In the context of ongoing research in tertiary care centres, serial TVUS examinations performed during the follicular phases of one or more menstrual cycles have helped to define both normal and abnormal patterns of follicular growth and development.2,3 Although the logistical challenges of serial TVUS examinations can be somewhat daunting, a consistent pattern will usually become apparent within the span of a few cycles.
Normal Follicular Growth and Development
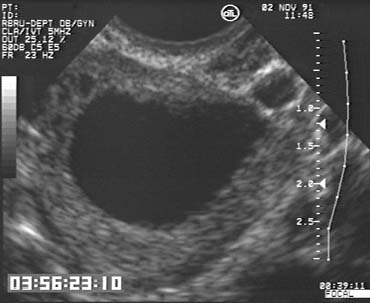
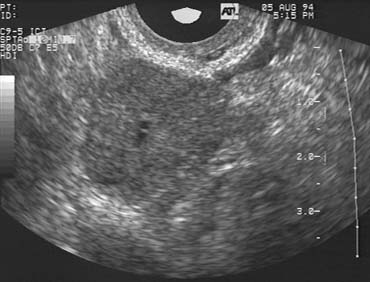
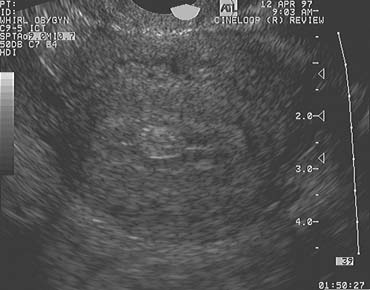
The population of ovarian follicles can be evaluated at any time during the menstrual cycle, but the interval of greatest interest spans the late follicular phase during the final stages of preovulatory follicular development. The dominant follicle destined to ovulate can be identified by its size on approximately cycle day 7 and thereafter grows at an average rate of 2 mm per day until it reaches full maturity at a diameter of approximately 22 mm (Fig. 1).2,4,5,6,7,8
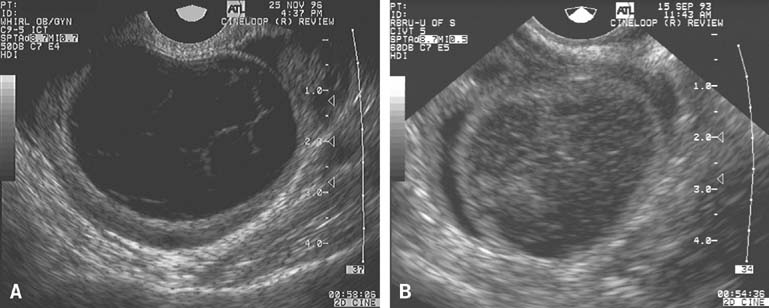
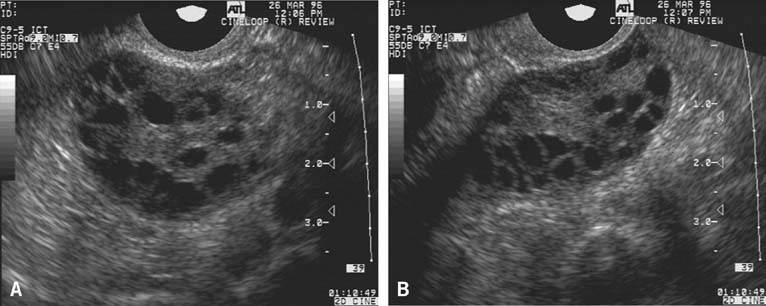
In the classically normal menstrual cycle, ovulation occurs on or at approximately cycle day 14 and menses begins approximately 14 days thereafter.2,4,5,6 Although ultrasound has been used to detect ovulation in women for a number of years,9 the ability to observe the physical rupture of the preovulatory follicle, escape of the follicular fluid, and extrusion of the cumulus/oocyte complex have only recently been clearly demonstrated (Fig. 2).10 The site at which ovulation will occur can be readily identified up to a week in advance.5,10 The sequence of ultrasonographic events that surrounds ovulation occurs over an average of approximately 10 minutes, but the interval varies, lasting from less than 1 minute to more than 20 minutes in duration. After ovulation, in normal cycles, the collapsed follicle gives rise to the corpus luteum. The corpus luteum then becomes the dominant ovarian structure and can be easily recognized across the remainder of the cycle (see Fig. 2).5,10
Use of Transvaginal Ultrasonography to Determine the Optimum Time for Artifical Insemination
Increasingly, TVUS monitoring of follicular development is being used to better and more precisely define the time of ovulation and the optimum time to perform IUI, when indicated, in both spontaneous and stimulated cycles. The accuracy of TVUS in defining the time of ovulation is far superior to estimates based on results of monitoring basal body temperature (BBT) or urinary luteinizing hormone (LH) excretion. Given the relatively brief interval for which sperm remain viable after IUI and the considerable costs involved, serial TVUS examinations may help to maximize both the effectiveness and efficiency of such advanced forms of treatment.
