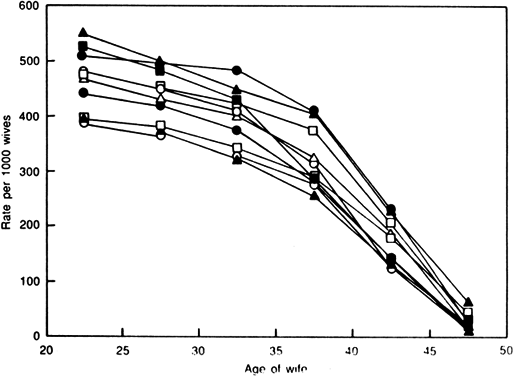
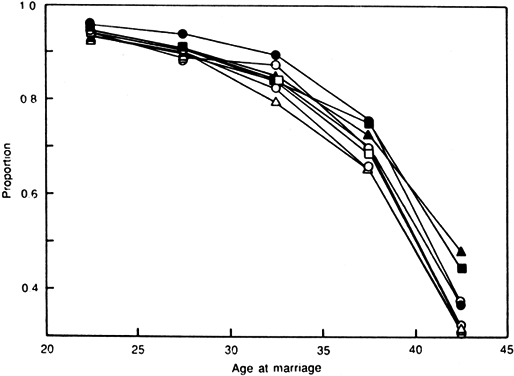
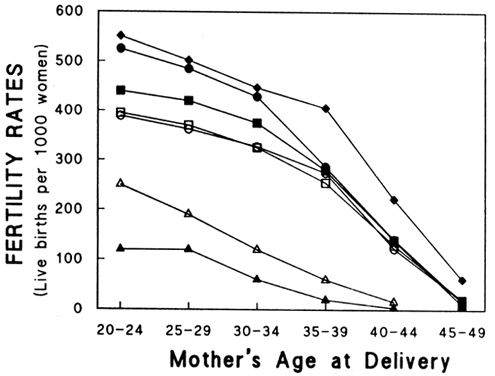
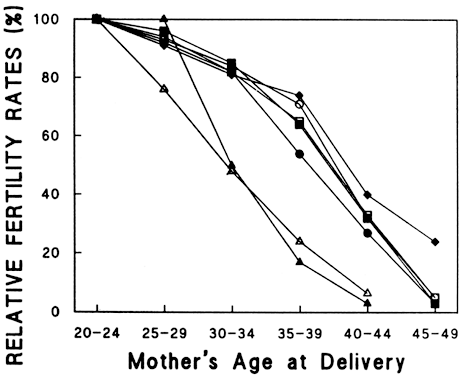
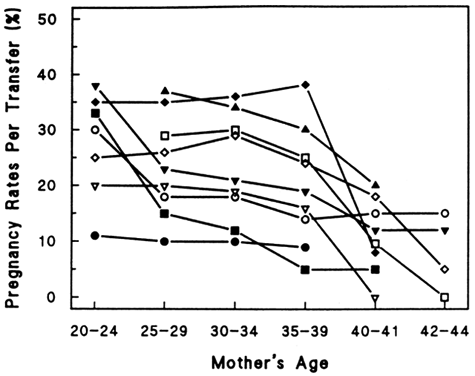
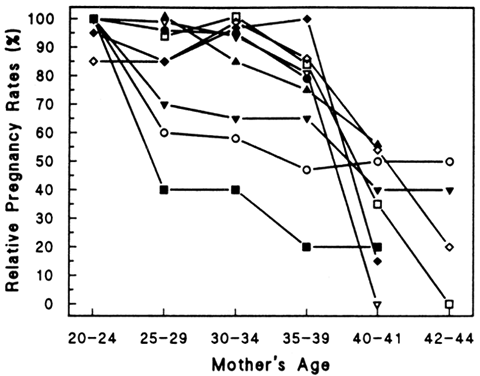
Aging affects all organs of the human body, but each organ is affected
to different degrees. Some organ systems, although adversely affected
by age, may be functionally restored through medical intervention. Multiple
theories explaining the mechanisms of aging have been described
over the years. No single theory provides an inclusive explanation. Although
a thorough discussion of each of the theories is beyond the scope
of this chapter, some include age-related changes in chromosome exchange
and gene expression, programmed cell death and apoptosis, reactive
oxygen species and mitochondrial DNA damage, and telomere shortening. These
theories and their application to general physiologic aging and
ovarian aging continue to receive considerable attention within the
research community. In the near future, one or more of these theories
may enhance our understanding of female reproductive aging. In the following sections, we consider the role of genetics in female reproductive
aging. We then examine various general pathologic conditions
and environmental exposures that occur with increasing frequency as
women age that may influence reproductive aging. We evaluate the age
effect of the organs that are directly related to the reproductive system, such
as the adrenal glands, hypothalamus, pituitary gland, fallopian
tubes, uterus, and ovaries. Genetics So complex are the determinants of female fecundity that even the intrauterine
environment in which a female fetus develops may profoundly affect
her reproductive potential and the timing of her onset of menopause.35,36 In a comprehensive update on premature ovarian failure (POF), Anasti summarized
the relation of genetic disorders and future fecundity.37 He emphasized that many of the causes of POF are related to chromosomal
abnormalities that may exert their effect in utero on germ cell migration, oocyte proliferation, oocyte depletion, and other
complex mechanisms. For instance, the POF associated with Turner's
syndrome is caused by accelerated follicular atresia during late
fetal development. Other chromosomal abnormalities may lead to follicle
dysfunction, enzyme deficiencies, or signal defects. Numerous other
inherited genetic abnormalities have long been known to cause infertility
and POF. Moreover, investigators have shown a correlation between
the age of onset of menopause and more subtle genetic factors through
family studies. For instance, Torgerson and colleagues showed a correlation
between mothers and daughters and the age of onset of menopause.38 Likewise, Cramer and coworkers demonstrated an association between sisters
with POF and the age of onset at menopause.39 The cumulative data rather convincingly suggest that a woman's endowed
follicular pool, which provides the foundation for her reproductive
potential, is established by events occurring at the time of fetal
formation. From then on, her reproductive potential is modified continuously
throughout her life. Pathologic Conditions As women age, they experience an increase in exposure to an assortment
of pathologic processes. For example, the number of women who develop
uterine fibroids increases with age. As many as 20% to 50% of women may
have uterine fibroids, and the incidence increases with age.40 Uterine fibroids have been implicated in infertility.41 As increasing numbers of women delay childbearing until later years, fibroids
are becoming an increasingly important problem. Fertility increases
after surgical removal of uterine fibroids in natural populations.42–44 Women who have submucosal or intramural fibroids demonstrate a significant
reduction in implantation rate and pregnancy rate when undergoing
ART.45 By working through the logic of such arguments, it becomes clear that
these pathologic processes are likely to adversely influence a woman's
ability to conceive. Similarly, endometriosis represents another
pathologic process related to aging and impaired fertility. Endometriosis is a common gynecologic condition that is found in 3% to 10% of
the general population in the reproductive-age group and 25% to 35% of
an infertile population.46,47 The relation between endometriosis and infertility is well supported by
the literature.48 The incidence of endometriosis increases with increasing age.49,50 Physicians can expect to see an increase in endometriosis-associated infertility
as women delay childbearing. Westrom identified another interesting
finding associated with impaired fertility and aging through
his work on pelvic inflammatory disease.51 He found that the incidence of postinfection infertility after the first
exposure was significantly higher in women 25 to 34 years of age compared
with women between the ages of 15 and 24. This age difference was
eliminated, however, when women had multiple episodes of infection. Still
another interesting age-related problem is demonstrated through
the increased incidence of ectopic gestation found in women of advancing
maternal age. There is a threefold increase in ectopic pregnancies
in women between the ages of 35 and 44 compared with women aged between 15 and 24 years
old.52,53 Over time, cumulative exposure to these pathologic conditions and more
likely contribute to the age-related decline in female reproductive capacity. In addition to specific pathologic processes directly related to the reproductive
tract that lead to impaired fertility, a host of medical conditions
caused by the effects of aging on other endocrine organ systems
may indirectly lead to impaired fertility. An example is provided by
diabetes. The incidence of type II diabetes increases with advancing
age.54,55 We know that even impaired glucose tolerance, a condition often present
before the diagnosis of overt diabetes is made, has a negative impact
on ovarian function and ovulation.56,57 Another example is thyroid disease. The incidence of thyroid disease in
women increases significantly with age.58,59 And thyroid disease is known to be associated with oligomenorrhea, amenorrhea, menorrhagia, and
anovulation, leading to impaired fertility.60,61 A theme is established that demonstrates the negative influence of aging
on reproduction. This theme continues as we examine the impact of continually
increasing exposure to various external environmental factors
as women age. Effects of Environmental Exposures With increasing age, women are exposed to potential environmental toxins
that are likely to impair fertility. An increasing number of women are
exposed to toxins in the workplace and the environment.62 More than 50 synthetic chemicals that are ubiquitous in the environment
have been implicated as reproductive toxins.63 Sharara and colleagues describe the biologic basis of adverse effects
of environmental toxins on female reproduction and discuss the mechanisms
of action of environmental toxins. Although they acknowledge that
some environmental chemicals are present in low concentrations or have
low affinities to induce biologic responses, they highlight the fact
that many environmental toxins are lipid soluble, and over decades, exposure
may be significant because of bioaccumulation in the food chain. Chronic
exposure as women age may result in adverse reproductive outcomes. They
also discuss endocrine disruptors, heavy metals, solvents, industrial
chemical, and pesticides. Perhaps most importantly, they discuss
cigarette smoking and the effects of active and passive smoking
on female reproduction. Active and passive exposure to cigarette smoke increases with age, and
to quote Sharara, “Cigarettes represent the ultimate legal delivery
system of hundreds of reproductive toxicants and carcinogens. Cigarette
smoke contains more than 4000 chemical compounds, including 43 carcinogens
or poisons and more than 300 polycyclic aromatic hydrocarbons.” Smoking
among women is beginning at an earlier age, and they
are smoking more cigarettes on average.64 The effect of cigarette smoking on female reproduction has consistently
demonstrated impairment of fertility and fecundity in natural populations
and populations undergoing ART. For instance, Van Voorhis and coworkers
reported, for women undergoing ART, a 50% reduction in implantation
rate and ongoing pregnancy rate in active smokers compared with
nonsmokers.65 In natural populations, fecundity decreases, and the incidence of spontaneous
abortion increases.66,67 Ovarian reserve appears to be adversely affected by cigarette smoking.68 Ovarian reserve in smokers between the ages of 35 and 39 is decreased
compared with that of nonsmokers between the ages of 35 and 39.69 Women who smoke reach menopause 1 to 4 years earlier than their nonsmoking
age-matched counterparts.70 These last two factors imply that smoking accelerates follicular loss
and results in earlier entry into the perimenopause, accelerating the
age effect on fertility and fecundity. Additional adverse exposures include radiation and chemotherapeutic agents, which
