Adhesiolysis
Pelvic adhesions have been identified as a major contributing cause of infertility. In these patients, goals of reconstructive pelvic surgery include lysis of adhesions that interfere with physiologic processes of ovum release, pickup, and transport and minimal subsequent development of postoperative adhesions.
Classification of the type or extent of pelvic adhesion has been described in several previous reports.30,31,32,33,34,35 Jessen34 described an improved pregnancy rate in the presence of low-grade compared with high-grade adhesions (64% versus 22%, respectively). Subsequently, Hulka and colleagues31 classified the extent and type of adnexal adhesions, and later the same authors demonstrated their prognostic value.33 Pregnancy was more likely to occur when more than 50% of the ovary was visible and when adhesions were filmy and avascular, rather than dense and vascular. Similarly, Caspi and coworkers32 demonstrated that pregnancy rates were higher in association with fine, avascular as opposed to fibrous, coarse adhesions. In these reports, as well as those of Young and associates,35 the significance of adhesions (as assessed by the pregnancy rate) was clouded by the classification systems, which also relied on tubal patency (and performance of fimbrioplasties and salpingostomies).
The report that has most closely assessed the role of periadnexal adhesions alone on pregnancy outcome is that of Bronson and Wallach.30 They scored adhesions at varying locations (right and left ovaries, fallopian tubes, pelvic sidewalls, broad ligaments, cul-de-sac, and rectosigmoid) and observed that pregnancy rates were lower among women with high adhesion scores, although the difference was not significant. Importantly, however, in these studies looking at pregnancy outcome,30,31,32,33,34,35 the incidence, extent, and severity of adhesions at the time the women were trying to conceive is not known, nor were there extensive controls for other factors that may contribute to infertility.
Adhesiolysis has traditionally been performed by sharp dissection or electrosurgery; more recently, it has also been performed using the CO2 and argon lasers36,37 and linear cutters. A comparison of pregnancy outcome after adhesiolysis at laparotomy and at laparoscopy with various techniques is shown in Table 2.38,39,40,41,42,43,44,45,46 In general, the use of microsurgical techniques has led to a trend for improvement in pregnancy outcome after adhesiolysis. As shown, in women in whom there is no distal tubal obstruction, adhesiolysis can be expected to lead to pregnancy in 50% to 60% of cases. Use of the CO2 laser, compared with nonlaser techniques, has not resulted in improvements in pregnancy outcome,43 nor has it resulted in consistent reductions in the adhesions observed at the time of second-look laparoscopy.47 Consistent with these findings is the report of Pittaway and colleagues,48 which described no reduction in postoperative intraperitoneal adhesion formation in rabbits and rats, respectively, with the CO2 laser as compared to electrocautery.48a
TABLE 2. Pregnancy Outcome Following Adhesiolysis
| Laparotomy/ | Macrosurgery/ |
| Total No. | No. Ongoing |
| Laparoscopy | Microsurgery | Laser | Patients | Pregnancy (%) |
Wallach38 | Laparotomy | Macro | - | 94 | 43(46) |
Jansen30 | Laparotomy | Macro | - | 64 | 26(41) |
O'Brien40 | Laparotomy | Macro | - | 41 | 16(42) |
Betz13 | Laparotomy | Macro | - | 29 | 20(69) |
Grant41 | Laparotomy | Macro | - | 268 | 94(35) |
Caspi32 | Laparotomy | Micro | - | 101 | 38(38) |
Donnez42 | Laparotomy | Micro | - | 42 | 27(64) |
Diamond16 | Laparotomy | Macro | - | 220 | 55(25) |
| Laparotomy | Micro | - | 140 | 80(57) |
Tulandi43 | Laparotomy | Micro | - | 33 | 17(52) |
|
| Micro | + | 30 | 16(53) |
Frantzen44 | Laparotomy |
| - | 49 | 20(41) |
Gomel45 | Laparoscopy | Micro | - | 92 | 54(59) |
Fayez46 | Laparoscopy |
| - | 50 | 23(46) |
As a result of technological advances, it is now possible to perform adhesiolysis (and other reproductive pelvic surgical procedures) at laparoscopy. Although relatively minor procedures can be performed by most laparoscopists, treatment of more extensive disease requires advanced training and experience. In experienced hands, laparoscopic treatment (usually involving multiple-puncture techniques) appears to yield results similar to those achieved with laparotomy.45,46 However, no well-controlled studies examining this issue have been performed.
