The equipment, procedures, and hazards of laparoscopic sterilization have been dealt with elsewhere. The following is a review of the various approaches to tubal occlusion, along with a discussion of their specific advantages and disadvantages. These techniques are presented in chronologic order of their introduction in the medical literature.
Electrocoagulation
UNIPOLAR.
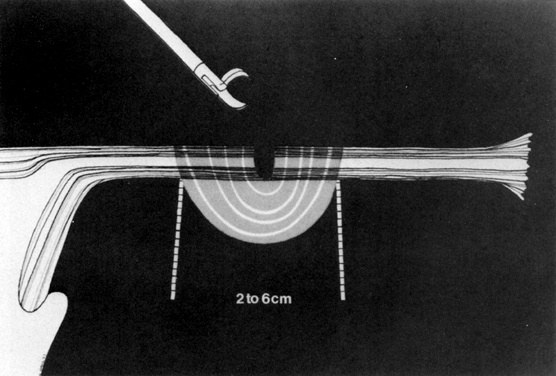
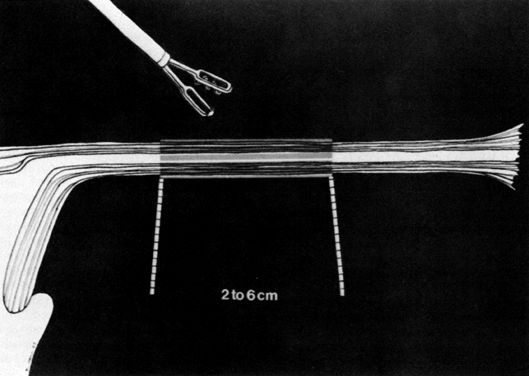
Although gynecologic laparoscopy was flourishing in Europe (only gastroenterologists were using the technique in North America), it was Anderson,4 an American, who in 1937 proposed laparoscopic electrocoagulation of the fallopian tubes to achieve female sterilization. However, it is Palmer5 of France who is credited with describing the modern technique of laparoscopic tubal electrocoagulation in 1962. The first instrument employed for this purpose was the Palmer biopsy forceps, because it was already adapted for electrosurgical capabilities. Powerful, grounded, high-voltage generators producing unipolar electric current were employed. Under such circumstances, the electrical energy is concentrated at the site where the fallopian tube is grasped by the jaws of the forceps. Once current is applied, it then has to travel through the patient's body, from whence it exits and is collected by a ground plate or return electrode to complete the electrical circuit. The original technique involved electrocoagulation of the tubal isthmus, followed by tubal transection and recoagulation of the cut edges (Fig. 1).6
|
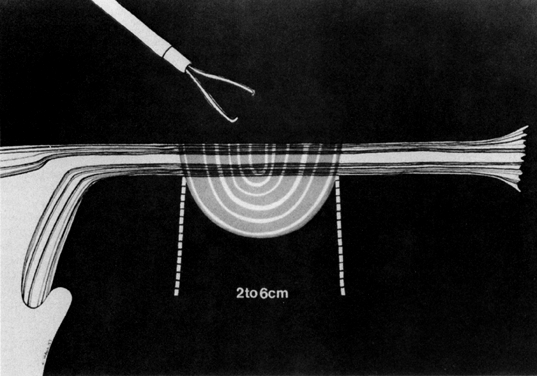
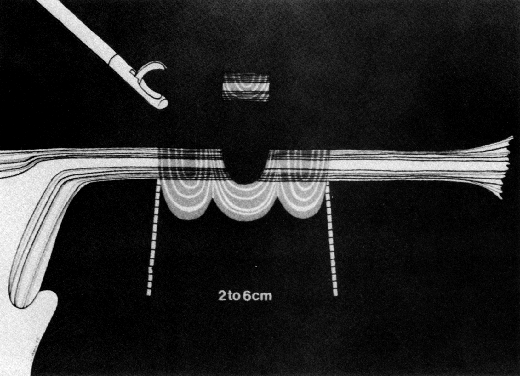
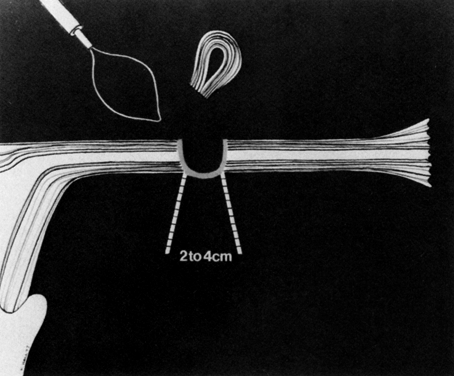
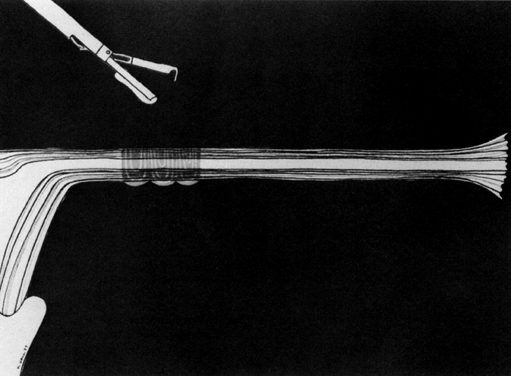
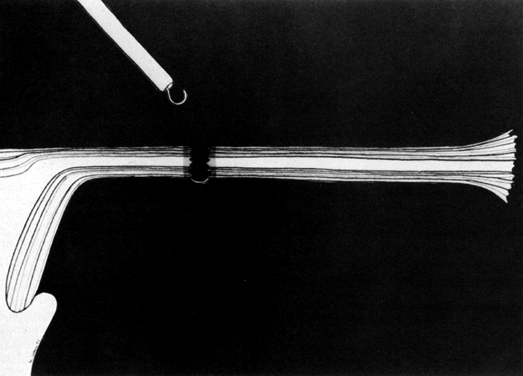
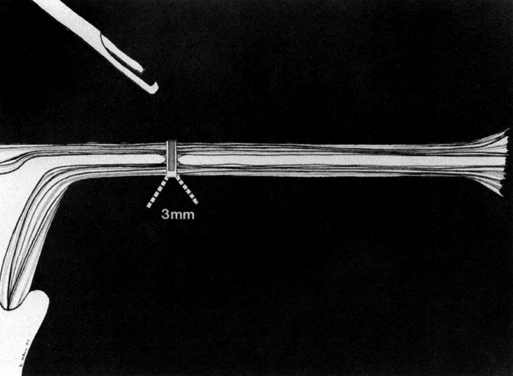
Some authors subsequently indicated that coagulation alone without tubal transection is equally effective, provided the surgeon is experienced and confident that the coagulation is complete (Fig. 2).7 Others recommended that a specimen of the tube be excised for pathologic confirmation.8 This step appears to have evolved as much for medicolegal reasons as for scientific ones. Unfortunately, the frequently charred tissue specimen cannot be identified as originating from the fallopian tube, thus defeating the primary purpose of the biopsy (Fig. 3). With this in mind, Soderstrom and Smith described a technique whereby a loop of tube