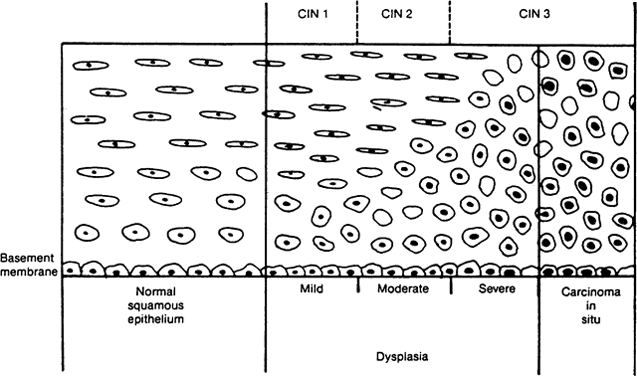
Cervical intraepithelial neoplasms are atypical proliferations of immature squamous epithelium that do not penetrate the basement membrane of the epithelium. Mitotic figures, both normal and tripolar or even tetrapolar, are seen above their usual position among the reserve cells at the base of the epithelium. Nuclear abnormalities are characteristic and include a coarse chromatin pattern, abnormal chromatin distribution, pleomorphism, and hyperchromaticity. The nuclear-cytoplasmic ratio is increased. Progressive squamous differentiation is diminished, being restricted to the upper levels of the epithelium; in the most advanced lesions, differentiation is absent, and the full thickness of the epithelium is composed entirely of neoplastic cells.
Table 1 compares the systems for classification of CIN that have been used as understanding of cervical carcinogenesis has changed. The modified Papanicolaou system was developed to distinguish cancer and carcinoma in situ (CIS) from other lesions and now is obsolete. The dysplasia and CIN systems were standard until recently and remain the histologic descriptive terms of choice. They are especially useful for clinicians who elect to treat mild dysplasia or CIN I. The Bethesda system is used most commonly for cytopathologic description and is most appropriate when clinicians plan to treat only high-grade lesions. Multiple descriptors can appear on single reports.
TABLE 1. Comparison of Classification Systems for Precancerous Lesions
of the Cervix
Modified Papanicolaou | Dysplasia | CIN | Bethesda |
I | Normal | Normal | Normal/Benign |
II | Atypia | Atypia | ASCUS/LGSIL |
III | Mild dysplasia | CIN I | LGSIL |
III | Moderate dysplasia | CIN II | HGSIL |
III | Severe dysplasia | CIN III | HGSIL |
IV | Carcinoma in situ | CIN III | HGSIL |
V | Cancer | Cancer | Cancer |
CIN, cervical intraepithelial neoplasia; ASCUS, atypical squamous cells of uncertain significance; LGSIL, low-grade squamous intraepithelial lesion; HGSIL, high-grade squamous intraepithelial lesion.
History
Although cervical CIS was described in the early 1900s,1 the clinical importance of these lesions was not appreciated until useful means for detecting these asymptomatic, invisible lesions were developed. Beforehand, cervical cancer detection relied on inspection and palpation, with biopsy of obvious invasive cancers.2 Schiller developed a technique for iodine staining as a gross means for detecting areas of abnormal epithelium,3 but this test could not distinguish metaplastic from neoplastic areas of the cervix and could not distinguish small areas of invasion present in a field of diffuse nonstaining epithelium. In this era, the nature of intraepithelial lesions was controversial, often described at the margin of invasive lesions but at times noted as a precursor to invasion.3,4 Nevertheless, the description in the 1920s and 1930s of what came to be known as CIN provided the foundation for the development of cytologic study.