Adhesion reformation after reproductive pelvic surgery occurs frequently, even with the use of microsurgical techniques and surgical adjuvants.49 Although adhesion reformation was defined in one report49 as a redevelopment of adhesions at sites that were subjected to adhesiolysis at the time of the initial procedure, other authors have used the same term to refer to different situations, thereby creating considerable confusion in the literature.50 To provide a common terminology for comparison of efficacy of different techniques and adjuvants in reducing postoperative adhesions, a classification system has been proposed that takes into consideration the presence or absence of adhesions initially, and whether a surgical procedure other than adhesiolysis was performed at that site (Table 3). Importantly, this classification also includes the concept of de novo adhesion reformation (the development of adhesions, observed at the time of the second-look procedure, for which adhesiolysis was not performed at the time of the initial procedure). As shown in Table 4, de novo adhesion formation can be a frequent problem after microsurgery performed at laparotomy, occurring in 58% of available ovaries and at almost one third of all available sites in those affected.51
TABLE 3. Classification of De Novo Adhesion Formation and Adhesion Reformation
Type 1 initially | De novo adhesion formation--development of adhesions at sites which did not have adhesion |
| No operative procedure at site of adhesion formation |
| Operative procedure performed at site of adhesion formation |
Type 2 performed | Adhesion reformation--redevelopment of adhesions at sites at which adhesiolysis was |
| No operative procedure at site of adhesion reformation (other than adhesiolysis) |
| Operative procedure performed at site of adhesion reformation (in addition to adhesiolysis) |
TABLE 4. De Novo Adhesion Formation*
|
|
|
| Sites |
|
Available |
|
|
|
|
|
|
|
|
| Available for | Sites with |
Sites with |
|
|
|
|
|
|
| Adhesions at |
| De Novo | Occurrence of |
De Novo |
|
|
|
|
|
| Total No. | Initial | Structures | Adhesion | De Novo |
Adhesion |
|
|
|
|
|
| Sites | Procedure | Excised | Formation | Adhesions |
(%) |
|
|
|
|
|
Ovaries | 164 | 84 | 8 | 72 | 42 |
58 |
|
|
|
|
|
Tubes | 164 | 50 | 10 | 104 | 26 |
25 |
|
|
|
|
|
Omentum | 82 | 4 | 0 | 78 | 13 |
17 |
|
|
|
|
|
Small bowel | 82 | 8 | 0 | 74 | 19 |
26 |
|
|
|
|
|
Colon | 82 | 11 | 0 | 71 | 13 |
18 |
|
|
|
|
|
Cul-de-sac | 82 | 26 | 0 | 56 | 16 |
29 |
|
|
|
|
|
Sidewall | 82 | 27 | 0 | 55 | 30 |
55 |
|
|
|
|
|
Total | 738 | 210 | 18 | 510 | 159 |
31 |
|
|
|
|
|
*Frequency of de novo adhesion identified at early second-look laparoscopy at sites throughout the pelvis after reproductive pelvic surgical procedures performed at laparotomy.
(Diamond MP: Adhesion prevention. In Gershenson DM, DeCherney AH, Curry SL (eds): Operative Gynecology, Chap 8. pp 147---158. Philadelphia, WB Saunders Company, 1993)
It had been suggested, anecdotally, that procedures performed by laparoscopy might be less likely to be followed by the postoperative development of pelvic adhesions. Theorized explanations included reductions in tissue drying, tissue manipulation, and introduction of foreign materials and lack of packing of bowel. However, in a multicenter study evaluating adhesion reformation at a second-look pro-cedure after laparoscopic adhesiolysis, adhesion reformation was identified in 66 of 68 subjects (97%).52 However, laparoscopic adhesiolysis was able to reduce significantly the extent of pelvic adhesions, to approximately half of what was present initially. De novo adhesion formation occurred in only 8 (12%) of these 68 women, and in 11 (23%) of 47 available sites in those affected. This suggests that de novo adhesion formation (not adhesion reformation) may occur less frequently after laparoscopic surgery, but confirmation of this hypothesis will require properly controlled studies.