are known to have deleterious effects on female reproduction, and
the insult is more profound with advancing age.71 Independent of any of the aforementioned exposures, all human organ systems
undergo adverse age-related changes. We focus on the age-related
changes of specific organ systems. Adrenal Gland Changes The adrenal gland is a vital organ to the endocrine system and the adrenal
gland synthesizes and secretes several hormones that are related to
the reproductive system. The adrenal glands produce and secrete many
of the same hormones that are concurrently produced and secreted by the
ovaries. Various hyperandrogenic adrenal disorders such as congenital
adrenal hyperplasia and late-onset adrenal hyperplasia have a detrimental
effect on female reproduction. However, little is known about the
age-related decrease in adrenal androgen production and its effect, if
any, on the age-related decline in female reproduction. Because adrenal
steroidogenesis is markedly different between species, it is difficult
to find an animal model to study that resembles human adrenal physiology. The
cause of the changes in adrenal activity that occur with
age are not clearly understood, but what little is known about the age-related
changes in adrenal function does not implicate the adrenal
gland as having a major role in the age-related decline in reproduction. Early in life, adrenal androgen production is quite low. Adrenarche marks
the onset of a significant increase in the production of adrenal steroids
and is independent of gonadarche.72 Once adrenarche occurs, adrenal androgen production rises steadily until
around the age of 25, at which time an irreversible decline ensues.73–75 The primary circulating androgens include androstenedione, testosterone, dehydrotestosterone (DHT), dehydroepiandrosterone (DHEA), and dehydroepiandrosterone
sulfate (DHEAS). The adrenal glands and the ovaries
in equal amounts produce androstenedione. Approximately 25% of testosterone
is produced by the adrenal glands, 25% is produced by the ovaries, and
the remaining 50% is derived from the peripheral conversion of
androstenedione to testosterone. Androstenedione and testosterone are
converted to DHT in the liver and the skin. About 90% of DHEA is produced
in the adrenal glands, and 10% is produced by the ovaries, whereas
DHEAS is produced almost exclusively in the adrenal glands. With the
adrenal glands contributing a significant amount of these steroid hormones
to the circulating pool of androgen hormones in the endocrine environment, age-related
changes in the adrenal glands may be expected to
contribute to the age-related changes in reproductive system. One of the principal secretory hormones of the adrenal glands is cortisol. Circulating
levels of cortisol do not change with age.76 The adrenal glands respond normally to corticotropin stimulation with
aging.77 On the contrary, the adrenal androgens do demonstrate age-related changes, but
it becomes important to try to separate the ovarian and adrenal
contribution to this decline before implicating either organ. For instance, beginning
in the mid-twenties, androstenedione levels progressively
decrease.78 Because the ovarian and adrenal contribution of androstenedione is split, oophorectomized
women were studied as well. Although androstenedione
levels decreased with advancing age, these levels did not change significantly
until women were in their early fifties at the time of the
menopausal transition.74 The levels also reach a nadir and plateau in women when they reach their
sixties. Androstenedione levels do not decrease significantly when
correlated to the time of last menses, thereby suggesting that the ovarian
contribution of androstenedione is already minimal by the time menopause
is reached.79 Testosterone levels decline with age. Most testosterone is derived from
the conversion of DHEA and DHEAS to testosterone, and although testosterone
levels change slowly through the perimenopause, the 24-hour testosterone
levels do change significantly with age.75 Circulating DHEA and DHEAS peak in the mid-twenties and begin to drop
independent of menopause or oophorectomy.80–82 The reason these adrenal androgens fall with age has been studied intensively, and
it does not appear to be caused by changes in the metabolic
clearance rate or the peripheral metabolism of these adrenal androgens.83–87 There is no change in adrenal androgen secretion because of a decrease
in the size of the adrenal cortex with age.88 Because oophorectomy does not increase the changes in adrenal androgen
secretion, it is unlikely that increasing gonadotropins are responsible
for the adrenal changes. Although elevated androgens are known to disrupt
the hypothalamic-pituitary-ovarian axis, the decreasing levels
of adrenal androgens do not seem to have a significant impact on the hypothalamic-pituitary-ovarian axis or reproductive aging as far as indirect
evidence suggests. Hypothalamic-Pituitary Changes The menstrual cycle is controlled by the neuroendocrine system. The cyclic
nature of the menstrual cycle requires an exquisitely coordinated
endocrine system that is intricately regulated through feedback signals. The
highest signals are generated through hypothalamic-pituitary interactions. In
the past, studies of the hypothalamicpituitary interaction
have been performed primarily in rodents, but rodents exhibit regulatory
mechanisms that are entirely different from humans. Because of
this, extrapolation of biologic mechanisms of hypothalamic-pituitary aging
characteristic of rodents to humans is somewhat limited. In humans, the
first sign of reproductive aging is a rise in follicle-stimulating
hormone (FSH) levels independent of luteinizing hormone (LH) levels (monotropic
FSH rise).89,90 Whether this change is caused by intrinsic aging at the level of the hypothalamic-pituitary
system or to a change in responsiveness of the hypothalamic-pituitary
system to feedback signals remains uncertain.During
the past few years, we have started to understand some of the characteristics
of hypothalamic-pituitary interactions in humans. In monkeys, the
monotropic FSH rise can be induced by altering the gonadotropin-releasing
hormone (GnRH) pulse generator.91 It has been postulated that changes in the frequency or amplitude of pulses
secreted from the GnRH pulse generator might explain this phenomenon
in humans, but Klein and coworkers were unable to demonstrate any
differences in LH pulse frequency, LH pulse amplitude, or mean LH levels
across menstrual cycle phases when comparing younger and older women.92 It is generally accepted that the LH secretion pattern accurately reflects
the secretion pattern of the GnRH pulse generator.93 Alexander and coworkers also examined basal pulsatile LH secretion by
monitoring pulse frequency and amplitude and failed to demonstrate a difference
between younger and older oophorectomized women.94 Other investigators have studied the stores of GnRH to determine whether
differences exist between younger and older women. Parker and coworkers
studied the hypothalamic content of GnRH stores in young premenopausal
women and postmenopausal women. Postmenopausal women demonstrated
significantly less GnRH secretion than the younger premenopausal women. This
finding implies a decrease in synthesis or storage of GnRH in
older women. However, the hypothalamic content of GnRH was also measured
in younger women who had undergone bilateral oophorectomy, and they
demonstrated lower GnRH content than that of the normal younger women.95 Other investigators have looked at the bioactivity of the FSH molecule, suggesting
that intrinsic changes in the molecule may demonstrate different
activity, but the bioactivity does not differ with advancing age.96 The study also found no difference in ovarian steroid secretion of estradiol
and progesterone; however, it did show accelerated recruitment
and ovulation of a dominant follicle. This observation is consistent with
Treolar's findings of a shortening of menstrual cycle length
in women of advancing reproductive age.97 This occurs before there is a lengthening of menstrual intervals, followed
by the eventual cessation of menstrual flow. Other investigators
have also shown shortening of the menstrual cycle.98 The hypothalamic-pituitary axis retains its responsiveness to ovarian steroids
and its sensitivity to positive and negative feedback mechanisms.99 The pituitary gland undergoes morphologic changes with aging, but these
changes do not alter the functional units of the gland.100,101 Several investigators have convincingly shown that the monotropic FSH
rise is associated with decreasing inhibin-B and inhibin-A levels in women, suggesting
a decrease in the negative feedback mechanisms of the
ovary on pituitary gonadotropin secretion.102–104 These changes do suggest that a deterioration of ovarian follicular function
lead to the monotropic FSH rise and the subtle changes in the menstrual
cycle. Ultimately, hypothalamic-pituitary changes do not seem
to initiate the cascade of reproductive failure that is associated with