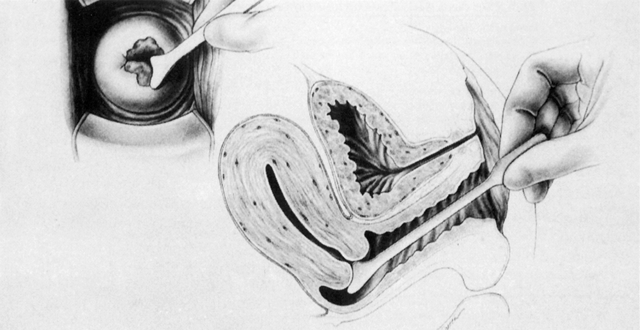
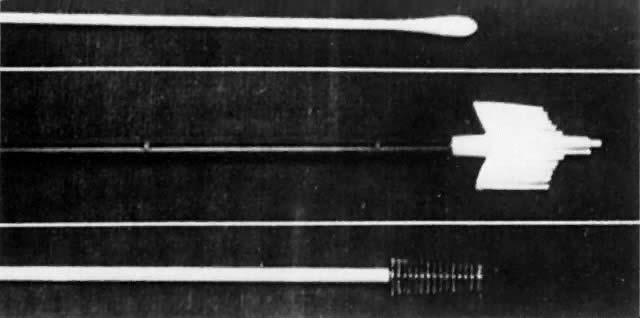
Although cytologic examination of exfoliated cervical epithelial cells was first described by Babes in 1928 in the French literature,5 only with the appearance in 1941 of the findings of Papanicolaou and Traut did this technique enter clinical practice as a means for the early diagnosis of cervical cancer.6 Like any epithelium, the cervical mucosa constantly is being sloughed and regenerated. When examined as thin smears, neoplastic cells from the cervical surface retain characteristic microscopic abnormalities that allow them to be distinguished after staining from normal squames, leukocytes, and glandular cells. Papanicolaou described a technique for aspiration of cells from the posterior vaginal pool, fixation, and cytologic staining that remains the foundation of current screening strategies. Whereas others identified unsuspected neoplasms after abnormal smears and suggested that CIS was a cancer precursor,7-11 Papanicolaou's findings generated significant controversy,12 and his technique required modifications. Over time, vaginal pool aspiration was dropped, to be replaced by spatula collection, as developed by Ayre to facilitate the sampling of cells directly from the ectocervix.13 Augmented with a cotton-tipped applicator for endocervical cell collection,14 this instrument remained the standard collection instrument until the development of endocervical brushes and one-step samplers during the 1980s.
Initially, in the United States, histologic evaluation of abnormal findings on cervical cytologic smears was achieved through blind biopsy of four quadrants of the cervix or through biopsy directed by the Schiller test.15-18 However, although such biopsies were useful when results were positive, the inherent high negative predictive value of this random procedure in identifying lesions invisible to the naked eye soon was recognized.18,19 Cone biopsy became and remains the definitive procedure for histologic evaluation of the cervical transformation zone, punch biopsy being reserved for patients with gross lesions or lesions detected colposcopically.17,18,20-23 This procedure, which involves the excision of the cervical transformation zone, requires anesthesia and hospitalization and carries a significant risk of complications, whereas later studies showed disconcertingly high rates of negative findings in cone biopsies from patients with cytologic tests showing abnormalities of lesser severity than CIS or invasive cancer.18 Alternative methods of diagnosis were sought.
Colpomicroscopy was proposed as an alternative offering magnification as
high as 480 , but technical factors precluded its wide acceptance.24,25 Hinselmann had described colposcopy in 1925, and the technique had become
incorporated into clinical care in other countries, but only in the 1950s
was intensive training in colposcopic techniques promoted in the
United States.26-30 Initially, a role for colposcopy as a screening modality for CIN was proposed,26,28,29 and it still is used as such in some parts of Europe, but the greater
simplicity and wider availability of cytologic screening led to retention
of cytologic examination as the standard screening test in the United
States, with colposcopy retained as the study of choice for evaluation
of women with abnormal cytologic findings. The value of colposcopy
for excluding invasive cancer or CIS in women with cytologic results
consistent with dysplasia was established,31,32 and studies comparing colposcopy with cone biopsy showed acceptable rates
of correlation.33,34 The American Society for Colposcopy and Cervical Pathology, dedicated
to promoting skills in colposcopy through research and education, was
founded in 1965 and, as a member of the International Federation of Cervical
Pathology and Colposcopy, remains a force for the development of
strategies for screening, diagnosis, and management of CIN.