Surgical adjuvants have been used frequently after adhesiolysis and other types of intra-abdominal reproductive pelvic surgical procedures. The rationale for use of adjuvants is to minimize development of postoperative adhesions and to maximize maintenance of tubal patency after the initial surgical procedure. As shown in Table 5, a variety of agents have been used for this purpose. This use has been described in detail in several reviews.15,53,54,55,56,57,58,59,60,61
Fibrinolytic Agents | Fibrinolysin |
| Papain |
| |
| |
| Hyaluronidase |
| Chymotrypsin |
| Trypsin |
| Pepsin |
Anticoagulants | Heparin |
| Citrates |
| Oxalates |
Anti-inflammatory Agents | Corticosteroid |
| |
| Antihistamines |
Antibiotics | Tetracyclines |
| Cephalosporins |
Mechanical Separation | Intraabdominal instillates |
| Dextran |
| Mineral oil |
| Silicone |
| Povidine |
| Vaseline |
| Crystalloid solutions |
| Carboxymethylcellulose |
| Barriers |
| Endogenous tissue |
| Omental grafts |
| Peritoneal grafts |
| Bladder strips |
| Fetal membranes |
| Exogenous materials |
| Interceed TC7 |
| Gore-Tex Surgical Membrane |
| Seprafilm |
| Repel |
| Adcon-P |
| Poloramer 409 |
Despite application of microsurgical technique and use of surgical adjuvants by experienced surgeons, the development of postoperative adhesions is an all too frequent occurrence.62,63,64,65,66,67,68,69 Currently, no serum marker or scanning technique is consistently able to identify adhesions, and a repeat operative procedure is required for evaluation. As shown in Table 6, pelvic adhesions were identified at the time of second-look laparoscopy in 55% to 100% of women who had undergone reproductive pelvic surgical procedures. Such adhesions represent both adhesion reformation and de novo adhesion formation.70 Inasmuch as such adhesions might impair the ability to conceive, there appears to be room for improvement in preventing postoperative adhesion development. In a poll of the members of the Society of Reproductive Surgeons, Holtz71 reported widespread use of agents for this purpose throughout the United States, including perioperative antibiotics, corticosteroids, and antihistamines and intraoperative instillation of 32% dextran 70 (Hyskon). The literature is replete with conflicting results for most adjuvants, and their efficacy is not well established. A potential explanation is differences in the pathologic conditions being treated.
TABLE 6. Pelvic Adhesions Noted as Second-Look Procedures
|
|
|
| Percentage |
| Time from Initial |
| Total No. with | with |
| Procedure | Total No. Patients | Adhesions | Adhesions |
Raj and Hulka69 | 1 wk to 2 yr | 60 | 51 | 85 |
Diamond62 | 1–12 wk | 106 | 91 | 86 |
DeCherney63 | 4–16 wk | 20 | 15 | 75 |
| 1–3 yr | 41 | 31 | 76 |
Surrey65 | 6–8 wk | 31 | 22 | 71 |
| >6 mo | 6 | 5 | 83 |
Pittaway66 | 4–6 wk | 23 | 23 | 100 |
Trimbos-Kemper67 | 8 d | 188 | 104 | 55 |
Daniell68 | 4–6 wk | 25 | 24 | 96 |
Diamond52 | <12 wk | 68 | 66 | 97 |
Three groups have demonstrated fundamental differences between adhesion formation and adhesion reformation in animal models. Holtz and associates72,73 described reduction in adhesion formation with 32% dextran 70, but a similar inhibition of adhesion reformation could not be achieved with higher doses of dextran. Similarly, Elkins and coworkers74,75 observed a greater extent of adhesion reformation than adhesion formation after dextran treatment. Finally, Diamond and associates76,77 compared adhesion formation and reformation models and noted a greater extent of adhesions in the latter.
In the survey of the members of the Society of Reproductive Surgeons, Holtz71 reported that 45% performed second-look laparoscopy on occasion. There are several benefits of second-look procedures after reconstructive pelvic surgery. Such procedures allow for assessment of the adnexal disease, allowing patients to assess their potential for fertility and plan accordingly. Second, they provide an opportunity to lyse adhesions that have reformed (or to perform additional procedures as needed, such as vaporization of endometriosis or deagglutination of fimbria). Third, based on the previously established principles that pelvic adhesions can cause infertility30,32,45 and that lysis of adhesions is associated with pregnancy establishment in many women,30,32,45 performance of second-look laparoscopy may improve the pregnancy rate after reproductive pelvic surgery, although this theoretical improvement has yet to be substantiated. Trimbos-Kemper and colleagues67 and Jansen78 reported that second-look laparoscopy was associated with a significant reduction in the subsequent observation of permanent pelvic adhesions, but the pregnancy rate was not improved. Trimbos-Kemper and coworkers67 did, however, note a reduction in the occurrence of ectopic pregnancies after salpingostomies in women who underwent early second-look laparoscopy. Finally, for the benefit of the surgeon, performance of second-look laparoscopy allows for feedback as to the surgical success of the procedures previously performed.