aging, but this idea requires confirmation. Fallopian Tube Changes The fallopian tubes represent another reproductive organ influenced by
aging, but they are not implicated as a major contributing factor associated
with reproductive failure. Studies have demonstrated an increased
incidence of ectopic gestation with advancing maternal age.105–107 One reason for this may be that tubal function is in part regulated by
cyclic ovarian steroid secretion and steroid secretion becomes increasingly
irregular as women approach the menopause. Several investigators
have studied the electrical activity and contractility of the fallopian
tubes, and they have demonstrated a progressive decline in tubal motility
with advancing age.108–111 Although there does seem to be changes in tubal function, the data also
suggest that these changes may be related to ovarian dysfunction and
inadequate luteal phase steroid secretion rather than inherent changes
in the fallopian tubes.108,112 The increased incidence of ectopic pregnancy seen with advancing age may
be more related to ovulatory dysfunction than inherent deterioration
of fallopian tube function. Uterine Changes There has been considerable debate about whether uterine receptivity deteriorates
with advancing age. Several explanations have been offered
implicating endometrial receptivity as a major contributor to declining
fecundity with advancing age. For instance, some investigators have
proposed that an age-related decline in uterine perfusion may contribute
to the decline in fecundity.113,114 Uterine vascular changes such as fibrosis may lead to changes in blood
flow through the uterus.115 Sterzik and coworkers reported an increasing number of out-of-phase endometrial
linings in women older than 35 years of age during unsuccessful
IVF cycles.116 Others have not found any differences in the expression of receptors, cellular
proliferative index, vascular patterns, histology, and other
characteristics in women of advancing age compared with younger women.117–119 A major obstacle facing investigators studying age-related effects on
human reproduction is to distinguish between intrinsic age-related changes
in endometrial receptivity and intrinsic changes in other organ systems
that result in changes in endometrial receptivity. Until the advent of donor egg programs, it was impossible to pinpoint the
organ system most likely responsible for the age-related changes in
fecundity in women of advanced maternal age. The reason for this is that
oocyte quality (ovarian derived) could not be separated from endometrial
quality (uterine derived) in women of advanced reproductive age
using autologous eggs. Convincing data support the hypothesis that oocyte
quality decreases with increasing age.120–122 Deriving oocytes from young women enabled investigators to study these
two variables independently by optimizing the egg quality for all age
groups, followed by an evaluation of endometrial receptivity. In the
late 1980s, programs using donor eggs for women with ovarian failure began
to publish their data on outcomes.123,124 Shortly thereafter, the use of donor eggs spread to perimenopausal women
and women who had failed multiple IVF cycles. Initially, investigators
used the data from their programs to explain the loss in fecundity
in women with advancing maternal age. Natural populations have not been
studied; only women undergoing oocyte donation have been studied. Initial reports were conflicting about whether endometrial receptivity
decreased with increasing age. Several independent investigators suggested
that the aging uterus and decreased endometrial receptivity were
responsible for the decline in fecundity in women of advancing maternal
age.125–128 At the same time, other investigators reported data suggesting that there
was no loss of endometrial receptivity in women of advancing maternal
age.129–133 Meldrum proposed that one of the reasons for the difference in outcomes
might be differences in the protocols used for hormone replacement.134 Differences in populations studied, sample size analyzed, and confounding
variables represent additional possible explanations for the conflicting
results. It has been substantiated in the literature that there
is no decline in endometrial receptivity with aging.120,135,136 Even when postmenopausal women are given appropriate hormone replacement, the
uterus maintains its ability to provide a receptive endometrium
and demonstrates normal histologic, ultrasonographic, and steroid receptor
response.137 Although the process of reproductive aging involves complex mechanisms
at the level of the hypothalamus, pituitary gland, fallopian tubes, and
endometrium, it is relatively clear that ovarian dysfunction and oocyte
depletion is primarily responsible for age-related reproductive failure. Ovarian Changes Throughout life, the functional activity of the ovary changes dramatically. Research
findings have enabled us to better understand the physiologic
basis for the decline in female fecundity and ultimately menopause. Once
thought to begin only a few years before menopause, ovarian dysfunction
begins well over a decade before the cessation of menses. Long
before the menopause, the ovary begins to undergo changes at the cellular
level that lead to abnormal steroid and glycoprotein production, abnormal
gamete formation, and impaired fertility in women as early
as their mid-thirties. Our understanding of the functional components
of ovarian follicular physiology has greatly advanced because of the
availability of granulosa cells from women undergoing ART.138 Our understanding of oocyte structural mechanics and physiology have improved
because of the availability of human oocytes from these women. Access
to human granulosa cells and oocytes has allowed investigators
to use in vitro methodologies to probe some of the mechanisms responsible for in vivo clinical outcomes. The quantitative changes of the ovarian follicular
pool associated with aging have been clearly demonstrated, and we are
able to demonstrate changes in quality as well. The changes that occur within the ovarian cortex throughout a woman's
life are primarily responsible for the decrease in her reproductive
potential, the increase in her menstrual cycle irregularities, and ultimately
the cessation of her menses. Tracing the embryologic development
of the ovarian cortex begins to highlight the intricate complexity
of this organ. Early in gestation, primordial germ cells, which are
of endodermal origin, migrate to the gonadal ridge. As the ovary forms, primordial
germ cells are converted to oogonia, which are localized
to the cortex. The ovarian cortex consists of tightly packed cells, throughout
which are scattered oogonia. Oogonia multiply many times through
mitotic activity. After the last mitotic division, DNA replication
occurs, and the oogonia are converted to oocytes as meiotic divisions
begin. Meiosis results in a reduction of the number of chromosomes, a
redistribution of chromosomes of maternal and paternal origin, and an
exchange of genetic material between some of the chromosomes. The prophase
of meiosis I has been divided into stages: leptotene, zygotene, pachytene, and
diplotene. It is in the diplotene stage of meiosis I that
the oocyte becomes arrested and remains quiescent for up to 50 years. Through continued mitotic and meiotic activity, the maximum number of oocytes
is reached at approximately 20 weeks' fetal gestation. That number
peaks at 6 to 7 million.139 Intervening cortical stroma, which is of mesodermal origin, migrates around
the oocytes and forms the primordial follicle.140 Early on, mitotic activity leads to the profound increase in oogonia, but
after meiosis and follicular formation has occurred, follicular atresia
begins and causes an enormous depletion of oocytes. Follicular atresia
is very similar to apoptosis (i.e. programmed cell death).141 By birth, only 1 to 2 million oocytes remain.142 All of the remaining oocytes progress through to the diplotene stage of
prophase meiosis I shortly after birth and remain there until the time
of ovulation.143 Follicular atresia continues, and approximately 300,000 oocytes remain
at the time of menarche.144 Of the remaining follicles, only 400 to 500 are ovulated over the next 30 to 40 years.145 At the time of menopause, nearly all of the oocytes have been depleted.146 The photomicrograph in Figure 7 illustrates the depletion of follicles occurring with advancing age.147 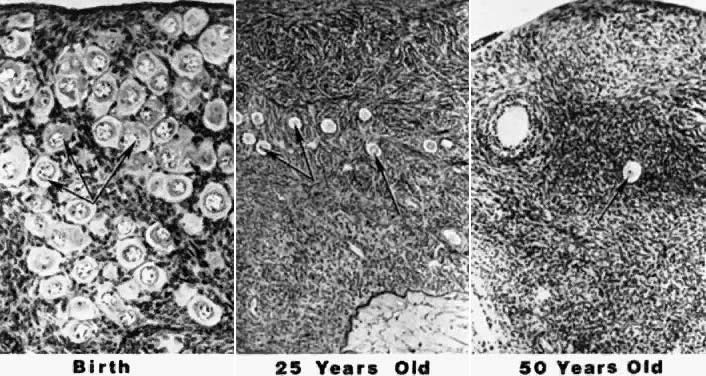 Fig. 7. Photomicrographs showing the progressive decrease in the number of primordial
follicles at different periods in the life of a woman. The process
of recruitment is the cause of the decrease.(From Erickson G: An analysis of follicle development and ovum maturation. Semin
Reprod Endocrinol 4:233, 1986.) Fig. 7. Photomicrographs showing the progressive decrease in the number of primordial
follicles at different periods in the life of a woman. The process
of recruitment is the cause of the decrease.(From Erickson G: An analysis of follicle development and ovum maturation. Semin
Reprod Endocrinol 4:233, 1986.)