could be passed through an electrified polyp snare.9 As the snare is squeezed tightly, current is applied; a loop of tube is mechanically excised and the tissue bed concomitantly coagulated. The excised segment, structurally unaltered, can then be evaluated pathologically as well as bacteriologically if indicated (Fig. 4). This method is technically complex and requires the use of an operating laparoscope as well as a second suprapubic puncture.
|
|
|
All of the laparoscopic procedures described up to this point employ the two-puncture technique (i.e., a viewing laparoscope introduced at the umbilicus, and an operating instrument introduced through a second, suprapubic puncture in the midline). In 1972, Wheeless10 adapted this method to the operating laparoscope, thus obviating the need for a second puncture. The one-puncture technique appears to be particularly suitable for use in conjunction with local anesthesia and requires few instruments with less surgical assistance.
Whether one or two punctures are employed, and regardless of the coagulation technique used (i.e., coagulation only, coagulation combined with tubal transection, or resection), it was discovered that the unipolar electrical system was associated with some inherent dangers. As early as 1973, Thompson and Wheeless11 reported 11 cases of bowel burns secondary to accidental electrical injury during the process of tubal electrocoagulation. Other reports followed, further emphasizing the fact that a potentially dangerous situation existed with the use of unipolar current.12 There generally was no rational explanation for such accidents and, as a consequence, it was difficult if not impossible to avoid them. We now know that some cases were simply secondary to faulty technique, but most were related to the inherent danger associated with high-voltage, grounded generators. Some of these units, when set in the coagulation mode, were capable of producing thousands of volts and hundreds of amperes of current. As the tubal tissue was being dehydrated, the tissue resistance increased, and the generator produced more current in an attempt to overcome this resistance. Today, more sophisticated generators are available; these are generally of solid-state construction, producing low-voltage, high-frequency current; they are not grounded, but rather isolated from ground. Although these have turned out to be much safer, some bowel burns unfortunately still occur.