If second-look laparoscopy is to be performed, Swolin79 recommended that it be done early (6 to 8 weeks) to improve the possibility of lysis of postoperative adhesions. Subsequently, Raj and Hulka80 examined second-look laparoscopies performed up to 2 years after the initial procedure and demonstrated that bleeding was more common if the procedure was performed after 12 weeks or before 2 weeks. In the former case, bleeding was attributed to increased density and vascularity of the adhesions; in the latter, bleeding was attributed to granulation tissue. Between 2 and 12 weeks, the adhesions were filmy and more amenable to lysis. Surrey and Friedman65 observed that attempted adhesiolysis 6 months after the initial procedure was associated with more dense and vascular adhesions than were present at 6 to 8 weeks. DeCherney and Mezer63 also compared early (4 to 16 weeks) versus late (approximately 18 months) second-look laparoscopy; in the former group, 60% of patients had thicker neovascular adhesions. Finally, Daniell and Pittaway,68 McLaughlin,64 and Diamond and coworkers62 have also observed that adhesions at “early” second-look laparoscopy are more likely to be filmy and avascular, making them more susceptible to easy lysis. However, in multiple studies involving an opportunity for early second-look laparoscopy after the initial surgical procedure, there appeared to be no difference in the type (filmy and avascular versus dense and vascular) of adhesions observed at second-look laparoscopy.47,80a,80b,80c
Distal Tubal Obstruction: Complete
The surgical treatment of complete fimbrial occlusion is a neosalpingostomy: the creation of a new tubal ostia. Performance of neosalpingostomy is associated with a subsequent tubal patency rate of approximately 90% to 95%;81 however, pregnancy rates are only about 30% (Table 7).82,83,84,85,86,87,88,89 With prolonged follow-up of 5 years, pregnancy rates of 42% have been achieved.90 Comparison of series of macrosurgical and microsurgical neosalpingostomies fails to demonstrate an advantage of microsurgery. Again, however, a study designed to directly compare the two techniques has not been performed. Both Mage and Bruhat86 and Tulandi and Vilos87 have compared microsurgical neosalpingostomies performed by laser and nonlaser techniques. Each group reported no difference in pregnancy outcome.
TABLE 7. Pregnancy Outcome After Surgical Treatment of Complete Distal
Tubal Obstruction
| Laparotomy/ | Macrosurgery/ |
| Total No. | No. Term | Pregnancy |
| Laparoscopy | Microsurgery | Laser | Patients | Pregnant | (%) |
Wallach38 | Laparotomy | Macro | - | 24 | 5 | 21 |
O'Brien40 | Laparotomy | Macro | - | 80 | 20 | 25 |
Betz13 | Laparotomy | Macro | - | 20 | 1 | 5 |
Grant41 | Laparotomy | Macro | - | 103 | 12 | 12 |
Gomel82 | Laparotomy | Micro | - | 41 | 11 | 27 |
DeCherney83 | Laparotomy | Micro | - | 54 | 14 | 26 |
Frantzen44 | Laparotomy | Micro | - | 85 | 12 | 14 |
Kitchin84 | Laparotomy | Micro | - | 103 | 26 | 25 |
Donnez42 | Laparotomy | Micro | - | 83 | 26 | 31 |
Boer-Meisel85 | Laparotomy | Micro | - | 108 | 22 | 20 |
Mage86 | Laparotomy | Micro | + | 38 | 9 | 24 |
| Laparotomy |
| - | 30 | 5 | 17 |
Tulandi87 | Laparotomy | Micro | - | 30 | 6 | 20 |
|
|
| + | 37 | 9 | 24 |
Daniell81 | Laparotomy | Micro | + | 48 | 9 | 19 |
Kelly88 | Laparoscopy | Micro | + | 28 | 2 | 7 |
Fayez46 | Laparoscopy |
| - | 19 | 0 | 0 |
Daniell89 |
|
| + | 22 | 3 | 14 |
Performance of neosalpingostomy at laparoscopy has been reported by several groups.36,40,89,91 Pregnancy results appear to be less than for comparable reports at laparotomy. However, the patient selection for neosalpingostomy at laparoscopy includes women who refuse laparotomy, and therefore the patient populations may not be comparable.