|
Follicular atresia is a process that causes follicular depletion, and it
occurs in two settings. Monthly follicular atresia, a gonadotropin-dependent
process, occurs as a cohort of up to 50 follicles is recruited
each month resulting in the selection of a dominant follicle that progresses
to ovulate while the remaining follicles are resorbed. This process
accounts for less than 1% of the loss of the total endowed primordial
oocyte pool. More significantly, a tonic gonadotropin-independent
process of atresia occurs, causing hundreds of thousands of primordial
follicles to be continuously resorbed. This type of atresia occurs
even in the presence of oral contraceptives and pregnancy.148 This process is responsible for the loss of 80% of the endowed primordial
oocyte pool from 6 months in utero to birth and is responsible for the loss of 95% of a woman's endowed
oocyte pool by the time puberty is reached. Postpubertal follicular
atresia is a lifelong process on which monthly cyclic ovulating attrition
is superimposed. The age-related depletion of a woman's endowed
follicular pool is accompanied by the disruption of follicular granulosa
cell function and ovarian steroid production, particularly estrogen. A
reduction of ovarian steroid synthesis leads to a disruption
of cyclic ovarian function and ultimately to menopause. This is caused
by significant age-related changes in ovarian follicular physiology. The ovarian cortex contains numerous follicles that vary greatly in stages
of growth and degeneration. Figure 8 demonstrates the typical architecture of an ovarian follicle at different
stages of development. The complexity and size of the follicles also
vary greatly with the stage of development, but each follicle contains
an oocyte surrounded by epithelial cells. In the young adult woman, the
most commonly found follicles are primordial follicles. They are
made up of a primary oocyte and a few flattened follicular cells, pregranulosa
cells, enclosed by a basal lamina. Remaining in a resting stage, these
follicles are quiescent and essentially unresponsive to gonadotropins. A
large number of follicles are continuously leaving this stage
and undergoing sequential maturation. After growth is initiated, primordial
follicles become primary follicles. The follicular cells differentiate
into a complete layer of cuboidal cells, or granulosa cells. The
basal lamina separates these cells and the oocyte from the rest
of the ovary. The oocyte increases in size but remains in prophase meiosis
I. Granulosa cells manufacture and secrete a glycoprotein extracellular
matrix, which accumulates between the oocyte and the granulosa
cells, ultimately forming the zona pellucida.149 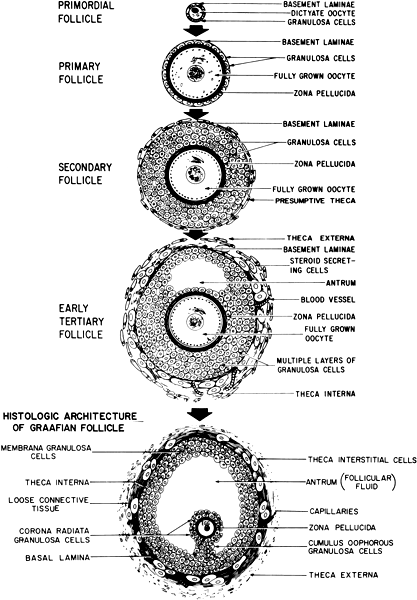 Fig. 8. Architecture and classification of ovarian follicles during folliculogenesis. Recruitment
occurs within the pool of nongrowing primordial follicles. Once
the oocyte is surrounded by a single layer of cuboidal cells, it
is reactivated and completes its growth and zona pellucida formation
early, at the primary-secondary follicle stage. Growth of the preantral
follicle is a consequence of granuloma proliferation and ends
with cavitation or antrum formation in the early tertiary stage. In response
to follicle-stimulating hormone, follicular fluid accumulates
in the antrum, and the early tertiary follicle becomes a graafian follicle. After
cavitation, the follicle develops a polarity and populations
of gran-ulosa become different from one another with respect to position.(From Erickson G: An analysis of follicle development and ovum maturation. Semin
Reprod Endocrinol 4:233, 1986.) Fig. 8. Architecture and classification of ovarian follicles during folliculogenesis. Recruitment
occurs within the pool of nongrowing primordial follicles. Once
the oocyte is surrounded by a single layer of cuboidal cells, it
is reactivated and completes its growth and zona pellucida formation
early, at the primary-secondary follicle stage. Growth of the preantral
follicle is a consequence of granuloma proliferation and ends
with cavitation or antrum formation in the early tertiary stage. In response
to follicle-stimulating hormone, follicular fluid accumulates
in the antrum, and the early tertiary follicle becomes a graafian follicle. After
cavitation, the follicle develops a polarity and populations
of gran-ulosa become different from one another with respect to position.(From Erickson G: An analysis of follicle development and ovum maturation. Semin
Reprod Endocrinol 4:233, 1986.)
|
The solid preantral follicle continues to grow as the oocyte increases
in size from 10 to 100 μm. At the same time, the granulosa cells are
increasing and become multilayered, and the zona pellucida thickens. Before
the end of the preantral stage, stromal cells in the cortex that
immediately surround the granulosa cells organize into distinct layers
called the theca interna and theca externa. The antral stage follows the preantral stage as the follicle develops a
fluid-filled space called the antrum. Follicular fluid originally appears
scattered throughout the follicle but coalesces to form the antrum. Granulosa, theca, and
interstitial cells all contribute to the production
of antral fluid. As the antrum grows in size, it separates the
granulosa cells into different layers. The layers of cells surrounding
the oocyte, the cumulus oophorus, is connected to the granulosa cells
lining the wall of the follicle, the mural granulosa cells, by a stalk
of cells between the two layers. The layer of cells immediately surrounding
oocyte and zona pellucida is commonly referred to as the corona
radiata. Important connections exist between these granulosa cells and
the oocyte.150 The mature antral follicle contains a large oocyte that is still arrested
in prophase of meiosis I. The cells of the corona radiata share gap
junctions with each other and the oocyte. The mural granulosa cells
maintain a stratified cuboidal epithelium. The basal layer consists of
low columnar cells overlying a dense basement membrane. The theca interna
becomes thicker and increasingly vascular. From the cohort of recruited
follicles follows a selection of even fewer follicles, leading
ultimately to the appearance of a dominant follicle destined to ovulate.151 Ovulation of the dominant follicle is preceded by a series of complex events. During
the preovulatory period, the oocyte completes diakinesis
within the nucleus, the nuclear envelope disperses, and metaphase spindle
organizes at the margin of the oocyte. The nucleus divides, separating
the homologous chromosomes and results in a reduction division with
very unequal dispersion of the cytoplasm. The result yields a secondary
oocyte with a small polar body adjacent to the oocyte, both of which
are encased within the zona pellucida. The secondary oocyte proceeds
to metaphase of meiosis II and remains there until fertilization occurs. Models of follicular depletion have been previously described. Gougeon
described one of the commonly accepted models of oocyte depletion.152 According to this model, at any given time, a given proportion of follicles
are moving through various stages of follicle maturation (Fig. 9). Gougeon described a class system for ovarian follicles. Eight classes
have been used to describe follicular development through two phases. The
phases are tonic (slow) growth phases and gonadotropin-dependent (rapid) growth
phases. The time required for a given follicle to pass
through all classes is approximately 85 days and spans the length of
three menstrual cycles (Fig. 10). 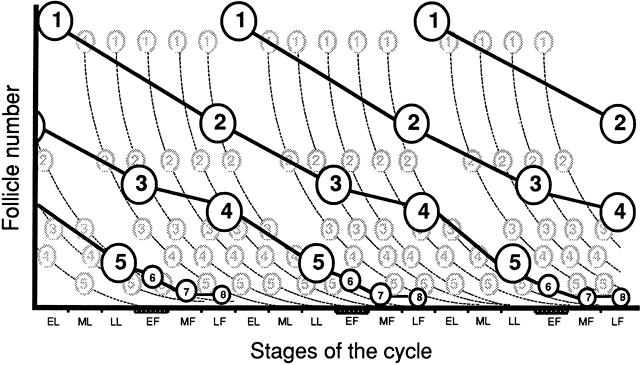 Fig. 9. Description of the progression of the “major wave” throughout
successive cycles, from the entry of follicles into the preantral (class 1) to
the preovulatory stage (class 8). Follicles that become preantral
during the early luteal phase of every menstrual cycle have been
endowed with selective advantages for further development. As growth
is progressing, the initial number of follicles belonging to the major
wave ( bold line) decreases by atresia, but in lesser proportion than do follicles from “minor
waves” ( dotted line ). Consequently, when follicles belonging to major waves and of a given
stage of their development ( e.g., class 1) enter the subsequent class ( e.g. class 2) during the late follicular phase, they are in larger numbers
than at any other time during the cycle, explaining why this passage is
morphologically discernible.(From Gougeon A: Regulation of ovarian follicular development in primates: Facts
and hypotheses. Endocr Rev 17:121, 1996.) Fig. 9. Description of the progression of the “major wave” throughout
successive cycles, from the entry of follicles into the preantral (class 1) to
the preovulatory stage (class 8). Follicles that become preantral
during the early luteal phase of every menstrual cycle have been
endowed with selective advantages for further development. As growth
is progressing, the initial number of follicles belonging to the major
wave ( bold line) decreases by atresia, but in lesser proportion than do follicles from “minor
waves” ( dotted line ). Consequently, when follicles belonging to major waves and of a given
stage of their development ( e.g., class 1) enter the subsequent class ( e.g. class 2) during the late follicular phase, they are in larger numbers
than at any other time during the cycle, explaining why this passage is
morphologically discernible.(From Gougeon A: Regulation of ovarian follicular development in primates: Facts
and hypotheses. Endocr Rev 17:121, 1996.)