, but technical factors precluded its wide acceptance.24,25 Hinselmann had described colposcopy in 1925, and the technique had become
incorporated into clinical care in other countries, but only in the 1950s
was intensive training in colposcopic techniques promoted in the
United States.26-30 Initially, a role for colposcopy as a screening modality for CIN was proposed,26,28,29 and it still is used as such in some parts of Europe, but the greater
simplicity and wider availability of cytologic screening led to retention
of cytologic examination as the standard screening test in the United
States, with colposcopy retained as the study of choice for evaluation
of women with abnormal cytologic findings. The value of colposcopy
for excluding invasive cancer or CIS in women with cytologic results
consistent with dysplasia was established,31,32 and studies comparing colposcopy with cone biopsy showed acceptable rates
of correlation.33,34 The American Society for Colposcopy and Cervical Pathology, dedicated
to promoting skills in colposcopy through research and education, was
founded in 1965 and, as a member of the International Federation of Cervical
Pathology and Colposcopy, remains a force for the development of
strategies for screening, diagnosis, and management of CIN.
Several technologic enhancements to colposcopy have appeared in the past two decades. Videocolposcopy allows for image recording in real time, with enhanced visualization and opportunities for patient and trainee education. Systems for videocolposcopy include those added to traditional optical colposcopes as well as video-only systems. A comparison study of optical and video systems shows that use of video systems by even experienced colposcopists requires a learning phase but that similar findings result.35 Computerized image analysis and image storage is a recent advance with the potential to minimize subjective analysis of lesions and offer the option of serial follow-up with quantification of changes in lesion size and character.36-38 In addition, computerized colposcopy allows the integration of nonvisible spectra and tissue fluorescence into image analysis—processes with the promise of replacing colposcopy altogether as a diagnostic test.39
An understanding of the natural history of CIN has developed progressively. The preinvasive nature of CIS was the subject of prolonged debate,3,40,41 resolved once the ability of CIS to progress or to regress after biopsy was recognized. After this, the importance of lesser degrees of neoplastic change was investigated. Both the graded severity of lesions of the cervical epithelium and the sometimes prolonged interval between diagnosis of dysplasia and development of invasive cervical cancer came to be understood.42-46 The concept of cervical dysplasia as a continuum of disease was elaborated in the 1950s,47,48 and the term dysplasia was agreed on at the first International Congress of Exfoliated Cytology in 196249 (Fig. 1). Recognizing that pathologic distinctions between severe dysplasia and CIS are difficult to reproduce,50,51 Richart developed the CIN classification system currently used for description of histologic specimens, describing CIN as a continuum of neoplastic change with progressively increasing risk of invasion.52
Once CIS was linked to invasive cancer, principles of management of CIS were derived from those established for cervical cancer, with radical and modified radical hysterectomy recommended for women with CIS.16,22,51,53 This probably was appropriate when no method for thoroughly evaluating the cervical transformation zone existed short of cone biopsy and when cone biopsies were not universally performed or exhaustively sectioned because numerous patients undergoing hysterectomy for CIS were found to have foci of invasive cancer.15,54 Once lesser dysplasias were recognized as cancer precursors, hysterectomy also was recommended for these lesions.55 However, data accumulated to show that intraepithelial neoplasms did not metastasize and that hysterectomy could be avoided when invasion was excluded by meticulous examination of cone biopsy specimens.56-60 Conservative office techniques for treatment of CIN were developed. These include, in order of their incorporation into practice, electrocautery, cryotherapy, carbon dioxide laser ablation and conization, and loop excision.61-67 Recently, studies of natural history suggest that some patients with early CIN may have spontaneous regression of lesions and may require no treatment.68,69
Cytologic and clinical observations led Ayre to the concept that viral infection of “halo cells” represents the first stage of cervical carcinogenesis.70 This concept was validated when Zur Hausen defined the link between HPV and cervical dysplasia in 1977,71 and the concept of high- and low-risk HPV subtypes was recognized in the years after.72-74 Through the 1980s, as HPV testing became more available, substantial epidemiologic and biochemical evidence accumulated supporting the role of HPVs as necessary but not sufficient factors for cervical oncogenesis. A full discussion of these and other areas in HPV research is presented in a separate chapter.