The relatively poor pregnancy outcome after neosalpingostomy, compared with other types of reconstructive pelvic surgical procedures, and despite re-establishment of tubal patency in most patients, is thought to be caused in large part by intraluminal tubal pathology. The presence of coexistent pelvic adhesions, which restrict tubal motility and ovum capture, has also been implicated in reduction of successful pregnancy outcome.
Intraluminal tubal abnormalities primarily represent the pathologic sequelae of infection, although changes can also occur in response to endometriosis or exposure to diethylstilbestrol (DES)92 in utero. Chronic postinfectious histologic intraluminal changes observed include the presence of intraluminal adhesions, loss of villous folds, and ciliary destruction. It is unclear whether the latter is caused by the increased hydrostatic pressure resulting from tubal occlusion or is an effect of the infectious process itself.
Classifications of pregnancy outcome after neosalpingostomy have been based on intratubal and extratubal characteristics. Screening for these characteristics, to assess the likelihood of establishing a pregnancy, is performed by hysterosalpingography and laparoscopy, respectively. Rock and colleagues93 developed a classification of distal tubal obstruction based on hydrosalpinx diameter, rugal pattern, extent of fimbrial damage, and extent of coexistent pelvic adhesions (Table 8); they were able to demonstrate a deterioration in outcome as the extent of disease progressed from mild to moderate to severe.
TABLE 8. Rock Classification of the Extent of Tubal Disease With Distal
Tubal Obstruction
Extent of |
|
Disease | Findings |
Mild | Absent or small hydrosalpinx |
| Inverted fimbria easily recognized when patency achieved |
| No significant peritubal or periovarian adhesions |
| Preoperative hysterogram reveals a rugal pattern |
Moderate | Hydrosalpinx 15–30 mm diameter |
| Fragments of fimbria not readily identified |
| Periovarian and/or peritubal adhesions without fixation, minimal cul-de-sac adhesions |
| Absence of rugal pattern on preoperative hysterogram |
Severe | Large hydrosalpinx |
| No fimbria |
| Dense pelvic or adnexal adhesions with fixation of the ovary and tube to either the broad |
ligament, pelvic |
|
| side-wall omentum, and/or bowel |
| Obliteration of the cul-de-sac |
| Frozen pelvis (adhesion formation so dense that limits of organs are difficult to define |
More recently, Boer-Meisel and coworkers85 used a system for classification of hydrosalpinges to establish intrauterine pregnancy rates with good, intermediate, and poor outcome (77%, 21%, and 3%, respectively). The factors used for this analysis were the nature and extent of pelvic adhesions, the hydrosalpinx diameter, the hydrosalpinx tubal wall thickness, and the macroscopic assessment of the condition of the endosalpinx.
After surgical correction of complete distal tubal obstruction, the most likely time to conceive is more than 1 year after the surgical procedure.42,82,94 This time lag is thought to be required because of the tubal mucosa needs to regenerate after the damage that occurred while a hydrosalpinx was present. According to two preliminary descriptions, the surgery-to-conception interval after neosalpingostomy can be reduced if the procedure is performed with the CO2 laser;81,95 however, no further substantiation of this possibility has been forthcoming.
New diagnostic techniques to predict the likelihood of establishing a pregnancy after distal tubal obstruction are tuboscopy and fimbrial biopsy. In the former technique, a narrow-diameter scope or fiber is passed into the fimbrial ostium at the time of laparoscopy or laparotomy, or through the tubal cornua, which is visualized hysteroscopically. This allows direct examination of the intraluminal anatomy and identification of adhesions, areas of deciliation, and flattening of the mucosal folds. In early studies with this technique, the findings correlated significantly with subsequent pregnancy outcomes and therefore may be helpful in referral of patients for tubal repair and ART.96,97,98,99,100,101 This may particularly be the case as technologies for transcornual viewing are improved, making this an office procedure. Therefore, tuboscopy may be beneficial in preoperative patient screening. Regarding fimbrial biopsy, Donnez and associates42 showed that the ciliation index obtained on microbiopsy was impaired in the presence of tubal disease and that the index was predictive of subsequent pregnancy outcome.