|
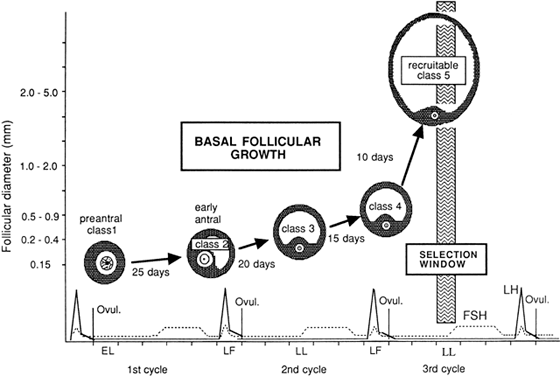 Fig. 10. Dynamics of the development of follicles belonging to the cohort from which
the follicle destined to ovulate will be selected. Growth commences
with the entry of follicles into class 1 during the early luteal phase (EL). Twenty-five
days later, during the late follicular phase (LF) of
the following cycle, entry of follicles into class 2 can be seen. The
end of the follicular phase is a favorable period for the appearance
of the antrum, because the circulating levels of follicle-stimulating
hormone have been high during the preceding days, and this hormone
promotes the initiation of antrum formation. Twenty days later, follicles
pass into class 3 during the late luteal phase (LL) and 15 days later
into class 4 during the late follicular phase (LF) of the subsequent
cycle. Follicles enter into class 5 (selectable stage) 10 days later, in
the LL phase, and constitute a population from which the follicle
destined to ovulate during the subsequent cycle will be selected.(From Gougeon A: Regulation of ovarian follicular development in primates: facts
and hypotheses. Endocr Rev 17:121, 1996.) Fig. 10. Dynamics of the development of follicles belonging to the cohort from which
the follicle destined to ovulate will be selected. Growth commences
with the entry of follicles into class 1 during the early luteal phase (EL). Twenty-five
days later, during the late follicular phase (LF) of
the following cycle, entry of follicles into class 2 can be seen. The
end of the follicular phase is a favorable period for the appearance
of the antrum, because the circulating levels of follicle-stimulating
hormone have been high during the preceding days, and this hormone
promotes the initiation of antrum formation. Twenty days later, follicles
pass into class 3 during the late luteal phase (LL) and 15 days later
into class 4 during the late follicular phase (LF) of the subsequent
cycle. Follicles enter into class 5 (selectable stage) 10 days later, in
the LL phase, and constitute a population from which the follicle
destined to ovulate during the subsequent cycle will be selected.(From Gougeon A: Regulation of ovarian follicular development in primates: facts
and hypotheses. Endocr Rev 17:121, 1996.)
|
Classes one to four make up the tonic growth phase and represent the transition
of preantral follicles up to the early antral follicles less
than 2 mm in diameter. Tonic growth is relatively slow but does not imply
gonadotropin independence. Gonadotropins are necessary for growth
during this phase, but they do not have the same influence on follicles
that they have on the gonadotropin-dependent growth phase. Classes 5 to 8 make
up the gonadotropin-dependent growth phase. Figure 11 depicts the eight classes and their corresponding developmental sequence. This
phase is highly regulated by gonadotropins, and they have profound
effects on the follicles. Class 5 follicles represent the group of
follicles from which the ovulatory follicle is recruited and selected, leading
to dominance and ovulation. The remaining follicles of any
given cohort undergo atresia anywhere along the process. Why follicles
undergo atresia when they do remains a mystery. 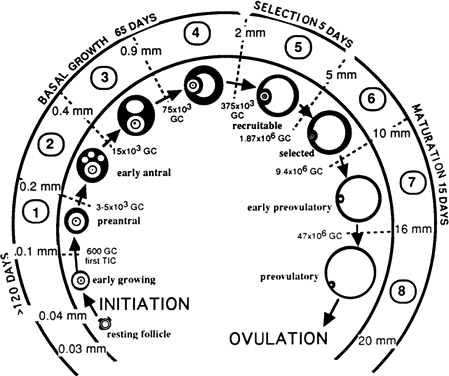 Fig. 11. Classification of follicles in the human ovary.(From Gougeon A: Regulation of ovarian follicular development in primates: facts
and hypotheses. Endocr Rev 17:121, 1996.) Fig. 11. Classification of follicles in the human ovary.(From Gougeon A: Regulation of ovarian follicular development in primates: facts
and hypotheses. Endocr Rev 17:121, 1996.)
|
Because of the continuous loss of follicles, the follicle pool begins to
deplete and show changes in response to the decreasing reserve of follicles. In
the past, we defined the perimenopause as the time when ovarian
function was so altered by this depletion of follicles that endocrine
changes, such as menstrual irregularities, appeared in women.153 We now understand that endocrine changes are occurring at a cellular level, perhaps
in response to the depletion of follicles, more than a decade
before this time. There is an accelerated loss of follicles from
the ovaries around the age of 37 years.154,155 At the same time, women experience an increase in the number of embryonic
chromosomal abnormalities, an increase in spontaneous miscarriages, a
decrease in bone mineral density,156 and a decrease in fertility rates. It is important for clinicians to identify
the progressive deterioration of ovarian function, because someday
we may be able to delay the some of the untoward effects of aging
in women. From accumulating data, progressive deterioration of ovarian function seems
to be more closely related to the number of follicles remaining in
the ovarian cortex than to ovarian age. Figure 12 demonstrates that when a woman enters her middle to upper thirties, the
rate of oocyte depletion increases. At that time, approximately 25,000 follicles
remain, and the rate of oocyte depletion accelerates. After
the oocyte pool reaches approximately 1000 follicles, menopause ensues. Shortly
after menopause, there are essentially no follicles remaining.157,158 As the rate of accelerated oocyte depletion begins, we also see an increase
in the incidence of aneuploidy, an increase in the incidence of
spontaneous abortions, and a decrease in fertility rates.159–161 The function of the follicular apparatus is changing during this time
and most likely leads to many of these adverse outcomes as women age. These
outcomes are similarly found in women who have infertility and are
undergoing assisted reproductive technologies.162–164 For example, as age increases in women undergoing IVF, so does the incidence
of spontaneous abortions, reaching as high as 50% in women older
than 40 years.163 Data suggest that these changes are likely to involve follicular cell
compromise. 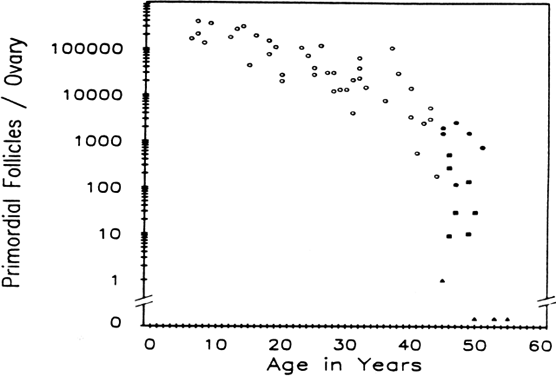 Fig. 12. Relations between age and primordial follicle number in Block's study
of 44 girls and women, (7 to 44 years old) with that of this study
of women (45 to 55 years old). Follicle depletion appears to accelerate
in the decade preceding the menopause. Open circles, women in Block's study; solid circles, women from the present study with regular menses; solid squares, perimenopausal women; solid triangles, postmenopausal women.(From Richardson S, Senikas V, Nelson J: Follicular depletion during the
menopausal transition: evidence for accelerated loss and ultimate exhaustion. J
Clin Endocrinol Metab 65:1231, 1987.) Fig. 12. Relations between age and primordial follicle number in Block's study
of 44 girls and women, (7 to 44 years old) with that of this study
of women (45 to 55 years old). Follicle depletion appears to accelerate
in the decade preceding the menopause. Open circles, women in Block's study; solid circles, women from the present study with regular menses; solid squares, perimenopausal women; solid triangles, postmenopausal women.(From Richardson S, Senikas V, Nelson J: Follicular depletion during the
menopausal transition: evidence for accelerated loss and ultimate exhaustion. J
Clin Endocrinol Metab 65:1231, 1987.)
