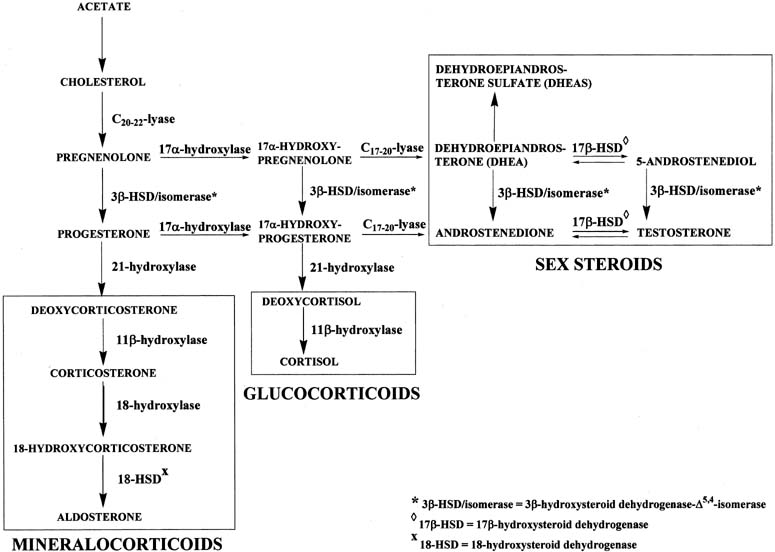
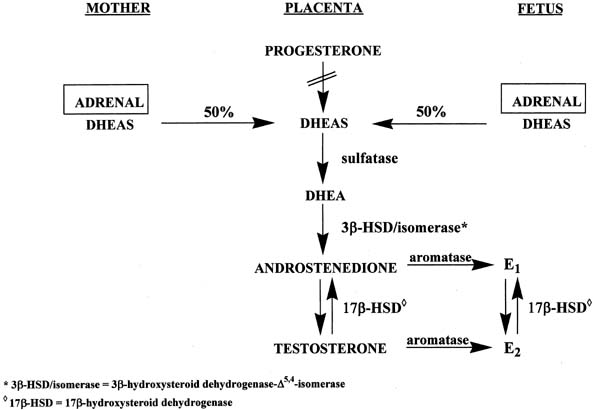
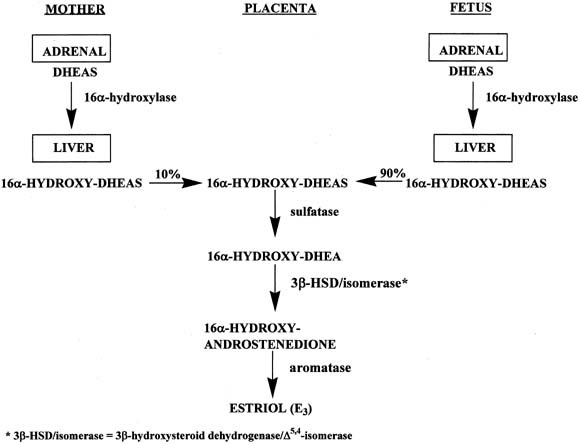
Figure 4 illustrates the biosynthetic pathways leading to the formation of androgens
and estrogens in the ovaries and testes. This occurs by one of two
series of biochemical reactions, which are often referred to as the Δ5 pathway and the Δ4 pathway. In the Δ5 pathway, all of the steroids have a double bond between carbons 5 and 6, whereas
the double bond is between carbons 4 and 5 in the Δ4 pathway. The relative importance of the two pathways is poorly understood. 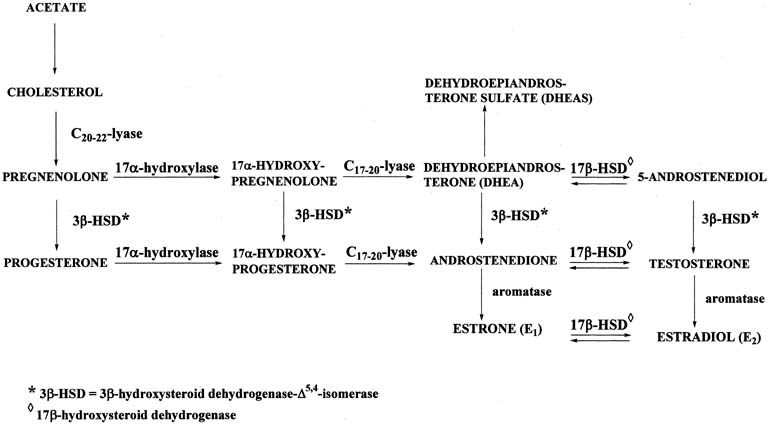 Figure 4. Biosynthesis of steroid hormones in the ovaries and testes. Figure 4. Biosynthesis of steroid hormones in the ovaries and testes.
