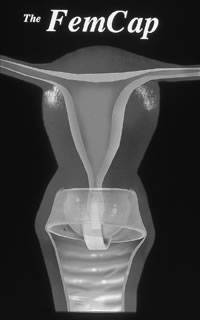
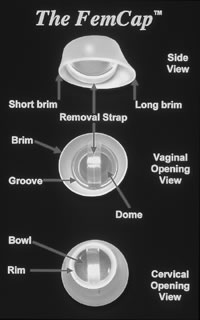
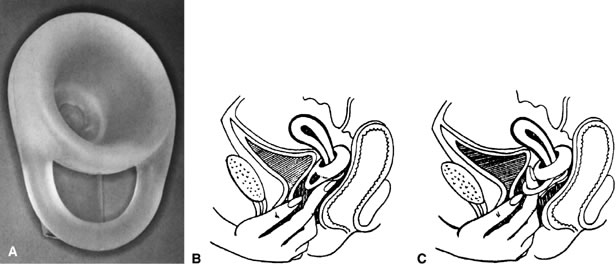
The condom (also known as a prophylactic, rubber, sheath, or French letter) is
the only male method of contraception available besides coitus
interruptus (withdrawal) and male sterilization. Its invention is attributed
to Gabriel Fallopio (1564).41 It is one of the oldest and most extensively used contraceptives. The
worldwide upsurge of STDs as well as the unstoppable pandemic of HIV/AIDS
contributed to dilute most of the reservations about condoms and its
use held by society at large. Because of its excellent record of infection
prevention and contraceptive protection, its availability, and
simplicity of use, the condom has become the darling of family planning clinics. It offers protection against unintended pregnancy
and serves as the greatest defense against acquiring an STD or
HIV infection; its historical role in the prevention of STDs is unmatched
by any other method of contraception. Protection, however, is not 100% in
either case. There is consistent clinical evidence from prospective
studies of discordant couples, in which one partner is infected
with HIV and the other not, that latex condoms used correctly during
every act of sexual intercourse are very effective, not only in preventing
unintended pregnancy but also HIV transmission STDs.42,43,44,45 When used correctly during each sexual act, condoms are very effective
in preventing unintended pregnancies and STDs. In this dual capacity, condoms
may be regarded as the contraceptive gold standard against which
all other contraceptive methods should be gauged. In strict comparison, most
other contraceptive methods fall short, in fact, considering
the specifications for an ideal contraceptive: high effectiveness, safe, reversible, inexpensive, free from side effects, light and inconspicuous, aesthetically
acceptable, self-administered, simple to use, requiring
no special skills or professional intervention, easy to store
and distribute, offering effective prevention against STD infection, long
shelf life, and use independent of sex. Male condoms answer with
ease all those requirements except the last.46 Condoms also have an important role to play in the rare cases when women
are hypersensitive (atopic allergy) to contact with semen. Condoms are widely available, easily obtainable, and reasonably inexpensive (even
provided without charge by many family planning and STD clinics
and some student health services). They do not require prescription
or fitting. They have virtually no contraindications or side effects, except
occasional allergy to the latex rubber47,48 or the powder or lubricant by either partner. Problems possibly related
to being powdered with talc have been reported.49,50 Latex allergy is estimated to be 1% to 3% in the general population and 6% to 7% in
persons frequently exposed to latex (health workers).7 Condoms are inconspicuous. They are simple to use, and their use is easy
to teach and understand, even by people with limited or no education. To
a certain degree, condoms constrict the penis and may attenuate
somewhat glans sensitivity. This might inhibit erection in some users; however, in
others, it may be a bonus that translates into prolonging
coitus by delaying ejaculation. Condoms are an ideal method for sporadic
or unanticipated coitus, for those who have sex infrequently, or for
those who are faced with an unexpected sexual encounter. Condoms are
a responsible alternative method for couples who wish to share the responsibility
for contraception, as well as when immediate assurance of
successful protection against conception is psychologically important
to either partner. Condoms are recommended as an interim method before
hormonal contraception may be started or an IUD inserted. Some women
like the condom because they prefer the man to take the contraceptive
responsibility and rely on his decision making or because they want
to avoid contact with the ejaculate or postcoital messiness. Table 3 lists the indications and contraindications for condom use. Table 3. Indications and Contraindications for Condom Use
Indications Male Male contraceptive control preferred
Genital or penile disease, cystitis, urethritis, including active sexually
transmitted disease
Sensitivity or allergic reaction to vaginal secretions
Premature ejaculation
Female Male control preferred
Unreliable sexual partner
Vaginitis even under treatment
Contraindications to systemic contraceptives and intrauterine contraceptives
devices (IUDs)
Wants proof that semen was not released in the vagina
Aversion to contact with semen or allergy to it
Rejects hormonal contraceptives and foreign body (IUDs) inserted in her
body
Psychological, cultural, or religious conflict with personal use of contraception
Temporary methods when: Sporadic coitus or a regular sexual relationship is not established