|
Granulosa cells provide essential metabolic support and participate in
intrafollicular communication with their accompanying oocyte. The life
cycle of a granulosa cell is composed of periods of proliferation and
differentiation followed by quiescence, senescence, or apoptosis. Granulosa
cells produce a number of steroids, glycoproteins, and proteins. Changes
in the cell cycle or the production of various factors may reflect
age-related changes in human granulosa cell competence accompanied
by a decline in female fecundity. These changes may also explain the
age-related decline in ART success. With increasing age, a woman's
estradiol response to ovulation induction, the number of oocytes
retrieved, the number of successful implantations, and pregnancy rates
decrease.163,165–172 There may be an increase in the resistance of follicles to exogenous gonadotropins, demonstrated
by a decreased ovarian response to an increased
amount of stimulation in women of advancing reproductive age. These clinical observations parallel specific physiologic endocrine alterations
of the aging ovary. A characteristic shortening of the overall
menstrual cycle occurs with advancing age because of a shortening of
the follicular phase.97 This is accompanied by a higher early follicular estradiol and rising
FSH level (i.e. monotropic FSH rise), as well as a premature rise in progesterone, leading
to an earlier LH surge and ovulation.173 Quantitative follicular changes occur within the aging human ovary because
of atresia; however, the mechanisms by which this occurs are not
well understood. We now believe that qualitative changes also occur in
the remaining follicles, contributing to the physiologic changes found
clinically. Ongoing clinical investigation has demonstrated that various
biomarkers may reflect these qualitative changes. Through intensive
study of various biomarkers, we continue to uncover complex physiologic
mechanisms that explain the age-related decline in female fecundity. Through the experience of assisted reproductive technologies, we now believe
that various biomarkers may be better predictors of a woman's
reproductive potential than her chronologic age. Ovarian reserve describes
a woman's reproductive potential as it relates to the processes
of follicular depletion and oocyte quality. The first biomarker
found to be correlated with predicting ovarian reserve was a measured
day 3 serum FSH level.174 Although there may be some degree of intercycle variability, women with
elevations of FSH in one cycle usually have elevations in subsequent
cycles.175 Generally, the success of ovulation induction and IVF diminishes with
declining ovarian reserve, as reflected by rising follicular phase FSH
levels.176,177 This is consistent with the fact that serum FSH levels (i.e. monotropic FSH rise) gradually rise in the early follicular phase 8 years
before the perimenopausal-menopausal transition in healthy women.90,178 Other investigators have reported that unexpected elevations of basal
FSH are associated with unexplained infertility despite regular menses.179 Another basal test that has been examined includes basal estradiol levels. Investigators
have consistently demonstrated that elevated day 3 estradiol
levels are predictive of poor outcomes in women undergoing ART.180–182 Added to these basal measurements, FSH assessment after provocative testing
may increase a clinician's ability to detect diminished ovarian
reserve. The use of the clomiphene citrate challenge test (CCCT) has possibly provided
additional sensitivity to the predictability of ovarian reserve. The
CCCT involves the use of serial measurements of various hormones
assayed on specific days before and after the administration of clomiphene
citrate. First described by Navot and coworkers in 1987, the CCCT
has become increasingly used as a screening test for ovarian reserve.183 The original test consisted of measuring FSH concentration on cycle day 3, administering 100 mg
of clomiphene citrate on cycle days 5 through 9, and
measuring the serum FSH concentration on cycle day 10. Day 3 FSH
levels served as basal levels, and day 10 levels served as provoked
levels. The premise behind the CCCT was based on the idea that the day 10 level
might unmask diminished ovarian reserve not detected by day 3 FSH
levels alone. Scott and other investigators performed follow-up
analysis of abnormal CCCT results and found that they were predictive
of diminished ovarian reserve and decreased pregnancy rates in women
in natural cycles and women undergoing ART.184–186 However, Scott also found that age is an independent predictor of poor
outcomes despite a normal CCCT. With this in mind, it becomes clear that
the CCCT may serve as a useful screening test but fails to explain
the physiologic basis for the abnormal rise in FSH levels. Investigators have identified the physiologic basis of the rising FSH levels
in women of advancing reproductive age. Known to modulate the secretion
of FSH, inhibin represents a serum marker that may be directly
related to functional ovarian reserve.187 Clinical work has shown significantly lower serum inhibin levels in women
older than 35 years who are undergoing IVF without showing significant
differences in estradiol levels.188,189 This suggests that an age-related reduction in inhibin during maximal
ovarian stimulation may be an early index of declining ovarian function
with advancing age. It is thought that controlled ovarian hyperstimulation
may unmask a condition of “compensated granulosa cell failure” reflected
by a decreased serum inhibin, compared with the
unchanged serum inhibin levels during spontaneous menses in women with
occult ovarian failure (i.e. regular menses and elevated FSH levels). Initial studies measured total
inhibin concentration because assays were unable to differentiate inhibin-A
from inhibin-B. As new and improved assays for the detection of
inhibin-A and inhibin-B have become available, investigators have identified
the serum marker responsible for rising FSH levels in women of
advancing reproductive age. Inhibin-B concentrations have been shown to decrease with increasing serum
FSH concentrations in women in the follicular phase.103 The physiologic basis of ovarian reserve screening using the CCCT is grounded
in the hypothesis that in women with normal ovarian reserve, inhibin
production by the developing follicles is sufficient to overcome
the effects of the clomiphene citrate on the hypothalamic-pituitary
axis. This leads to the suppression of the FSH levels back to within the
normal range by cycle day 10. Hoffman and coworkers confirmed this
hypothesis in women undergoing ovarian reserve screening.190 They demonstrated lower serum inhibin-B levels on day 3 and day 10 in
women with diminished ovarian reserve (elevated FSH levels) compared with
women with normal ovarian reserve (normal FSH levels). The data strongly
suggest that granulosa cell inhibin-B production in women with
diminished ovarian reserve is inadequate to maintain basal FSH levels
in the normal range and is unable to suppress FSH levels in the presence
of clomiphene citrate. As a result of the data accumulating on inhibin, some
investigators have proposed that inhibin-B screening may be
a more direct measure of ovarian function and a more sensitive indicator
of ovarian reserve than pituitary FSH secretion. Danforth and coworkers evaluated luteal phase inhibin-A and follicular
phase inhibin-B levels and found an inverse relationship with advancing
age.102 They also demonstrated a decline in inhibin-A and inhibin-B before the
monotropic FSH rise. This is consistent with the findings of Seifer and
colleagues that day 3 inhibin-B concentrations are predictive of IVF
outcomes.191 Additional data also support the hypothesis that day 3 serum inhibin-B
levels decline before the monotropic FSH rise.192,193 Seifer and coworkers studied women undergoing ART who demonstrated a poor
response to stimulation as measured by increased ampules of gonadotropins
for stimulation yielding a higher cancellation rate, a lower estradiol
response, fewer oocytes retrieved, and lower clinical pregnancy
rate. They found that this group of women also had lower inhibin-B
levels than women undergoing ART with normal ovarian responsiveness, despite
both groups having similar nonelevated day 3 FSH levels. In addition
to confirming the inverse relationship between declining inhibin
levels and rising FSH levels, Santoro and colleagues demonstrated an
increase in activin-A, which also contributes to the FSH elevation.194 In the future, day 3 inhibin-B screening may provide a more direct and
reliable measure of ovarian reserve. Our understanding of ovarian physiology
is evolving through the study of potential biomarkers of aging. The gonadotropin agonist stimulation test (GAST) is another ovarian reserve
test. First described by Padilla in 1990, the GAST is a measure of
ovarian responsiveness to GnRH-a gonadotropin stimulation.195 In the early follicular phase, estradiol levels are evaluated in response
to GnRH-a, and the data suggest a positive correlation with ART success. Although
there are concerns about the expense of the test and the
applicability of the test to the general population, some argue that
the GAST is excellent marker of ovarian reserve and a sensitive predictor
of IVF outcomes.196 Another test introduced as a measure of ovarian reserve is the exogenous
FSH hormone ovarian reserve test (EFORT).197 The EFORT test measures basal FSH levels and estradiol levels obtained
on cycle day 3, followed by the administration of 300 IU of purified
FSH. Twenty-four hours later, estradiol is measured and the change in
estradiol (delta E2) is calculated. Women with a low basal FSH level and a high delta E2 were considered normal, demonstrating a good response to gonadotropin
therapy. The EFORT, GAST, and CCCT all represent ovarian reserve tests
that require ovarian stimulation. Other tests of ovarian reserve have
been described that do not require stimulation testing. In the past, investigators have attempted to quantify potential ovarian