Distal Tubal Obstruction: Incomplete
Comparison of outcome after surgical treatment of incomplete distal tubal obstruction is difficult because of the diversity of pathology it may represent. The clinical findings range from slight fimbrial agglutination to peritubal bands to fimbrial phimosis causing near-total occlusion and can occur in association with varying degrees of pelvic adhesions. Therefore, analysis of efficacy of treatment of incomplete distal tubal obstruction must be carefully scrutinized. A summary of pregnancy outcomes after such procedures is shown in Table 9.102 These results tend to be better than those for complete distal obstruction, probably reflecting less intratubal damage. Although follow-up is limited, surgical treatments with the CO2,103 argon,36 KTP-532,104 and Nd:YAG105 lasers have been described; their use has not been associated with improved pregnancy rates. Few series describe pregnancy outcome after laparoscopic treatment of incomplete fimbrial obstruction; therefore, analysis of the relative efficacies of laparoscopy and laparotomy as methods of treatment is premature.
TABLE 9. Pregnancy Outcome After Surgical Treatment of Partial Distal Tubal
Obstruction
| Laparotomy/ |
| Total No. | No. Term | Percentage |
| Laparoscopy | Laser | Patients | Pregnant | Pregnant |
Frantzen44 | Laparotomy | - | 49 | 11 | 22 |
Patton102 | Laparotomy | - | 40 | 25 | 63 |
Donnez42 | Laparotomy | - | 132 | 79 | 60 |
Diamond103 | Laparotomy | + | 24 | 9 | 38 |
Fayez46 | Laparoscopy | - | 14 | 3 | 21 |
Endometriosis
Endometriosis has been reported to contribute to infertility, not only by causing mechanical barriers to sperm-oocyte interaction but by a diverse group of other mechanisms as well. These alternative mechanisms include effects on ovarian steroidogenesis, ovulation, tubal ovum pickup, tubal motility, tubal ciliary action, and peritoneal macrophage activity. In part, these effects are thought to be mediated by prostaglandins.
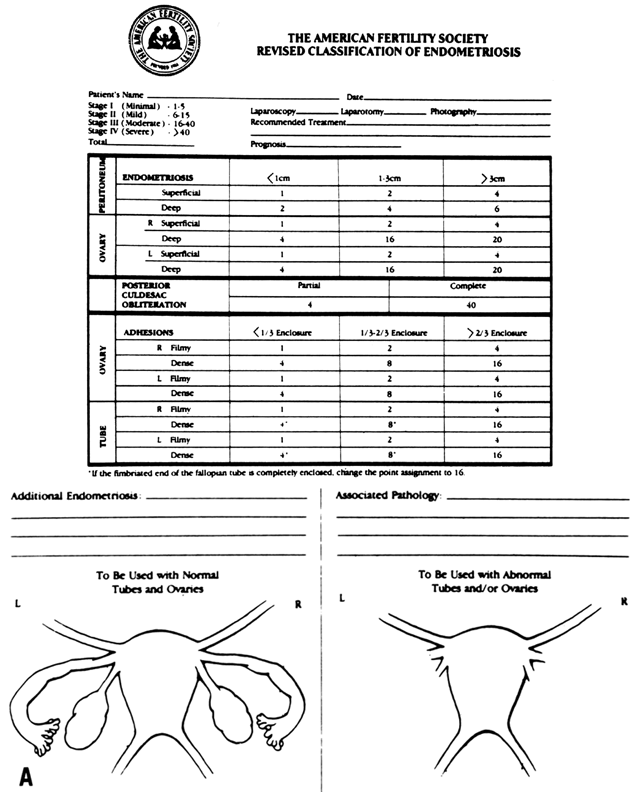
Various systems have been used to classify endometriosis.106,107,108,109 Most early reports used the Acosta classification,106 grouping cases into mild, moderate, and severe stages based on the location and extent of endometriosis and the degree of pelvic adhesions. Classifications by Kistner and associates107 and by the American Fertility Society (now called the American Society for Reproductive Medicine) were subsequently established,108 followed by a revised classification by the latter group in 1985,109 which is pictured in Figure 1. Use of classifications is advantageous because the scoring confers an extent of disease, allowing comparison of reports from different investigators and centers.
