Congenital CMV infection occurs by hematogenous spread to the placenta
followed by placental infection and subsequent fetal infection. Transmission
can occur anytime during pregnancy. The influence of gestational
age at the time of maternal acquisition of CMV infection is not as clear
as with other congenital infections. However, the worst fetal sequelae
have been associated with infection in the first trimester,26,48 and clinically significant handicaps are associated more often with primary
infection before 27 weeks' gestation.35 Infants born to mothers infected early in pregnancy also are more likely
to be small for gestational age and to have microcephaly and intracranial
calcifications, whereas infants born to mothers infected later
in pregnancy are more likely to have hepatitis, pneumonia, purpura, and
severe thrombocytopenia.60 As noted previously, primary maternal infection is more likely to result
in intrauterine transmission than recurrent infection. Prenatal Diagnosis Despite the common occurrence of primary maternal CMV infection and the
significant risk of sequelae for offspring of infected mothers, routine
maternal prenatal screening has not been advocated because a reliable
and easy method to diagnose fetal infection is not available. Unfortunately, patients
often must make decisions regarding pregnancy management
based on empiric risk figures only. Because the majority of primary and recurrent infections are asymptomatic, the
diagnosis of CMV infection in pregnancy often is missed. Suspicion
of maternal infection should be confirmed by appropriate serologic
tests because ascertainment of primary versus recurrent infection is
critical to predict neonatal prognosis. Seroconversion or rising titers
of IgG or IgM often are difficult to show, as most women do not know
their prepregnancy CMV serostatus. Paired samples run 1 to 4 weeks apart
may show rising titers, but the time necessary for this diagnostic
test prevents a rapid clinical decision. IgG avidity testing may assist
in the diagnosis of primary CMV, but these tests generally are only
available at research laboratories. Direct diagnosis of fetal infection is difficult and no consensus exists
regarding a gold standard for diagnosis. Clinical judgment is at the
core of accurate diagnosis. Integration of maternal symptoms, physical
findings and serology together with amniotic fluid culture and/or PCR
results, fetal IgM determination, and ultrasound findings form the foundation
of prenatal diagnosis. Unfortunately, definitive fetal diagnosis
often can be made only at autopsy or delivery. ISOLATION OF CYTOMEGALOVIRUS FROM AMNIOTIC FLUID. The first prenatal diagnosis of fetal CMV infection occurred in 1971 by
isolation of the virus from amniotic fluid.61 Because the renal tubular epithelium is a major site of viral replication, the
infected fetus presumably excretes CMV into amniotic fluid via
fetal urine.62 Studies have documented the utility of amniotic fluid cultures to detect
fetal infection, and many consider amniotic fluid culture to be the
optimal site to establish a prenatal diagnosis of congenital infection.63,64 Testing the amniotic fluid after 20 weeks' gestation is recommended to
reduce the number of false-negative culture results. Sensitivity of amniotic
fluid CMV culture ranges from 80% to 100%,64–66 and specificity is close to 100%.65 Recently, PCR of amniotic fluid has been added as a rapid means of detection, but
results must be interpreted with caution because of possible
false-positives. CHORIONIC VILLUS SAMPLING. Chorionic villus sampling (CVS) has been proposed as an option to diagnose
congenital CMV in the first trimester. Diagnosis of fetal CMV infection
in the first trimester would allow earlier, safer termination of
pregnancy if desired by the patient. Because the virus seems to infect
the placenta initially with subsequent amplification and passage to
the fetus,67 placental tissue theoretically offers a potential source to detect fetal
infection. However, limited data exist on the sensitivity and specificity
of culture or PCR of placental tissue or both. Hogge and colleagues68 performed CVS on four of six pregnancies complicated by primary maternal
CMV infection. All four had negative PCR results that were confirmed
at birth with negative neonatal urine cultures. More research is needed
before CVS is routinely used for first-trimester diagnosis of CMV
infection. CORDOCENTESIS FOR FETAL SEROLOGY. The fetal immune system is capable of a consistent response to certain
antigenic stimuli by 22 weeks' gestation, and evaluation of fetal sera
for organism-specific IgM has been used to diagnose certain congenital
infections. However, production of specific IgM appears to be related
to the infecting organism. For example, although the majority of fetuses
with congenital rubella infection produce specific IgM after 22 weeks,69 only 15% of fetuses with congenital toxoplasmosis produce specific IgM
between 24 and 29 weeks.70 Lynch and coworkers71 found that although total IgM levels were elevated in infected fetuses
sampled at 21 and 23 weeks' gestation, CMV-specific IgM was present in
only one of five fetuses tested. This is in contrast to a prevalence
of 50% to 95% among infected neonates72 and likely represents immaturity of the fetal immune system at this gestational