Abnormal Follicular Growth and Development
Careful serial TVUS examinations during the latter stages of preovulatory follicular development and during the interval immediately after ovulation in both normal fertile women and those with idiopathic infertility suggest that subtle but distinct abnormalities of follicular growth, development, and ovulation occur and may often go unrecognized. The luteinized unruptured follicle (LUF) and the follicular cyst are two distinctly abnormal patterns of follicular development that may represent subtle forms of subtle ovulatory dysfunction.
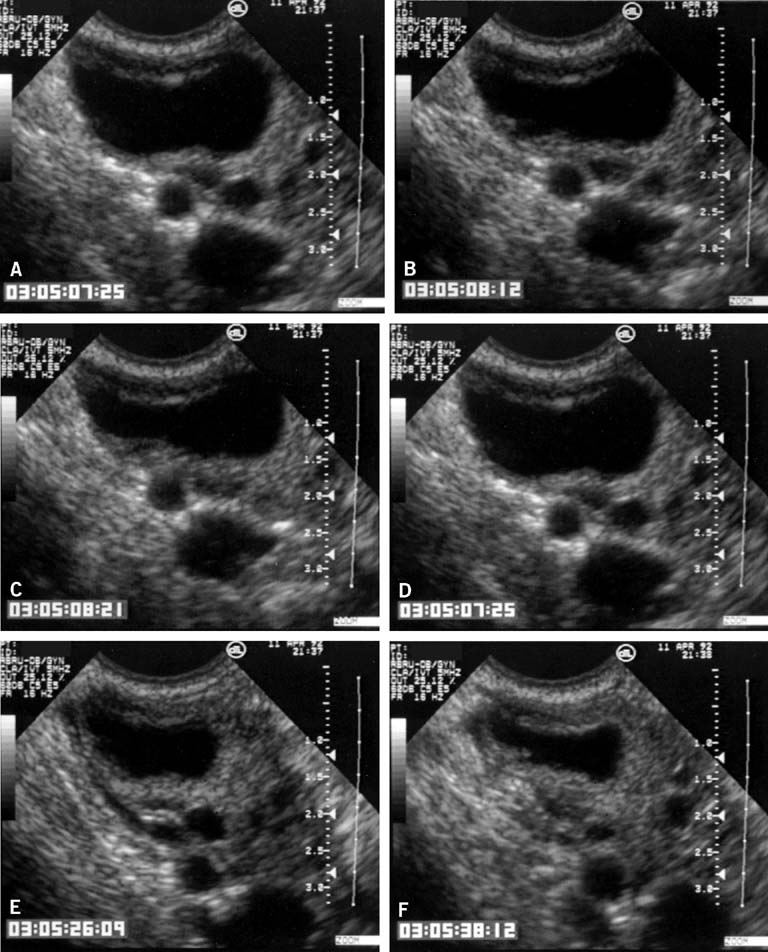
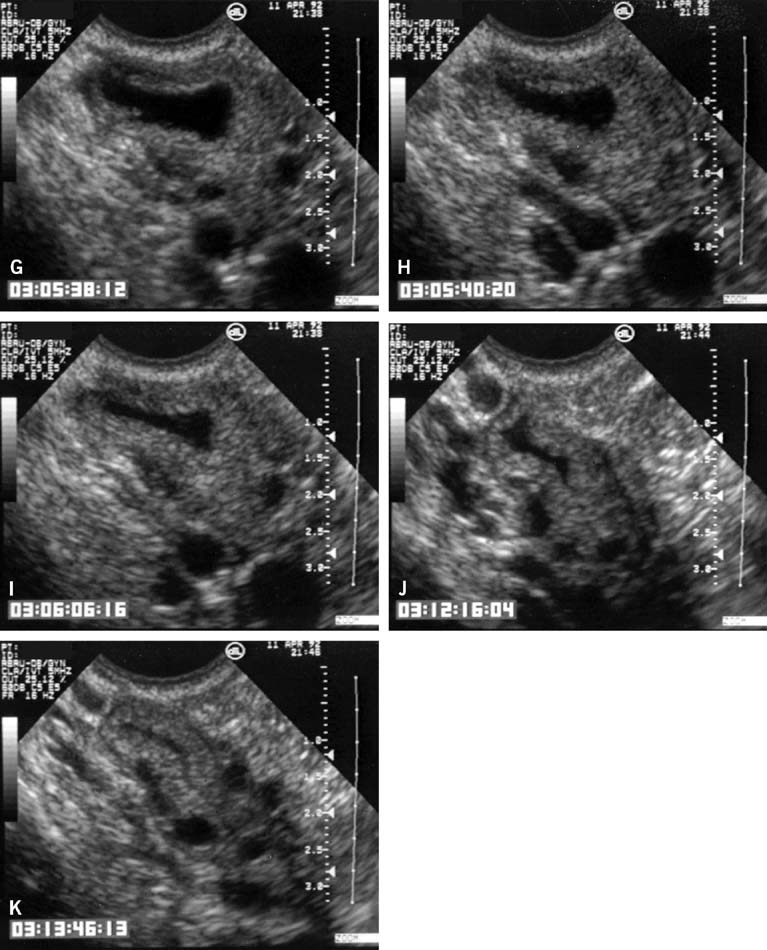
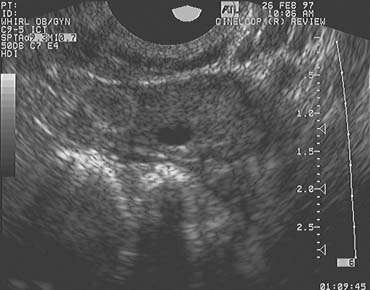
The LUF syndrome is characterized by a preovulatory follicle that grows and develops in a normal pattern but does not collapse at the expected time of ovulation. Ovulation does not, in fact, occur and the oocyte presumably remains trapped within the follicle. The follicular walls subsequently thicken and increase in density much like is seen after a normal ovulation, the follicular margins become less distinct, and the structure persists for the duration of the cycle, regressing in a manner very similar to that of a normally formed corpus luteum (Fig. 3).11,12 Other indices of ovulation (basal body temperature, serum progesterone concentration) remain normal; the time and character of the subsequent menses also are most often entirely normal.
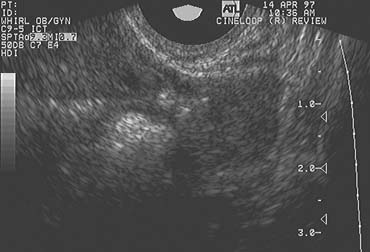
The follicular cyst is recognized when the preovulatory follicle grows to a diameter beyond those typically observed before ovulation but does not collapse and persists for an interval of several days before gradually regressing at a pace similar to that at which it grew. In contrast to the LUF, there is no sonographic evidence of luteinization. The walls of the follicular cyst do not thicken, increase in density, or become indistinct. Instead, they remain thin, and follicular margins are still sharply defined. Menses generally follow at or near the expected time, although the duration and character of flow is more variable.
Used in combination with serial TVUS examinations, serum estradiol determinations may provide even greater insights into the quality of follicular growth and development and reveal abnormalities that would otherwise go unrecognized by either method alone. TVUS examinations may demonstrate that normally rising serum estradiol concentrations reflect the combined contributions of a large number of small follicles measuring 5 to 10 mm diameter rather than the development of a single dominant preovulatory follicle. Conversely, a late follicular phase TVUS examination may reveal a follicle having the diameter normally expected immediately before ovulation, but an abnormally low estradiol concentration may indicate it is unlikely to contain a normal or mature oocyte and may be expected to regress (become irregular in contour and progressively decrease in size) over the ensuing week.
At present, such careful monitoring of follicular growth and development in natural cycles remains largely in the realm of ongoing clinical research and has limited applications in clinical care. Further research, to include investigations aimed at defining and correlating the microvascular characteristics of individual follicles with patterns of growth and ovulation or regression will surely expand our current understandings of follicular dynamics and ultimately may also help to explain the cause of reproductive failure in some women with otherwise idiopathic infertility. As technological capabilities and research continue to advance, TVUS may be expected to play an increasingly valuable role in the evaluation and treatment of infertility related to ovulatory dysfunction and failure.