|
The Δ5 pathway begins by formation of 17-hydroxypregnenolone from pregnenolone
via the enzyme, 17α-hydroxylase. Subsequently, 17α-hydroxypregnenolone is converted to dehydroepiandrosterone (DHEA) through
the action of C17-20-lyase. Both 17α-hydroxylase and C17-20-lyase reside on
a single protein and are encoded by a single gene, namely, CYP17.3,4 DHEA is then transformed to 5-androstene-3β,17β-diol (androstenediol) by 17β-hydroxysteroid
dehydrogenase (also referred to as 17β-hydroxysteroid
oxidoreductase [17β-HSD]). As the name oxidoreductase implies, the reaction in which DHEA is converted to androstenediol involves
reduction (addition of two hydrogens to the ketone group at
carbon 17 of DHEA) or oxidation (removal of two hydrogens from
the hydroxyl group at carbon 17 of androstenediol). Thus, the
conversion of DHEA to androstenediol is reversible. The Δ5 pathway stops after androstenediol is formed. The Δ4 pathway begins with the conversion of pregnenolone to progesterone through the action of two enzymes, namely, 3β-hydroxysteroid
dehydrogenase and Δ5,4-isomerase. The former enzyme is also re-ferred to as 3β-hydroxysteroid
oxidoreductase (3β-HSD). It is encoded by the 3β-HSD gene. There are two functional 3β-HSD genes (type 1 and type 2).5,6,7 Type 1 expression occurs primarily in the placenta, mammary gland, and
skin, whereas the type 2 isoform is expressed almost exclusively in the
adrenals and gonads. The enzymatic reaction involves oxidation, that
is, removal of two hydrogens from the hydroxyl group at carbon 3, forming
a ketone group. In contrast to the reversible formation of androstenediol
from DHEA, this reaction is not reversible to any significant
extent. Once the ketone group is formed, the double bond between carbons 5 and 6 is
rapidly shifted and becomes located between carbons 4 and 5 through
the action of the isomerase enzyme. As shown in Figure 4, in addition to pregnenolone, all of the other compounds in the Δ5 pathway can also be converted to the corresponding Δ4 compounds. Once progesterone is formed, the reactions in the Δ4 pathway proceed in the same manner as in the Δ5 pathway, with the same enzymes and genes. Thus, progesterone is first converted to 17α-hydroxyprogesterone, which undergoes
side-chain cleavage to form androstenedione. This androgen
is then transformed to the final product of the Δ4 pathway, which is testosterone, in a reversible reaction catalyzed by
the 17β-HSD enzyme. In humans, there are at least five isoforms of the 17β-HSD enzyme, encoded
by the 17β-HSD gene; they are designated types 1 through 5.8 Each type has cell-specific expression, substrate specificity, regulatory
mechanisms, and reductase or oxidative catalytic activity. The
affinity of the 17β-HSD type 1 isoenzyme is approximately 100-times
higher for C18 steroids than for C19 steroids, and
its catalytic preference is reduction. It is localized predominantly
in the ovary (granulosa cells) and placenta (syncytiotrophoblast). The 17β-HSD type 2 isoenzyme preferentially
catalyzes the oxidation of steroids with a hydroxyl group at carbon 17, for
example, testosterone, estradiol. The enzyme is distributed among
many extraglandular tissues, such as endometrium, placenta, and liver; however, it
is primarily expressed in the endometrium. The activity
of the type 2 isoenzyme is increased during the luteal phase of the
menstrual cycle in a manner that parallels circulating progesterone levels during the cycle. The 17β-HSD type 3 isoenzyme is expressed
in the testes and preferentially catalyzes the reduction of androstenedione
to testosterone at carbon 17. Relatively little is yet known about the 17β-HSD type 4 and
type 5 isoenzymes. It appears that the type 4 isoenzyme catalyzes the
oxidation of C18 steroids, for example, estradiol, to estrone, whereas
the type 5 isoenzyme catalyzes the reduction of C19 steroids, for example, androstenedione
to testosterone. Deficiency of the 17β-HSD type 3 isoenzyme causes a form of
male pseudohermaphroditism referred to as 17β-HSD deficiency, in which there is a defect in the biosynthesis of testosterone from androstenedione in the fetal testes.9 The deficiency is confined to individuals with a 46XY karyotype. These
individuals have testes, wolffian duct-derived male internal genitalia (with
the exception of a prostate), female external
genitalia, and gynecomastia. Deficiencies of 17α-hydroxylase and C17-20-lyase
in fetal testes have also been reported. As in the disorder of 17β-HSD
deficiency, individuals with a defect in one of these enzymes
also cannot produce testosterone and have incomplete masculinization. The two androgens, androstenedione and testosterone, can undergo a series
of complex reactions (aromatization) catalyzed by the aromatase
enzyme, forming the estrogens, estrone (E1) and estradiol (E2), respectively. This reaction is encoded by the CYP19 gene.10 |