The relative efficacy of different methods of treating endometriosis is not well defined; in some reports, pregnancy outcome was no different with observant management than it was with medical or surgical treatment.110,111 However, a recent large, well-designed, multicenter trial did identify improvement in pregnancy outcome after surgical treatment of mild endometriosis.111a The most widely used medical agents are danazol112,113 and gonadotropin-releasing hormone (GnRH) analogues.114,115 These agents are used alone for treatment of mild and moderate endometriosis, and in combination with surgery (both preoperative and postoperative) for treatment of these stages as well as severe endometriosis. The relative efficacies of these varied forms of treatment and the appropriate indications for concomitant therapy currently are unclear. An in-depth discussion of studies describing the medical and medical-surgical treatment of endometriosis is beyond the scope of this chapter; the reader is referred to published reports.111a, 112,113, 116,117,118
Pregnancy outcome after treatment is the most important end point for infertile couples; an assessment of outcome is given in Table 10.119,120,121,122,123,124,125,126,127,128,129,130 Considering the use of laparoscopy for reproductive pelvic surgery, more series have been described for the treatment of endometriosis than for other procedures. From the numbers in these reports (which are limited in part by lack of comparable life table analyses of outcome), it appears that, in experienced hands treatment at laparoscopy and at laparotomy are equally efficacious, not only for mild and moderate endometriosis but for severe and extensive endometriosis as well. This observation is consistent with a recent extensive meta-analysis of equivalent pregnancy outcome after treatment at laparoscopy and at laparotomy.130a Individual investigators131,132 have treated large endometriomas laparoscopically with high pregnancy rates resulting. In many series of laparoscopic treatments for endometriosis, the CO2 laser has been used.123,124,125,126,127,128,129 However, similar pregnancy rates obtained with nonlaser laparoscopic techniques have also been described.130 Other lasers that have been used for laparoscopic treatment of endometriosis are the argon,133 KPT-532,104 and Nd:YAG,134,135 types. Nevertheless, pregnancy outcome as an end point has its limitations, in that many factors can impair fertility after adequate treatment of endometriosis; furthermore, pregnancy can occur after inadequate treatment of endometriosis, and pregnancy can occur despite recurrence or persistence of endometriosis. Several investigators have tried to correct for other possible factors by looking at pregnancy outcome in women in whom endometriosis is the sole cause identified for infertility; slight improvements in pregnancy outcome have been identified.33
TABLE 10. Pregnancy Outcome After Surgical Treatment of Endometriosis
Click here to view Table
10.
Endometriosis is noted to be present in 2% to 47% of women after surgical or medical treatment,136 the actual likelihood depending, in part, on the initial severity of endometriosis and the treatment undertaken. However, it has been difficult to differentiate recurrent from persistent endometriosis. Three types of reports suggest that persistence may be more likely than previously recognized. First, microscopic implants of endometriosis not visible to the naked eye (or to the eye with magnification) are frequently present.137,138,139 Second, many implants do not have the gross purplish macroscopic appearance that has become clinically recognized as endometriosis; they may be clear, yellow, red, or black, or they may be represented by converging vascular patterns or white scarring.140 Third, recognized implants of endometriosis may extend from the identified lesion beneath the peritoneum.141 For the latter, it has been suggested that visualized lesions be treated so as to include the immediately surrounding (normal-appearing) peritoneum. The actual surface area that should be treated, and whether such treatment affects rates of recurrence or persistence or pregnancy outcome, have not been established.
The extent of recurrence or persistence of endometriosis is best assessed at second-look laparoscopy; however, this requires performance of an additional operative procedure. Clearly, a nonoperative method of assessment would have tremendous advantages. A potential candidate is the serum marker is CA-125, a membrane antigen that is present in many conditions, including epithelial serous ovarian carcinoma.142,143 The antigen concentration is elevated in women with endometriosis, and initial studies suggest the level correlates with the severity of disease and with its clinical course.142,143 Another technique that has been evaluated is an endometrial antibody assay.144 Although only a small number of subjects were examined, the antibody level did appear to correlate with observations at second-look laparoscopy. Additional studies are required to further examine the potential of these markers.
Tubal Anastomosis and Implantation for Tubal Obstruction
An overview of pregnancy outcome after tubal anastomosis and tubal implantation procedures is shown in Table 11.145,146,147,148,149,150,151,152,153,154,155,156 Compared with other types of reproductive pelvic surgical procedures, pregnancy outcome is best after tubal anastomosis, approaching 70% in many series. Many factors affect the outcome of surgical procedures designed to re-establish tubal continuity, including the cause of the obstruction, its location, the extent of coexistent pelvic disease, the length of the resulting tube after patency is restored, and the length of time since tubal blockage.