age. Despite the variability of fetal production of IgM, CMV-specific IgM determinations
by ELISA can be attempted on fetal blood obtained by cordocentesis. To
increase the sensitivity of this assay and to eliminate
false-positives due to rheumatoid factor, sera should be preabsorbed with
a modified staphylococcal protein A preparation.50 The few studies performed on the diagnostic utility of fetal CMVspecific
IgM have had variable results. Hogge and coworkers68 performed cordocentesis along with amniocentesis on three pregnant women
with primary maternal CMV infection. Compared with neonatal culture, all
three cordocentesis results were incorrect with two false-negatives
and one false-positive at 22.5 to 25 weeks. In contrast, Hohlfeld
and coworkers64 reported only two false-negatives and no false-positives in 16 patients
evaluated. Notably, in both series, the diagnosis was accurately made
by amniocentesis, and fetal IgM determinations added little further
information. The true sensitivity and specificity of fetal IgM determination
are not known, but sensitivity has been between 69% and 75% in
small series.65,73 Based on these findings, it is evident that fetal IgM alone cannot be
used to exclude or confirm congenital CMV infection. Although fetal IgM determination may not add useful diagnostic information, fetal
blood obtained at cordocentesis may yield prognostic information
if sent to evaluate hematologic and hepatic parameters. Extreme
thrombocytopenia, anemia, or elevated liver function test results documented
in fetal blood suggest more severe disease; however, how these
parameters relate to childhood handicaps remains unknown.64 ULTRASOUND. Often, congenital CMV infection is suspected because of abnormal findings
on routine obstetric ultrasound. Ventricular dilation, microcephaly, cerebral
calcifications, and hydrops are considered suspicious for
CMV infection. This information is derived from case series that evaluated
fetuses of mothers with documented primary CMV or fetuses with abnormal
findings on routine ultrasound that led to a workup for congenital
infection. Ultrasound findings associated with CMV infection are diverse
and can change over time in a given fetus. For example, ascites
or pericardial or pleural effusions can resolve; however, this does not
necessarily predict an improved prognosis.62,71 Any fetus with evidence of unexplained hydrops should be evaluated for
CMV.71 Abnormalities such as ventricular dilation, oligohydramnios,71 pericardial effusions,62 hydrops, and ascites documented by ultrasound have been associated with
more severe syndromes.64 LIMITATIONS OF PRENATAL DIAGNOSIS. Although amniotic fluid culture is sensitive, its use as the sole mode
of diagnosis is problematic. PCR applied to amniotic fluid samples further
increases the sensitivity of amniotic fluid analysis and provides
a more rapid diagnosis. However, because of reports of false-negative
amniotic fluid culture and false-positive amniotic fluid PCR results, a
combination of amniocentesis and fetal blood sampling may provide
the optimal means to diagnose fetal CMV infection. Cordocentesis also provides fetal blood for testing to determine fetal
prognosis. Hematologic evidence of systemic infection appears associated
with a worse prognosis.74 Similarly, abnormalities on ultrasound suggest a poor prognosis; however, several
cases in the literature indicate that systemic fetal CMV does
not universally result in a severely handicapped neonate.63,75,76 Thus, counseling the mother regarding a possible poor prognosis for her
infant must be framed appropriately. Further study is necessary to define
fetal indicators predictive of severe handicap. Neonatal Diagnosis of Congenital Infection At birth, the majority of CMV-infected infants are asymptomatic and only
about 10% show signs of CID. Clinical features of CID most frequently
include hepatosplenomegaly, microcephaly, jaundice, and petechiae. Hepatosplenomegaly
is caused by extramedullary hematopoiesis and mild hepatitis, which
usually is associated with elevated liver enzymes and
direct and indirect hyperbilirubinemia. Petechiae are the result of thrombocytopenia
and usually resolve within a few weeks to months. Other
less-common findings include purpura, pneumonia, chorioretinitis, cerebral
calcifications, hemolytic anemia, and inguinal hernias in males.13 Because the signs and symptoms of symptomatic CMV infection in newborns
so often overlap with those of other neonatal diseases, a definitive
diagnosis requires laboratory testing. Viral isolation from infants within
the first 3 weeks of life is considered proof of congenital infection
and the most sensitive means of diagnosis. The usual site for isolation
is the urine, but the virus has also been recovered from cerebrospinal
fluid, saliva, buffy coat, and biopsy specimens or autopsy tissue.77 Prognosis The incidence and severity of sequelae in congenitally infected infants
are related to the presence or absence of symptoms at birth and to the
stage (primary or recurrent) of maternal CMV infection experienced during
pregnancy. SYMPTOMATIC VERSUS ASYMPTOMATIC INFECTION AT BIRTH. Table 2 presents the incidence of sequelae in symptomatic infants compared with