During menstruation if coitus is to occur
During midcycle for extra protection while using a diaphragm, cap, or the
rhythm method
While waiting to start a systemic contraceptive or the insertion of an
IUD
When on oral contraceptive pills, injections, or several were missed
During the first cycle on minidose pill
Postpartum until the involution of the uterus and cervix permits the insertion
of an IUD, a cervical cap, the vagina permits fitting of a diaphragm, or
the stopping of lactation allows the use of hormonal combined
oral pills, injections, or implants
Both sexual partners Male control favored
Casual sex encounter or infrequent intercourse
At risk STDs/HIV infection
Active or suspected STD, including history of or inactive viral STD
Urinary tract infection (UTI) until treated
Interim method until a reversible method can be prescribed, an IUD inserted
or sterilizationis performed
Contraindications Either partner or both sexually irresponsible; cannot be trusted will use
the condomwell and consistently
Allergy to latex by either partner
Disruption or interruption of sexual play inhibits sexual interest or expression
STD, sexually transmitted disease; HIV, human immunodeficiency virus
Modified from Sobrero AJ Sciarra JJ: Contraception, In Cohn HP (ed): Current
Therapy 1978, Baltimore, WB Saunders Company, 1978
Drawbacks or perceived negative attributes of the condom are its historic
connection with illicit sex, promiscuity, and distrust of the partner's
health. Like all barrier contraceptives, condoms necessitate
persistent, recurrent motivation to achieve dependable protection against
pregnancy, STDs, and HIV infection. It must be used correctly at
each sexual engagement. An often-mentioned drawback is the need to interrupt
lovemaking to place it; however, resourceful couples may surmount
this by making the placement of the condom part of sexual foreplay
and a more erotic experience. Condoms are made of latex rubber, polyurethane, and natural membranes (Lamb's
cecal pouch). Most commercially available condoms are made
of latex rubber. They come in an astounding variety of shapes (e.g., blunt
or with a reservoir tip, ribbed, speckled, peppered with dots, contoured, with
a helix or spiral rib, with a loose pouch over the glans
or the penile shaft). Condoms come in a kaleidoscopic variety of colors, combinations, even
fluorescent, and flavored, with up to 16 different
tastes. They come in different sizes: standard (170 mm long and 50 mm
wide); long, 30% larger; 45%, extra-large; 6%, narrower; 15%, shorter
and 6%, narrower. They come as extra-strength and extra-thin. They
may be lightly powdered or lubricated with silicone or a water-soluble
spermicide or without spermicide, or a desensitizing product. Their
variety seems to be limited only by the imagination of the manufacturers. Indications
are that there is a market for such diversity and attesting
to the contemporary losing of old social restrains about sexual
intimacy. Usually, condoms are neatly rolled and packaged flat in paper, plastic, or
aluminum foil. They have a long shelf-life, especially
if they are protected from direct sunlight, heat, oily substances, and
ozone, all of which contribute to rapid latex decay.51 Latex condoms lubricated with a spermicide and packaged in aluminum foil
should be favored. However, there are no studies that show that condoms
lubricated with a spermicide are more effective than those unlubricated. Condoms
with spermicide decrease the risk of PID (pelvic inflammatory
disease), infertility, and ectopic pregnancy. Lubrication may
increase slippage.52,53,54,55 Rear entrance that is lengthy and vigorous coitus increase ruptures. Approximately
one condom break occurs per 100 acts of coitus; however, reported
figures indicate a range between 0% and 12%, with most publications
giving figures of 2% to 5%.52,53,54,55 In a U.S. survey with a breakage rate of one to 12 per 100 episodes of
vaginal intercourse, one pregnancy resulted from every three condom breakages.56 During manufacture, condoms are individually tested electronically for
holes and imperfections and must meet the stringent standards set by
the American Society for Testing Materials and the U.S. Food and Drug
Administration (FDA).59 In the United States, condoms are imprinted with the date of manufacture
or an expiration date. Aging seems to be the best predictor for condom
breakage. However, just as important is how they are packaged and
stored and how are they handled when used. Latex condoms form a continuous, impervious
barrier to bacteria and viruses. In vitro laboratory tests have shown that latex condoms provide effective protection
against gonorrhea, nongonococcal urethritis, Chlamydia trachomatis, cytomegalovirus, human papillomavirus, herpes simplex virus, HIV, and
the much smaller hepatitis B virus.6,7,57,58,59 One FDA study found that fluorescent polystyrene microspheres similar
