperbilirubinemia, but this risk is low with
the presently available short-acting sulfonamides. Trimethoprim, a
dihydrofolate reductase inhibitor, has been shown to have toxic
effects in experimental animals, although no teratogenicity has been reported
in pregnant humans. Tetracycline is contraindicated in pregnancy
because of potential maternal liver damage and permanent discoloration
of the deciduous teeth of the child. Fluoroquinolones are to be avoided
because cartilage abnormalities have been reported in animal studies. Fosfomycin
has not been shown to have teratogenic effects in animal
studies, but no well-controlled studies have been performed
in pregnant humans, and fosfomycin is not recommended for use during
pregnancy. A 7-day course of antibiotic therapy is recommended to treat asymptomatic
bacteriuria or uncomplicated acute UTI in pregnancy. Single-dose
therapy for asymptomatic bacteriuria in pregnancy is less
effective than in a comparable nonpregnant group, with failure rates of 30%.89 Three-day courses of antibiotic therapy are effective if only one
episode of infection has occurred and there are no complicating factors
or evidence of renal involvement. It is unclear whether shorter courses
of therapy eradicate the vaginal reservoir of uropathogenic bacteria
in pregnancy. While 33% of bacterial strains causing acute
uncomplicated UTI and pyelonephritis show resistance to amoxicillin, it
is still useful in pregnancy when the infecting organism is know to
be susceptible, and for Gram-positive organisms like group B Streptococci. Patients should have a follow-up urine culture 2 weeks after completing
therapy and monthly urine cultures thereafter. From 16% to 23% of
women will have recurrent asymptomatic bacteriuria during
the same pregnancy.81 For persistent asymptomatic bacteriuria, nitrofurantoin 100 mg nightly
is recommended for the remainder of pregnancy and 12 weeks postpartum
for suppressive therapy. Ampicillin 250 mg twice per day would be a reasonable
alternative. Antibiotic suppression is also recommended after
two acute episodes of UTI in the same pregnancy. Pregnant women with acute pyelonephritis should be hospitalized. Sepsis
is reported to occur in 10% of pregnant women with pyelonephritis. Intravenous
fluids, urine output monitoring, and parenteral antibiotics
are generally recommended. Urine and blood cultures should be obtained
in all pregnant women suspected of having pyelonephritis before
initiating antibiotic therapy. Twenty to 30% of organisms that
cause pyelonephritis are resistant to amoxicillin and first-generation
cephalosporins in vitro; therefore, these antibiotics are no longer used alone for empirical treatment. Parenteral ceftriaxone, gentamicin (with or without ampicillin), aztreonam, or
trimethoprim-sulfamethoxazole are recommended
for initial therapy until culture and sensitivity results are available. Response
to parenteral antibiotics is usually rapid, with 85% of
pregnant women becoming afebrile within 48 hours and 97% becoming
afebrile within 4 days of initiating treatment. Intravenous antibiotics
should be continued until the patient is afebrile and can tolerate
oral medications. A 10- to 14-day course of antibiotics
should be completed. If the patient does not have improvement
after 4 days of therapy, resistant organisms, obstructive uropathy, and
perinephric abscess should be considered. The incidence of recurrent acute pyelonephritis during the same gestation
is 10% to 18%.90,91 Without suppressive antimicrobial therapy, 60% of acute pyelonephritis
patients had a recurrent episode in one study.90 Patients maintained on suppressive antibiotics had a 2.7% recurrence
rate for the duration of the pregnancy.90 Antimicrobial agents used for suppression include nitrofurantoin 100 mg, sulfisoxazole 500 mg, or ampicillin 250 mg at bedtime daily. An acceptable alternative to suppressive therapy
is evaluation every 2 weeks in the clinic, with urine cultures and
prompt treatment of asymptomatic bacteriuria. Recurrent pyelonephritis
occurred in 7% of the group on suppressive antibiotics and 8% of
the group monitored with frequent urine cultures.92 Long-term follow-up of women with bacteriuria in pregnancy
reveals that 25% to 35% have evidence of renal infection
or urinary tract abnormalities including vesicoureteral reflux, IVP
evidence of chronic pyelonephritis, or recurrent bacteriuria.93,94 |