asymptomatic infants at birth. Prognosis is poor for infants with CID
at birth. More than 90% of symptomatic infants experience serious sequelae
such as death (5.8%), chorioretinitis (20.4%), sensorineural hearing
loss (58%), and mental retardation (55%). Boppana and coworkers,78 based on data from 106 neonates with symptomatic congenital CMV infection, confirmed
the multisystem nature of the disease and its associated
morbidity and mortality. Clinical and laboratory abnormalities indicated
involvement of the heptobiliary, hematopoietic, and central nervous
systems in the majority of infants studied in the first month of life. Greater
than two thirds of these infants had at least one neonatal
sign of central nervous system disease (e.g., microcephaly, lethargy/hypotonia, poor suck, seizures). These signs have
been found to correlate with mental retardation in school-age children.74 TABLE 2. Sequelae in Children After Congenital CMV Infection According
to Symptoms at Birth* and Type of Maternal Infection†
Sequela | Symptomatic % (No.) | Asymptomtic % (No.) | Primary % (No.) | Recurrent % (No.) |
Sensoneural hearing loss | 58.0 (58/100) | 7.4 (22/299) | 15.0 (18/120) | 5.0 (3/56) |
Bilateral hearing loss | 370.0 (37/100) | 2.7 (8/299) | 8.0 (10/120) | 0 (0/56) |
Speech threshold 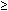 60dB† 60dB† | 27.0 (27/100) | 1.7 (5/299) | 8.0 (9/120) | 0 (0/56) |
Chorioretinitis | 20.4 (19/93) | 2.5 (7/281) | 13.0 (9/68) | 0 (0/32) |
IQ < 70 | 55.0 (33/60) | 3.7 (6/159) | 6.0 (7/112) | 2.0 (1.54) |
Microcephaly, seizures, or paralysis | 51.9 (54/104) | 2.7 (9/330) | 6.0 (8/125) | 2.0 (1/64) |
Microcephaly | 37.5 (39/104) | 1.8 (6/330) | 5.0 (6/125) | 2.0 (1/64) |
Seizures | 23.1 (24/104) | 0.9 (3/330) | 5.0 (6/125) | 0 (0/64) |
Paresis/Paralysis | 12.5 (13/104) | 0 (0/330) | 1.0 (1/125) | 0 (0/64) |
Death§ | 5.8 (6/104) | 0.3 (1/330) | 2.0 (3/125) | 0 (0/64) |
IQ, intelligence quotient.
*Data from Stagno S, Whitley RJ: Herpesvirus infections in neonates and
children: Cytomegalovirus and herpes simplex virus. In Holmes KK (ed): Sexually
Transmitted Diseases, p 1197, McGraw Hill, 1999.
†Data from Fowler KB, Stagno S, Pass RF et al: The outcome of congenital
cytomegalovirus infection in relation to maternal antibody status. N
Engl J Med 326:665, 1992.
‡For the ear with better hearing.
§After the newborn period.
Mortality in the most severely affected infants can be as high as 30%. Death
within the first month of life usually is caused by multiorgan system
disease that produces severe hepatic dysfunction, disseminated intravascular
coagulation, and secondary infections. Among the infants
studied by Boppana and coworkers,78 12% died during the first 6 weeks of life. Death occurring outside this
period but within 1 year of life usually is caused by progressive liver
failure and failure to thrive. Death after 1 year of life usually
occurs from malnutrition and infection among those with central nervous
system infection and neurologic disability.79 By contrast, prognosis is relatively good for congenitally infected infants
who are asymptomatic at birth. Only 5% to 15% of asymptomatic congenitally
infected infants have long-term sequelae develop that become
apparent within the first 2 years of life. Sensorineural hearing loss
is the most common sequelae in these infants, occurring at an incidence
of approximately 7.5% of infected infants asymptomatic at birth.80–84 The impairment is bilateral in nearly half of the cases and is of sufficient
severity to impair verbal communication and learning. Other neurologic
complications such as mental retardation, microcephaly, seizures, and
paralysis nevertheless occur in approximately 2% to 3% of congenitally
infected infants who were asymptomatic at birth.85 In summary, the likelihood that infants who survive symptomatic congenital
CMV infection will have normal intellectual development and hearing
is small.1 In contrast, the prognosis for infected infants who are asymptomatic at
birth is good. PRIMARY VERSUS RECURRENT MATERNAL INFECTION. Most infants with reported sequelae have been born to mothers with primary
infection during pregnancy.51 Congenital CMV infections in children of women with immunity acquired
before pregnancy are less likely to be symptomatic at birth than those
resulting from a primary infection.33,35 These children also are less likely to have severe sequelae (see Fig. 1). Fowler and coworkers51 compared the outcomes of 125 CMV-infected infants born to mothers who
acquired primary CMV infection during pregnancy with 64 CMV-infected infants
born to mothers with recurrent disease. The findings, presented
in Table 2, indicate that pre-existing maternal antibody to CMV protects the fetus
and lessens the severity of the sequelae of congenital CMV infection. Children
infected after primary maternal infection were more likely
to have functionally important sensorineural hearing loss and more likely
to have intelligence quotient scores less than 70 than children infected
after recurrent maternal infection. The children in the primary
group also were more likely to be severely damaged as indicated by the
prevalence of multiple sequelae (6%) in this group compared with that
in the recurrent group (0%). | 
 60dB†
60dB†