Evaluating the efficacy of procedures such as GIFT, ZIFT, and TET depends
largely on the comparison of their pregnancy rates to that of IVF-ET
in patients with different medical indications. Laparoscopy is more
invasive, riskier, and more costly than transcervical uterine embryo transfer; therefore, tubal
transfer procedures must demonstrate unequivocal
superiority over IVF-ET. Most assisted reproduction programs in the United States and Canada prefer
IVF-ET treatments, and fewer patients undergo laparoscopic tubal procedures.29,30 Table 2 shows the number of IVF and GIFT cycles and pregnancy and delivery rates
in the United States. Interpreting Table 2 is quite complicated. Undoubtedly, between 1988 and 1993 both IVF and GIFT
pregnancy rates have improved. Although the number of IVF cycles reported
has almost doubled, the number of GIFT cycles has increased less
than expected. This observation is intriguing because GIFT pregnancy
rates were higher than IVF-ET pregnancy rates. The unaware observer
may conclude that GIFT is superior to IVF. However, biases introduced
by dropout rates after the first and subsequent cycles, life table analysis, and
bias introduced from higher fecundity in the first 3 to 6 months
of observation may affect the final pregnancy rates. Indications
for GIFT may differ from the indications for IVF, resulting in the selection
of GIFT patients who are more likely to conceive.31 Comparison of GIFT to conventional treatments among women with unexplained
infertility generates an initial calculation of a sevenfold improvement
when GIFT is applied. However, taking into consideration only three
months or less of observation for GIFT, compared to longer periods
of observation for patients without any treatment, reduces the apparent
superiority of GIFT to an odds ratio of only 1.79 with a wide confidence
interval, making it statistically insignificant compared to no
treatment.31,32 If GIFT is compared to superovulation combined with intrauterine insemination (IUI) in
patients with unexplained infertility, and only prospective
studies are evaluated, the cycle fecundity of GIFT ranges between 0.12 to 0.28, and
the cycle fecundity of patients who underwent IUI
and superovulation ranges between 0.01 and 0.36.32 The very large overlapping pregnancy odds ratio does not support the superiority
of GIFT over less invasive and less expensive interventions
such as superovulation and IUI. TABLE 2. The Number of IVF and GIFT Cycles, Pregnancy, and Delivery Rates
in the United States
| IVF/GIFT | IVF/GIFT | | IVF/GIFT | IVF/GIFT |
| | Pregnancies | IVF/GIFT | Pregnancy | Deliveries |
| | per Retrieval | Deliveries per | per cycle | per cycle |
Year | Cycles (#) | (%) | Retrieval (%) | (%) | (%) |
1988 | 17,496/3,949 | 16/27 | 12/21 | 13/21 | 9/17 |
1990 | 19,070/4,439 | 19/29 | 14/22 | 16/25 | 12/19 |
1993 | 31,900/4,992 | 23/35 | 19/28 | 20/29 | 16/24 |
IVF, in vitro fertilization; GIFT, gamete intrafallopian transfer.