in diameter (110 nm) to the HIV virus (90 to 130 nm) could pass through 29 of
unlubricated latex condoms (p< .03). The harsh physical conditions of the in vitro test under which the study was performed included 30 mL of a watery suspension
of the particles at a concentration 100 times that reported for
the average normal human ejaculate (3 mL) with a pressure of 90 mmHg
over 30 minutes. The authors acknowledge the superior capacity of the
latex condoms in containing HIV virus.55a Seamen on leave in a port with a high prevalence of infected commercial
sexual workers produced striking proof: none of 29 sailors who reported
using condoms became infected, but 71 (14%) of 499 sailors who did
not use condoms became infected with gonorrhea or nongonococcal urethritis.3 In a project that counseled HIV-discordant couples every 6 months, over 6 years
there was no HIV seroconversion.58 Women who are partners of condom users are less likely to become HIV positive.60,61,62 One study followed 245 HIV-discordant couples for an average of 20 months. Among 124 who
related using condoms at every coitus, no new cases
of HIV were detected, even though approximately 15,000 sexual intercourses
were reported. Among the 121 couples who used condoms irregularly, 12 new
cases of HIV resulted, a 4.8 incidence rate per 100 person-years. Couples
who used condoms more than half the time had approximately
the same number of HIV seroconversions among the spouses previously
HIV negative than among those who rarely used a condom.63 Most users of condoms are more worried about avoiding unwanted conception
than preventing an STD; however, this important personal and public
health aspect of contraception never should be relegated to a secondary
role. It is worthwhile to emphasize that the prevention of STDs may
prevent future infertility17,18,26,27,28,29,30 and cancer of the cervix.20,21,22,23,24 Barriers to condom use among women ages 15 to 30 years attending four
Planned Parenthood clinics were related to two restricting factors: pleasure
and intimacy, and low perceived need.64 Nonoxynol-9 (N-9), the spermicide used in most lubricated condoms, may
cause the release of a natural rubber latex protein that may increase
the chance of subjects having a hypersensitivity to latex develop. Natural
rubber latex protein levels leached from latex condoms vary from
brand to brand: condoms lubricated with N-9 have a fourfold to fivefold
increase of natural rubber latex protein compared to the same brand
without the spermicide.47 For latex-sensitive persons, even condoms without N-9 may cause an allergic
reaction.47,48 Latex allergies and urinary tract infections (UTIs) associated with barrier
contraceptive use are problems that only a few people experience. Although N-9 may protect against HIV infection, it is unclear whether the
genital irritation associated with latex allergy or N-9's local
effect may increase the individual risk of infection. Such sensitivity
may preclude correct use of condoms by susceptible persons; hence, they
may be exposed to infection because of failure to use condoms rather
than as a direct effect of using them. Condoms lubricated with N-9 have
been associated with an increased frequency of vaginal and UTIs
with Escherichia coli versus condoms without the spermicide.68,69 UTIs increased with frequency of condom exposure from 0.1 (95% confidence
interval, 0.65 to 1.28) for weekly or less during the previous month
to 2.11 (95% confidence interval, 1.37 to 3.26) for more than once
a week. Exposure to N-9-lubricated condoms produced a higher risk of UTI
with odds ratios increasing from 1.09 (95% confidence interval, 0.58 to 2.05) for
use weekly or less to 3.05 (95% confidence interval, 1.47 to 6.35) for
more frequent use.59 Data on pregnancy rates with condoms are limited. Reported condom failures
range from 1.2% in the United Kingdom to 60% in the Philippines, with
most studies between 4% and 14% in the first year.59 In the various National Surveys of Family Growth, high-parity women aged 25 to 34 years
who smoked and had used the method for less than 2 years
had a pregnancy risk of 14.7.13,70 The contraceptive effectiveness of the condom is acknowledged to be less
than that of oral contraceptives, hormonal injections and implants, and
the IUD; however, when condoms are used consistently and correctly, more
so with an adjuvant (e.g., contraceptive cream, jelly, foam, or
suppository) placed precoitally in the vagina, their use effectiveness
is very high. They may even match the use effectiveness of the pill, which
requires daily motivation, even when sexual activity is sporadic. Kestelman
and Trussell34,79 made a computer model of the use of condoms and spermicides simultaneously
and arrived at a probability failure during perfect use of both of 0.05%, which
is lower than that reported by perfect use of combined
oral contraceptives. Latex condoms fail most frequently because they are