responsiveness by using ultrasound measurements of various morphologic
ovarian characteristics. One of the characteristics showing some correlation
to ART outcomes is antral follicle counts. Investigators have
observed that the number of ultrasonically visible antral follicles decreases
with advancing age.198 Chang and colleagues showed that, in women undergoing ART, antral follicle
counts correlate well with ovarian responsiveness to stimulation
and pregnancy outcomes.199 Transvaginal ultrasound ovarian volume measurement represents another morphologic
test of ovarian reserve. Syrop and colleagues examined ovarian
volumes and found that total ovarian volume and the volume of the
smallest ovary were predictive of a woman's response to gonadotropin
stimulation and ART success.200 Large ovarian volumes were predictive of good ART outcomes, and small
ovarian volumes were associated with poor outcomes. Sharara examined the
relationship between ovarian volume and age and FSH levels but did
not find any significant correlation.201 However, they also found higher cancellation rates and therefore poorer
outcomes in women with smaller ovaries. They suggested that ovarian
volumes may be an earlier predictor of diminished ovarian reserve than
basal FSH or basal estradiol levels. Research is ongoing to discover the ideal biomarker of ovarian reserve, with
the hope of providing clinicians the tools to accurately predict
the impact of age on a couple's chance for success when undergoing
ART. Which of the aforementioned tests holds the greatest promise for
predicting diminishing ovarian reserve awaits further study. Table 1 summarizes these potential ovarian reserve tests. TABLE 1. Methods to Detect Ovarian Reserve
Basal tests Day 3 FSH174,175
Day 3 Estradiol180–182
Day 3 Inhibitin B190–194
Stimulation tests Clomiphene citrate challenge test (CCCT)183–186
Gonadotropin agonist stimulation test (GAST)195,196
Exogenous follicle-stimulating hormone ovarian reserve test (EFORT)197
Morphologic tests Antral follicle counts198,199
Ovarian volume measurement200,201
Uncovering the physiologic basis for ovarian reserve tests has led to an
improved understanding of follicular physiology and the follicular dysfunction
that occurs in women with advancing age. Although concerns
remain as to the predictive value of these ovarian reserve tests, they
do provide investigators with physiologic probes to assess the mechanisms
behind problems associated with advancing reproductive age such as
the increase in anovulatory cycles, chromosomal abnormalities, and spontaneous
abortions. According to the Society for Assisted Reproductive
Technology Registry, the data collected for all women undergoing IVF
in 1996 showed that the number of deliveries per retrieval for women
younger than 35 years of age was 31.2%, compared with 11.2% for women 40 years
of age or older.164 The miscarriage rate for women younger than 35 years of age was 12.8%, compared
with 31.9% for women 40 years of age or older. Investigators
have reported a greater incidence of aneuploidy in oocytes from women
of advanced reproductive age (i.e. women older than 38 years).202,203 More mitochondrial deletions are found in oocytes from women of advanced
reproductive age.204 Genetic abnormalities in oocytes provide a portion of the basis of explaining
the deleterious effect of maternal age on the reproductive system. We
have learned through the study of ovarian reserve biomarkers that
there is an evolving theme, suggesting that a breakdown in the ovarian
follicular apparatus is directly responsible for the clinical manifestation
of the age-related decline in female fecundity. Our understanding of the physiology of the follicular apparatus continues
to unfold. Follicular somatic cells, such as mural and cumulus granulosa
cells, nourish, sustain, and communicate with their accompanying
oocytes through molecular signals through gap junctions.205 This two-way communication is understandable in light of the embryology
of the granulosa cells. Granulosa cells are mesodermal in origin and
migrate to oocytes at 6 months in utero as pregranulosa cells. Later, the cells that come into close contact and
are attached to the outer surface of the oocyte differentiate into
granulosa cells.206 Oocytes that are of endodermal origin are known to be essential participants
in follicular development, because granulosa and theca cells do
not form in the absence of oocytes.207 This communication is changing throughout life and controls the fate of
the oocyte. The impact that granulosa cells and oocytes have on each other is highlighted
by the maintenance of meiotic arrest of the oocyte during the diplotene
stage of the first meiotic prophase. The determination of the
mechanism and agents responsible for this meiotic arrest is of great
interest because the resumption of meiosis does not occur until after
puberty, when there is stimulation by the gonadotropin surge. The resumption
of meiosis represents the final event that leads to full maturation
of the oocyte, and fortunately, we do understand much of the molecular
biology behind this crucial event. The resumption of meiosis probably
results from a FSH-induced reaction, causing the cascade of meiotic
events within the oocyte. Cyclic adenosine 3',5'-monophosphate (cAMP) is one of the primary
agents involved in this process. It has been proposed that cAMP
synthesized by cumulus cells surrounding the oocyte is responsible for
maintaining meiotic arrest.208 The oocyte itself cannot synthesize cAMP because it lacks adenylate cyclase, but
it does contain phosphodiesterase. Cyclic AMP enters oocytes
through gap junctions from the cumulus cells. This intercellular communication
is terminated by gonadotropin stimulation at the time of ovulation. The
oocyte, deprived of the necessary cAMP levels for meiotic
arrest, resumes division. A growth factor that may be involved in the
influence of cAMP on maintaining arrest of the human oocyte is inhibin. As
a product of granulosa cells and a member of the transforming growth
factor-β family, inhibin may inhibit oocyte meiosis and granulosa
cell mitosis.209 Oogonia enter the first meiotic prophase shortly after birth and remain
arrested until sometime after puberty. The factors that cause this to
happen are unknown. At some point, oocyte growth and cytoplasmic maturation
begins, and ultimately, meiosis resumes for final maturation of
the oocyte. As a result of gonadotropic stimulation, preovulatory follicles
undergo a series of changes that are essential for the ovulation
of normal oocytes capable of fertilization. Oocytes undergo a maturation
process that includes nuclear and cytoplasmic maturation. These processes
are highly coordinated and depend on one another for normal development.210,211 Nuclear maturation involves the resumption of meiosis in prophase meiosis
I through to the extrusion of a polar body and arrest at metaphase
meiosis II. Cytoplasmic maturation includes the preparation of the oocyte
for fertilization, activation, and early embryo development. Cytoplasmic
and nuclear maturation do not always result in mutual normal development, as
has been shown by studies evaluating oocytes from different
size follicles and evaluating their preimplantation embryo development.212,213 The follicular apparatus involves an intricate communication with the
oocyte during maturation. Another observation demonstrating the impact that oocytes and granulosa
cells have on each other is the modulatory role of the oocyte on granulosa
cell steroidogenesis. Oocytectomized cumulusoocyte complexes demonstrate
an enhanced progesterone production compared with intact cumulus-oocyte
complexes. These data suggest some type of oocyte-cumulus cell-inhibiting
factor.214,215 The basic mechanisms responsible for the poor oocyte quality found in
women of advanced reproductive age may be related to the accompanying
granulosa cells as well. The perimenopause describes compromise of the follicular apparatus within
the ovary. Diminished ovarian reserve may be explained by granulosa
cell compromise. The ovaries of women with diminished ovarian reserve
have fewer developing follicles, and each follicle contains fewer granulosa
cells. Seifer and coworkers evaluated the granulosa cells from
women with normal and diminished ovarian reserve who were undergoing superovulation
for assisted reproduction.216 Granulosa cell counts and the percentage of apoptotic cells harvested
from these women were elevated. Women with diminished ovarian reserve
had fewer luteinizing granulosa cells, and a higher percentage of those
cells were undergoing apoptosis. This type of aberration in follicular
development accounts for a 25% to 30% reduction in the number of luteinized
granulosa cells in the follicles of older women. This contributes
to the development of a dysfunctional follicular apparatus in older
women. Other investigators have looked at granulosa cell survival factors deficiencies, such
as insulin-like growth factor-1 (IGF-1), epidermal growth
factor, and progesterone, because each of these factors act directly
on granulosa cells to inhibit them from becoming apoptopic.217,218 Pellicer and associates investigated the effects of chronologic age on
granulosa steroid and glycoprotein production.219 Immunoreactive progesterone and α-inhibin (total inhibin) were examined in vivo and in vitro in two populations of women of different ages (mean age, 40 years versus 32 years) preparing
for ART. Serum and cell culture media immunoreactive α-inhibin
levels were lower in the group of older women compared
with the group of younger women. Cell culture progesterone was also
lower in the group of older women. These results also support the data
suggesting that granulosa cells from older women have reduced function. However, this
study was confounded by the absence of information
regarding ovarian reserve, because it is known that women of different
chronologic age may have similar ovarian reserve and that there is variability
in age of onset of this diminished ovarian reserve that parallels
a decline in reproductive potential. Investigators have cultured luteinized granulosa cells from two groups