In a prospective randomized study comparing IVF, GIFT, and TET in 150 couples
with unexplained infertility, male factor infertility, and minimal
endometriosis, the pregnancy rates per transfer were 46% for IVF, 38% for
TET, and 26% for GIFT. If the number of eggs and embryos transferred
were controlled for, GIFT had a significantly lower implantation
rate of 7% compared to IVF with 13%.33 This report concluded that implantation rates per embryo transferred are
not superior in tubal procedures compared to IVF.34 A prospective single-cycle IVF-ET nonrandomized study with patients who
underwent a previous IVF cycle demonstrated a higher pregnancy rate
with GIFT than with IVF.35 If one accepts that patients with unexplained infertility demonstrate
higher fertilization rates during IVF but lower implantation rates than
patients with tubal disease, then it would be preferable to perform
IVF rather than GIFT.31 The costs of tubal procedures also determine the final selection in patients
with normal fallopian tubes. If one to four cycles of superovulation
and IUI are as effective as one cycle of IVF in producing a pregnancy, three
non-IVF cycles are superior to IVF or ZIFT and comparable
to GIFT. Using the same assumptions, four cycles of superovulation and
IUI are superior to IVF or GIFT. If the average costs of one cycle of
superovulation with human menopausal gonadotropins (hMG) and IUI are
compared to one cycle of IVF, one would prefer four cycles of hMG combined
with IUI over IVF, GIFT, or ZIFT.36,37 The absence of the expected rise in the use of GIFT procedures over time (see Table 2) reflects a similar understanding of practitioners. Other considerations, such
as IVF's high costs and lack of insurance coverage for GIFT
procedures, may have also resulted in the preferential use of superovulation
with IUI over IVF, GIFT, or TET. Therefore, IVF or GIFT is
performed only in patients who can either afford this procedure or have
insurance coverage.37 The percentage of IVF-related ectopic pregnancies is higher than in the
general population. However, there is no indication that tubal transfer
procedures result in an ectopic rate higher than that of IVF-ET. This
observation was confirmed in several reports.29,30 Both IVF and GIFT pregnancies demonstrate a relatively high proportion
of preterm births and low-birthweight infants.38,39 Some of those preterm deliveries are induced because of multiple gestations. However, even
singleton term intrauterine gestations obtained either
by IVF-ET or GIFT have higher prematurity rates than in the general
population.39 This finding raises major concerns about the use of both IVF and tubal
transfer procedures, because the use of assisted reproductive technologies
increases significantly the medical expenses per live-born “take-home” baby. The increased percentage of twins and triplets
is associated with the obvious risks of prematurity, neonatal morbidity
and mortality, and increased risks of cardiovascular disease, diabetes
mellitus, and subsequent obstructive lung disease in adult life. Therefore, not
only pregnancy rates but also cost-effectiveness and the
well-being of the offspring must be included in the final assessment
of the role of tubal transfer procedures in the treatment of nontubal
infertility. Only a few studies have tested prospectively the hypothesis that tubal
transfer of gametes and embryos is superior to uterine transfer in patients
with nontubal factor infertility.40,41 Prospective randomization of patients did not demonstrate any superiority
of either procedure when standard outcome parameters were used in
relatively small studies. It can be argued that increasing the sample
sizes of those prospective randomized studies would have brought statistical
significance, and large studies would have demonstrated the superiority
of tubal transfer procedures. However, if power analysis requires
very large studies to demonstrate statistically significant differences
in pregnancy rates, then the magnitude of the difference is probably
of minor or no clinical importance. Until the superiority of tubal transfer procedures is unequivocally established, they
cannot be advocated without considering the IVF-ET alternative. Only
a few patients who demonstrate cervical stenosis or uterine
cavities inaccessible to catheters are unequivocal candidates for
GIFT, ZIFT, and TET. Even those patients may achieve an IVF-ET pregnancy
using a transmyometrial embryo transfer system42 (Fig. 3). 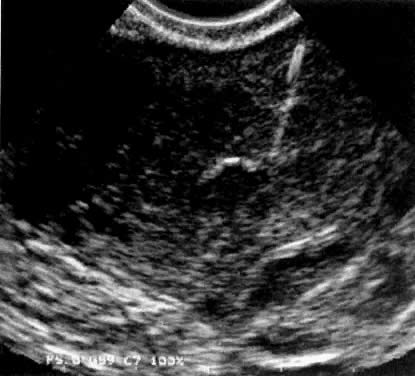 Fig. 3. Transmyometrial embryo transfer procedure in a patient with cervical stenosis. The echogenic line represents the needle equipped with an embryo transfer catheter protruding
into the uterine cavity. Fig. 3. Transmyometrial embryo transfer procedure in a patient with cervical stenosis. The echogenic line represents the needle equipped with an embryo transfer catheter protruding
into the uterine cavity.
|
The added cost of laparoscopy during tubal transfer procedures mandates
a careful consideration of health-care resources. Laboratory improvements
and the delivery of high-quality embryos into the uterine cavity
may eventually render tubal transfer procedures obsolete. The final role
of tubal transfer procedures in clinical practice remains unclear. The
early need to compensate for inferior laboratory skills is decreasing. Like
many other procedures, tubal transfer procedures will eventually
find their role in patients with nontubal factor infertility. |