not used than because of imperfections. Skin condoms made from the cecum of some lambs are also available. They
are preferred by some users, who claim higher sensitivity. Their thickness
is similar to that of rubber latex condoms (approximately 0.07 to 0.08 mm). They
are considerably more expensive and not as easy to find. No
use-effectiveness data are available on natural membrane condoms. They
represent less than 1% of the condoms sold. They must be kept
moist to prevent cracking, so they come with an excess of lubricant. They
are reliable for contraception and prevention of bacterial STDs, but
their ability to impede the passage of all types of viral STDs has
produced conflicting results in laboratory studies. They are considered
acceptable for the prevention of bacterial diseases but not reliable
for the prevention of most STDs of viral cause. Nonlatex condoms have been developed to obviate the problem with latex
allergy. Male synthetic condoms are manufactured using a dipped process
similar to latex condoms or a cut-and-seal process using a synthetic
elastomeric film. They are less elastic and wider than latex condoms, making
them less constrictive. Avanti is the only commercially available
synthetic male condom. It is made from Duron, a thermoplastic polyester
polyurethane. Avanti looks like a straight-sided, reservoir-tipped
condom. It is wider, approximately 65 mm when lying flat versus 52 mm
for the standard latex condoms. Avanti had a larger breakage rate than
latex condoms. Tactylon, another plastic condom made by the dipping
process from another synthetic elastomer, has been cleared by the FDA, but
it is not found commercially. Three Tactylon models were manufactured (standard, baggy, and with a wider closed end of 80 mm) to allow
greater comfort by diminishing glans constriction and to provide a more
elastic, standard shape condom. Tactylon also had a larger breakage
rate than latex condoms. Ezon, a synthetic elastomeric condom, not cleared
by the FDA, has a new design. Rather than being rolled on like
latex condoms, Ezon is slipped onto the penis; it can also be donned from
either end. Ortho McNeill Pharmaceutical of Canada and Carter-Wallace, Inc, are
two other companies involved in the manufacture of synthetic
elastomeric condoms. According most reports, they brake and slide
more often than latex condoms.71,72,73,74,75,76,77,78,79 It is unlikely that a physician will have to provide instructions on condom
use, but in some situations, as when dealing with the sexually uninitiated, full
discussion of this method, even its demonstration, may
prevent anguish and needless hardship. Occasionally, it may be important
to teach the social skills required to ensure condom use by a reluctant
partner. Condom use can be shown using one of the available wooden
or plastic models, a test tube of adequate size, or any other appropriate
object. Never assume that because proper condom use seems so obvious, it
will be used correctly. The following advice should be offered
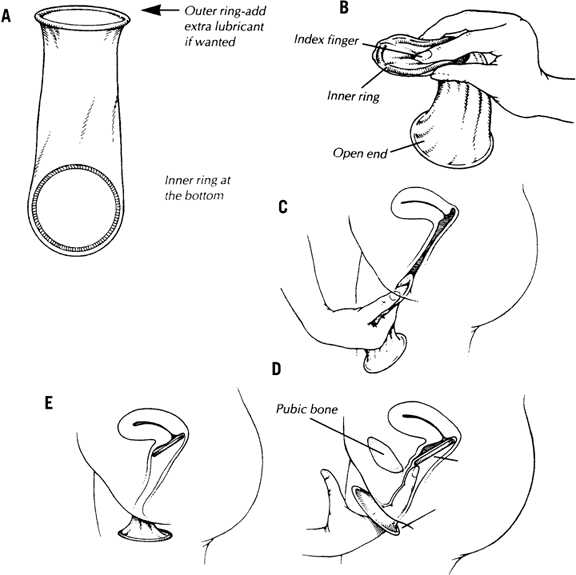
to the potential user. The condom should be applied over an erect penis
before any contact with the partner's body or genitals takes
place. If the man is uncircumcised, the foreskin should be pulled back
before condom placement. A small length may be unrolled over one or
two fingers and then placed on the penis. The rolling rim of the condom
should be outside. Care should be taken that no air is trapped at the
condom tip. The closed end of the condom should be pinched and held
with the fingers of one hand; this space allows for penile thrusts and
preserves glans sensitivity while allowing room for the ejaculate. The
rest of the condom is rolled completely over the shaft of the erect
penis. After ejaculation and before penile detumescence, the penis should
be withdrawn from the vagina while holding the sheath with the fingers
pressing against the base of the penis to keep it from slipping off
and to avoid spilling semen into the vagina. Buy condoms of good quality, packed in aluminum foil, preferably lubricated
with spermicide (unless allergic to them). Use a new condom every
time, and also if there is a suspicion that the condom may be damaged
or has not been placed properly, even while using it. Do not unroll the
condom or inflate it for testing, because very likely it will be ruined. All
condoms are tested electronically during manufacturing. Throw
away any condom that is or looks dry or brittle; one that seems to have
been damaged by fingernails, rings, or so forth during placement; or
one that was not placed correctly. After ejaculation, withdraw the
penis before it becomes soft while holding the condom against the base
of the penis; take special care to avoid spilling semen. If desired, after
intercourse, inspect the condom for tears or holes by urinating
into it before removal. Dispose of the condom in an appropriate, discreet
manner. |