of women with different ovarian reserves, as reflected by day 3 serum
FSH and examined the culture media for total and dimeric inhibin A.220 The total and dimeric inhibin media concentrations were approximately
twice as high in the low day 3 FSH group than that of the high day 3 FSH
group. These data suggest that a quantitative decline in immunoreactive
and bioactive inhibin produced by granulosa cells is associated with
a declining ovarian reserve, further suggesting that rising day 3 serum
FSH is an indirect bioassay of declining bioactive inhibin production
at the granulosa cell level. Klein and coworkers examined follicular
fluid from normal young women (20 to 25 years old) and from older
women (40 to 45 years old) with regular ovulatory cycles and no history
of previous infertility.221 The follicular fluid estradiol to testosterone ratios were higher, but
the IGF-1 concentrations were lower in the group of older women compared
with the group of younger women. Their findings suggest that older
women form an earlier dominant follicle (which contains higher estradiol
but lower androgen and IGF-1 levels) than younger women. Vascular endothelial growth factor (VEGF) represents another potential
biomarker that has been studied. An oocyte has no direct vascular supply
and depends on oxygen diffusion through the follicular fluid from the
granulosa cells. At the time of the LH surge, there is an increase
in microvascular remodeling. It has been proposed that as women age, their
developing follicles are surrounded by a deficient microcirculation
that may be related to errors in meiosis.222 It has been reported also that an increase of chromosomal scattering occurs
in oocytes obtained from follicles with reduced oxygen content.223 VEGF is one of many angiogenic cytokines secreted by granulosa cells that
are undergoing microvascular remodeling during the resumption of meiosis. Friedman
and colleagues measured the VEGF produced by follicles
from women broadly categorized by age.224 They found increased VEGF levels in follicular fluid from women of advanced
age. This supports the hypothesis that follicles maturing in aging
women may be sustained in a relatively hypoxic environment. Studies of granulosa cells have examined aging in the context of the cell
cycle, proliferation, steroidogenesis, glycoprotein, and growth factor
production. The cell cycle is of particular interest because it represents
the reproductive cell cycle of the individual cell itself. The
cell cycle can be defined as a sequence of events that begins with the
completion of mitosis in the parent cell and ends with the subsequent
mitosis that occurs in the daughter cell. Its purpose is to maintain
DNA constancy from parent to daughter cell. When Seifer and coworkers
examined apoptosis, they found 70% of the apoptotic cells to be in the
proliferative phase of the cell cycle.216 This observation suggests that most of the cells destined to become apoptotic
have entered the cell cycle. Under normal conditions, cells probably
are stimulated to undergo mitosis in the presence of mitogens and
other survival factors. Under optimal conditions, cells complete the
cell cycle, and the population doubles. If cells are stimulated to traverse
the cell cycle in the absence of survival factors, mitosis is
then blocked, and the cells become apoptotic.225,226 Because cells isolated from women with elevated basal FSH levels show
a fourfold increase in the percentage of apoptotic cells, it is likely
that many follicles within the ovaries of these women are deficient in
granulosa cell survival factors. Seifer and coworkers examined the proliferative index (PI)—defined
as the sum of the percentage of cells in the synthetic, postsynthetic, and
mitotic growth phases (G2M)—of luteinized granulosa cells as a function of chronologic age.227 Despite the differences in ages (mean age, 28 years versus 41 years) but
with similar follicular phase serum FSH levels, the PI did not differ
between the groups. Chronologic age did not have a significant independent
influence on the PI. This was followed by a study that examined
whether the PI of luteinized granulosa cells varied as a function of
day 3 serum FSH levels. Ages were similar between two groups of women
undergoing ovulation induction in preparation for ART (mean age, 32 years), but
day 3 serum FSH values were different (mean serum FSH, 4.8 IU/L
versus 25 IU/L) between these groups of women. This study demonstrated
that luteinized granulosa cells obtained from preovulatory follicles
in women with high day 3 serum FSH had a 25% decreased PI compared
with luteinized granulosa cells obtained from women of the same chronologic
age who had a low day 3 serum FSH.228 This suggests that women with diminished ovarian reserve have a smaller
percentage of their luteinized granulosa cells in the proliferative
portion of the cell cycle compared with women with normal ovarian reserve. This
finding may explain in part the more favorable response to ovulation
induction protocols that younger women demonstrate compared with
women of more advanced reproductive age. Luteinized granulosa cells obtained from women with different ovarian reserves
appear to vary with regard to their PI, steroidogenesis, and immunoreactive
and bioactive inhibin secretion. The compromised endocrine, paracrine, and
autocrine signals in the form of steroid, glycoproteins, and
growth factors associated with ovarian follicles of decreasing
competence, likely leads to an altered crosstalk between cells of the
follicular apparatus—granulosa cells and oocytes. It is speculated
that cytoplasmic and nuclear maturation of the oocyte may become
impaired because of altered granulosa signals. Such oocytes may begin
to develop meiotic spindle and microtubule abnormalities. Battaglia and colleagues looked at the meiotic apparatus to understand
meiotic nondisjunction in women of advancing maternal age and found abnormalities
of spindle formation and chromosome alignment.229,230 In one study, they used high-resolution, confocal microscopy to evaluate
oocytes retrieved from naturally cycling women. Oocytes from two groups, young (20 to 25 years) and old (40 to 45 years), were evaluated
with respect to meiotic spindle and chromosome placement during various
stages of meiosis. They found a higher frequency chromosomal alignment
abnormalities in the older age group. For instance, in the older women
they found a 79% incidence of abnormal tubulin placement in the spindles
with one or more displaced chromosomes at the metaphase plate during
the second meiotic division. In contrast, only 17% of the oocytes
from the younger women displayed similar spindle abnormalities. Most
oocytes displayed a well-ordered, bipolar meiotic spindle, with chromosomes
aligned at a distinct fully formed metaphase plate. However, most
oocytes recovered from the older group displayed poor connections of
the microtubule matrix and displaced chromosomes in addition to microtubule
nucleating sites in abnormal places within the oocyte remote from
the spindle poles. Angell studied the karyotypes of unfertilized eggs
and found more chromosomal abnormalities in women of advanced reproductive
age.231 There is also an increase in chromosomal abnormalities in women of advanced
reproductive age undergoing preimplantation genetic diagnosis.203 Spindle elements and microtubule assembly are responsible for chromosomal
alignment during meiosis, and abnormalities in the regulatory mechanisms
of oocyte maturation may result from a failing follicular apparatus
as the communication between the granulosa cells and the oocyte are
disrupted. As a result of increasing maternal age, clinicians see an
increase in chromosomally abnormal fetuses and miscarriages.232 Elevated day 3 serum FSH levels are predictive of fetal aneuploidy.233 Whether chromosomal abnormalities are caused by a hypoxic environment, an
abnormal communication between the oocyte and granulosa cells, or an
abnormal oocyte cytoplasmic and nuclear maturation remains to be determined. However, the
ovarian follicular apparatus undergoes progressive
deterioration that parallels age and leads to adverse clinical problems
for patients long before menopause. In summary, the perimenopause begins in the mid-thirties with decreasing
number and competence of the ovarian follicular apparatus. Within the
aging ovary, there are fewer developing follicles and within those follicles
are fewer granulosa cells. Individual granulosa cells demonstrate
diminished production of steroids and glycoproteins. The granulosa
cells demonstrate decreased mitosis and increased apoptosis. The compromised
endocrine, paracrine, and autocrine signals appear to result
in an altered communication between the somatic (granulosa) cells and
the germ (oocyte) cells. Because of altered signals, there may be abnormal
nuclear and cytoplasmic maturation within the oocyte. Because of
the failing follicular apparatus with aging, clinicians see a decrease
in fertility rates and an increase in chromosomal abnormalities and miscarriages.234 Pregnancy Outcomes Although multifactorial and not related to a single contribution, pregnancy
outcomes also deteriorate with advancing age. Maternal morbidity
and mortality increase with age. For instance, pregnancy conditions such
as preeclampsia, gestational diabetes, and placenta previa occur with
higher frequency among older women, as do labor abnormalities such
as induction of labor, fetal distress, operative vaginal deliveries, and
postpartum hemorrhage.235,236 Cesarean deliveries are also increased in women of advancing age.237 Maternal age is an independent risk factor for unexplained fetal death.238 Moreover, the maternal mortality ratio for women over 30 is 2.5 times
greater than that for women under 30.239 Because of advancing maternal age, pregnancy rates decrease and spontaneous
abortion rates increase. For those women fortunate enough to get
beyond the first trimester, they face a myriad of potential pregnancy
and labor complications at a higher rate than their younger counterparts. Age
is unforgiving when it pertains to human reproduction. |