Another and most logical theory to account for the bowel burns when a single puncture technique was employed is the role played by capacitative induced current. As we have seen, Wheeless popularized the use of the operating laparoscope for sterilization. If one adds to that the appearance of fiberglass sheathes through which the laparoscope is passed, one has all of the elements necessary to create an electrical capacitor. When high-frequency current is passed through the coagulating instrument within the operating channel of the laparoscope, up to 50% of the current is transferred or leaks to the laparoscope itself, despite the fact that the instrument is insulated. If a metal sheath is used rather than an insulating or fiberglass sheath, this current is dispersed within the abdominal wall, which is a much larger surface, and thus will cause no harm. However, if the fiberglass sheath or a metal sheath surrounded by a plastic anchor is used, the current will not be allowed to leak out and will instead charge the laparoscope, which now becomes a capacitor. This current can be discharged to a loop of bowel or other structure, should it come into contact with the laparoscope during the procedure without the knowledge of the operator.
BIPOLAR.
In 1973, after scattered reports of electrosurgical injury in the medial literature, Rioux and Cloutier13 devised the bipolar technique for tubal electrocoagulation. This method differed from the conventional unipolar system in that the operating forceps carried both the active and the return electrode.14
By completely isolating the two jaws of the forceps from one another, high-frequency current can be passed through one jaw and retrieved and returned to the generator through the other. The current thus passes selectively through the tissue grasped between the jaws of the forceps. Although high-frequency current is employed, it is required to travel only a short distance: that is, the thickness of the tube that is grasped within the jaws (1 to 2 mm). The bipolar procedure appears to be safer than the unipolar system, since the dangers of sparking are eliminated. Capacitative current is not produced because of a “canceling out” of the electric waves (i.e. the current enters and exits via the same instrument). Inadvertent electrosurgical injury to the bowel with bipolar forceps can, therefore, only occur if the bowel is grasped directly with the forceps. If such a situation were to occur, the complication would obviously be a result of surgical error as opposed to instrument failure. A further advantage of bipolar electrocoagulation is that the resulting burn is discrete and localized, selectively destroying only the tube that is in the grasp of the forceps jaws, leaving the mesosalpinx unaffected (Fig. 5).
|
At this point, a word of caution is essential: Bipolar instruments should be employed only with compatible electrosurgical units as recommended by the manufacturers, or with a generator possessing both limited output and isolated circuitry.
The complication rate for laparoscopy is directly proportional to the experience of the operator and his or her attention to the appropriate safety guidelines. Soderstrom and Butler15 achieved a significant reduction in the complication rate, from 13% to 3% within 1 year, after the introduction and implementation of an organized operative protocol. Electrosurgical complications have become considerably less frequent since the advent of the bipolar technique, as well as with the use of low-voltage, high-frequency generators. Furthermore, education and familiarization of physicians and operating-room personnel with electrosurgical instruments have resulted in fewer complications. Of further interest is the fact that Soderstrom and Butler15 demonstrated that many suspected bowel burns were actually the result of sharp trocar injuries, rather than electrical injuries.
Thermocoagulation
The technique of thermocoagulation originated in Germany in the 1970s. Frangenheim16 and Semm17 devised instruments that literally “cook” the fallopian tube by means of a small element heated by electric current (Fig. 6).
|
The advantage of this technique is that the current is markedly reduced from the exit potential of the standard electrical wall outlet and is therefore much safer. The apparatus employed for this thermocoagulation procedure requires no more than 4 or 5 V to produce the desired effect. With some of the sophisticated and automated thermocoagulation generators, the desired temperature and duration of current application may be preselected. Other generators provide an audible signal that varies according to the temperature of the heating element, indicating whether the instrument is in the heating or cooling cycle. Thermocoagulation now appears to be less dangerous than electrocoagulation; however, data regarding the efficacy of thermocoagulation are not yet available.