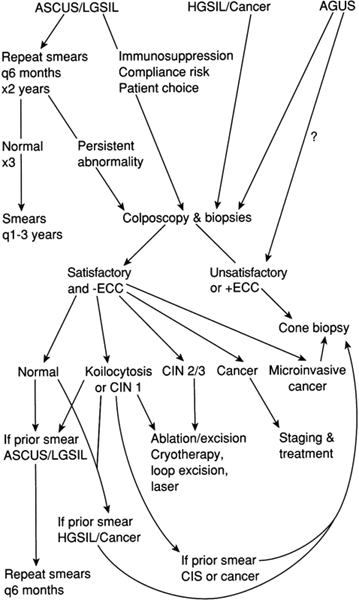
The following ideas and events—that with careful follow-up not all women with CIN require therapy, modification of classification systems, the idiosyncratic interpretations made of classification criteria, a growing appreciation of the malpractice risk inherent in a missed diagnosis of cervical cancer, publicized cases of cervical cancer developing after findings on smears were negative, and reports of cytopathology centers using suboptimal quality controls—all combined in the late 1980s to produce confusion among clinicians about the proper management of women with abnormal findings on cytopathology reports, especially those consistent with early lesions.
One attempted solution to these problems was the development of automated cytologic systems based on image recognition software.75 Adjunctive measures, such as HPV typing, and new instruments were developed to improve the sensitivity of cytologic study as a screening test for cervical cancer, especially for women with borderline abnormalities.76-80
The National Institutes of Health addressed these problems through consensus conferences charged with the development of standards for diagnosis and management of abnormal cytologic findings.81-83 First, the Bethesda system for cervical cytology interpretation was promulgated. Afterward, interim guidelines for management were devised. Recently, criteria for diagnosis have been published to standardize use of the various categories of the Bethesda system. The results of this group have been widely accepted, but not without criticism.84-86
Finally, in the last two decades, molecular biologists have explored the changes that lead cells in the cervical epithelium to become neoplastic. In addition to the inactivation of tumor suppressors from the p53 and retinoblastoma genes by HPV proteins, investigators have identified alterations in the expression of the epidermal growth factor receptor and the ras and myc oncogenes.87-91 Allelic loss follows the induction of tetraploidy and aneuploidy by p53 inactivation.92,93 Rates of apoptosis fall as proapoptotic proteins are down-regulated.94,95 Loss of heterozygosity analyses suggest that other important tumor suppressor genes may be deleted in preneoplastic cervical epithelium,96,97 including alterations in expression of the fragile histidine triad gene on chromosome 3.98,99 Angiogenesis is activated, and matrix interactions are altered as lesions advance.100,101 Despite these advances, however, the precise sequence of genetic alterations after HPV infection that leads to neoplastic transformation in the cervix remains to be elucidated.
The incidence of cytologic abnormalities in the United States has been estimated at approximately 5%,83 implying that CIN develops in hundreds of thousands of women each year. Despite this, the incidence of invasive cervical cancer in 2000 is estimated to be only 12,500.102 Given the magnitude of this public health problem and multitude of poorly understood factors that determine the progression of CIN to invasive cancer, it is understandable that controversy still surrounds the classification, diagnosis, and management of CIN.