TABLE 11. Pregnancy Outcome After Tubal Anastomosis/Tubal Implantation
|
|
| No. Term |
Percentage |
|
|
|
| Macrosurgery/Microsurgery | Total No. Patients | Pregnant |
Pregnant |
|
|
|
Anastomosis |
|
|
|
Wallach38 | Macrosurgery | 18 | 9 |
50 |
|
|
|
Siegler145 | Macrosurgery | 17 | 5 |
29 |
|
|
|
Hodari146 | Macrosurgery | 14 | 7 |
50 |
|
|
|
Jones147 | Macrosurgery | 12 | 10 |
84 |
|
|
|
McCormick148 | Macrosurgery | 53 | 25 |
47 |
|
|
|
Diamond14 | Macrosurgery | 12 | 3 |
25 |
|
|
|
| Microsurgery | 28 | 16 |
57 |
|
|
|
Frantzen44 | Microsurgery | 28 | 12 |
43 |
|
|
|
Donnez149 | Microsurgery | 82 | 36 |
44 |
|
|
|
McComb150 | Microsurgery | 26 | 15 |
56 |
|
|
|
Diamond151 | Microsurgery | 46 | 25 |
54 |
|
|
|
Gomel152 | Microsurgery | 118 | 76 |
64 |
|
|
|
Meldrum153 | Microsurgery | 32 | 21 |
84 |
|
|
|
DeCherney10 | Microsurgery | 129 | 73 |
57 |
|
|
|
Lavy154 | Microsurgery | 25 | 9 |
36 |
|
|
|
Implantation |
|
|
|
Wallach28 | Macrosurgery | 7 | 0 |
0 |
|
|
|
Grant41 | Microsurgery | 73 | 18 |
25 |
|
|
|
Williams155 | Macrosurgery | 681 | 70 |
10 |
|
|
|
Levinson156 | Macrosurgery | 35 | 21 |
60 |
|
|
|
Winston7 | Microsurgery | 14 | 3 |
21 |
|
|
|
Among women desiring reversal of previous tubal ligations, the method of tubal occlusion is a major prognostic factor in subsequent pregnancy outcome. The best results are obtained with Hulka clips or fallope rings; the worst outcome follows unipolar cautery, and intermediate pregnancy rates are achieved after segmental resection or bipolar cautery. These differences may be partially explained by the resultant length of tube after anastomosis, increasing tubal length correlating directly with improved pregnancy outcome. An additional factor may be the extent of damage to the tubal blood vessel arcade; both human and animal studies have suggested a possible role of this network in ovulation and pregnancy outcome. In tubocornual anastomosis, an additional advantageous prognostic factor is preservation of as much of the intramural segment of the fallopian tube as possible.149
The length of time between tubal ligation and reversal has been shown to be a prognostic factor, correlating inversely with subsequent pregnancy outcome. However, it is likely that this relation reflects the natural decline in fecundity with advancing maternal age, as opposed to an effect on the tubes per se.
DeCherney and coworkers10 examined the causes of failure to conceive in 35 women who underwent midsegment tubal anastomosis. A surgically related factor (bilateral tubal reocclusion) was noted in only 20% of these couples. In the remaining couples, ovulatory dysfunction was present, the husband had not previously fathered a child, or the infertility was unexplained.
Location of tubal occlusion is another major prognostic factor. Those women with tubal blockage that allows anastomosis to be performed generally have improved conception rates, compared with those requiring tubal implantation. In their in-depth review of surgical management of uterotubal obstruction, Musich and Behrman157 concluded that microsurgical anastomosis has replaced implantation as the primary method for re-establishment of tubal patency under most circumstances. Exceptions were surgeon preference and complete intramural occlusion. In a review of all series in the literature from 1965 to 1982 describing pregnancy outcome after uterotubal implantation and tubocornual anastomosis, Haseltine and Von Arras158 demonstrated improved pregnancy rates after anastomosis. Additionally, anastomosis obviates the decision of whether a conception occurring after a tubal implantation procedure should always be delivered by cesarean section and reduces the risk of uterine rupture at the site of implantation, a complication that has been described with the implantation technique.159
Women who have undergone fimbriectomy for sterilization represent a special category because the surgical correction required is a neosalpingostomy. In two small series of such women, pregnancy occurred in 57% (4/7)13 and 44% (4/9) of patients.160
Lasers have seen limited application in tubal anastomosis. Kelly and Roberts88 described excision of blocked tubal stumps with the use of a CO2 laser. It is unclear whether this procedure has any advantages over nonlaser techniques. Other investigators have attempted to anastomose the fallopian tube by “welding” with the CO2 laser but have noted this technique to be of little value.161
The feasibility of laparoscopic tubal anastomosis has now been demonstrated by Gray et al.205 It remains to be determined whether pregnancy outcome in such patients can approach the success observed at laparotomy.
 15 mm diameter
15 mm diameter 30 mm diameter
30 mm diameter