Electrocautery
The Waters Instrument Company18 devised another approach that employs the heating principle to produce coagulation as well as transection of the tube. This apparatus possesses a small, metallic hook that is heated by electric current produced in a battery-operated power source. The tube is grasped with the hook and pulled into an insulated outer sheath. The tube is coagulated and transected with hemostasis achieved by cauterization. Tissue destruction with this technique is minimal, but because sectioned ends of the tube remain in close proximity, recanalization may theoretically occur more easily than with some of the other techniques described (Fig. 7). Unfortunately, data for evaluating the true failure rate of this method are not available.
|
Mechanical Techniques
CLIP.
Two nonelectrical (mechanical) laparoscopic tubal occlusive techniques have become popular in the past few years. Hulka and colleagues19 developed the spring-loaded clip. Aware of the high failure rate associated with the use of a previously developed tantalum clip (up to 27% according to Wheeless20), yet still fascinated by the simplicity of the concept, Hulka and associates spent years developing a clip of Lexan (Richard Wolf Instrument Corp., Rosemont, IL) plastic jaws held in place by a gold-plated stainless steel spring, which is thought to reduce peritoneal reaction. The basic principle involved in this technique is that the plastic jaws with interlocking teeth leave no spaces. The maximal pressure on the tube is applied over a period of time, thus lessening the acute crushing effect that could result in fistula formation (Fig. 8). This plastic clip, as opposed to the tantalum clip, supposedly leaves no room for recanalization.
The original clip applicator was subsequently adapted to the operating laparoscope for single-puncture application. Later, Lieberman and co-workers,21 favoring the two-puncture technique, devised an appropriate applicator.
Another clip, devised by Filshie and colleagues22 and introduced into the United States in 1997 (more than 10 years after its introduction into Canada and many other countries), is made up of titanium jaws lined with silicone rubber. Locking of the device is achieved by a flattening effect of the upper jaw, which locks into the lower hooked jaw. This locking occurs through the mesosalpinx and is easily visualized at the time of application and enclosure of the clip. This confirms that the entire diameter of the tube has been included within the jaws. Visualization of the hooked end of the lower jaw can actually be confirmed before locking. When locked in place, the clip leaves no space at all between the jaws, thus achieving complete tubal occlusion. Compared to the Hulka clip, the Filshie clip appears to be easier to position properly before closure. In addition, because of its length, the Filshie clip can be placed on almost any fallopian tube, regardless of thickness or shape, including postpartum tubes.
FALOPE RING.
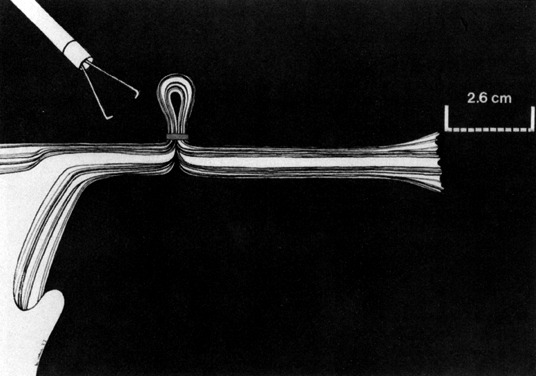
The Falope ring, devised by Yoon and King,23 is ingeniously simple. A loop of tube is drawn into the central hollow cylinder of the ring applicator forceps (single- or double-puncture technique) by gentle squeezing of the applicator handle. The loaded Silastic band is then forced down over the loop of tube by the forward action of an outer cylinder by a further squeezing of the applicator handle. This results in the release of the band from the applicator onto the loop of tube, thus achieving tubal occlusion. The portion of the tube encircled by the ring gradually becomes sclerosed, and the proximal and distal segments of tube become occluded (Fig. 9).
|
When the tubes are slender and lie free (i.e., are not involved in an adhesive or old inflammatory process), they are easily drawn up within the inner cylinder. When a thick or edematous tube is encountered, however, a slow “milking up” of the tube by means of a to-and-fro motion of the applicator handles is usually necessary in order to draw the tube into the hollow central cylinder. In doing so, the serosa may be damaged, or the tube may be totally transected (“click sign” of Levinson), thus increasing the risk of fistula formation, as with the Madlener technique. In addition, there is also the potential for bleeding from the transected tube.